Resarch Updated
Diunggah oleh
api-210258673Deskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Resarch Updated
Diunggah oleh
api-210258673Hak Cipta:
Format Tersedia
UNIVERSITY OF WISCONSIN-LA CROSSE Graduate Studies
GASTROINTESTINAL TOXICITIES FOLLOWING RADIATION THERAPY FOR PROSTATE CANCER DELIVERED BY 3D-CRT OR IMRT
A Research Project Report Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Medical Dosimetry
Krzysztof Karteczka
College of Science & Health Medical Dosimetry Program
June 2012
GASTROINTESTINAL TOXICITIES FOLLOWING RADIATION THERAPY FOR PROSTATE CANCER DELIVERED BY 3D-CRT OR IMRT
By Krzysztof Karteczka
We recommend acceptance of this project report in partial fulfillment of the candidate's requirements for the degree of Master of Science in Medical Dosimetry
The candidate has met all of the project completion requirements.
Nishele Lenards, M.S. Graduate Program Director
Date
The Graduate School University of Wisconsin-La Crosse La Crosse, WI
Author: Title:
Karteczka, Krzysztof. Gastrointestinal Toxicities Following Radiation Therapy for Prostate Cancer Delivered by 3D-CRT or IMRT
Graduate Degree/ Major: MS Medical Dosimetry Research Advisor: Nishele Lenards, M.S.
Month/Year: June 2012 Number of Pages: 45 Style Manual Used: AMA, 10th edition Abstract Prostate cancer in men is comparable to breast cancer in women; both cancers are ranked first, respectively, in incidence and are generally responsive to radiation therapy (RT). The advance and enhancement of prostatic specific antigen (PSA) has resulted in early detection of low-stage localized prostate cancers. This has created debate over the proper management of localized prostate cancer. To date there have not been any controlled, prospective, randomized trials of sufficient power to compare the various local therapies. Based on the current available data, the three commonly used local modalities: surgery, external beam radiation therapy and brachytherapy (radioactive seed implant) ensure similar efficacy governing the disease up to 10 years in many patients. Technological advances in treatment delivery and planning have improved the treatment of prostate cancer with external-beam radiotherapy using threedimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiation therapy (IMRT). The choice of the optimal RT treatment for the individual is still a topic of discussion, and the argument concentrates on two important outcomes namely, cancer control and reduction of side-effects. The aim of this paper is to compare acute and late gastrointestinal (GI) toxicities following radiation therapy for prostate cancer delivered either by 3D-CRT or IMRT.
The Graduate School University of Wisconsin - La Crosse La Crosse, WI
Acknowledgments A special thanks to Guru Prasad, as well to Pawel Dudziak, and Yuri Tabrizi from the Medical Physics Department of NorthShore University Health Systems for their knowledge, support, and patience.
Table of Contents .................................................................................................................................................... Page Abstract ............................................................................................................................................2 List of Tables ...................................................................................................................................4 List of Figures ..................................................................................................................................5 Chapter I: Introduction ....................................................................................................................6 Statement of the Problem ...................................................................................................11 Purpose of the Study ..........................................................................................................12 Assumptions of the Study ..................................................................................................12 Definition of Terms............................................................................................................12 Limitations of the Study.16 Methodology ......................................................................................................................16 Chapter II: Literature Review ........................................................................................................17 Chapter III: Methodology ..............................................................................................................32 Subject Selection and Description .....................................................................................32 Instrumentation ..................................................................................................................34 Data Collection Procedures................................................................................................34 Data Analysis .....................................................................................................................35 Limitations .........................................................................................................................35 Summary ............................................................................................................................35 Chapter IV: Results ............................................................................................................................ Item Analysis ........................................................................................................................ Chapter V: Discussion ....................................................................................................................... Limitations ............................................................................................................................ Conclusions ............................................................................................................................ Recommendations .................................................................................................................. References ......................................................................................................................................42
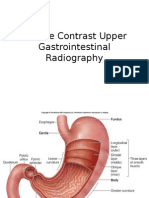
List of Figures Figure 1: The location of prostate and nearby organs................32 Figure 2: AP view of treatment borders.................32 Figure 3: Lateral view of treatment borders..33 Figure 4: 3D conformal treatment plan of a 6-field prostate.33 Figure 5: IMRT beam arrangements used for prostate irradiation: (a) 7-field technique; (b) conventional 5-field IMRT (CFIMRT) technique; (c) the opposed matched field (OMF) technique34
List of Tables Table 1: Primary tumor..35 Table 2: Regional lymph nodes.36 Table 3: Distant metastasis36 Table 4: Acute gastrointestinal complications according to the Radiation Therapy Oncology Group (RTOG)37 Table 5: Late gastrointestinal complications according to the Radiation Therapy Oncology Group (RTOG)37
Chapter I: Introduction Each year, the American Cancer Society evaluates the numbers of new cancer cases and deaths expected in the United States in the current year and collects the most recent data on cancer incidence, mortality, and survival.1 These numbers are based on incidence data from the National Cancer Institute, the Centers for Disease Control and Prevention, and the North American Association of Central Cancer Registries; mortality data is collected from the National Center for Health Statistics. A total of 1,638,910 new cancer cases and 577,190 deaths from cancer are projected to occur in the United States in 2012.1 Prostate cancer is the most common cancer diagnosed in North American men, apart from skin cancers. It is projected that in 2012, approximately 241,740 new cases and 28,170 prostate cancer-related deaths will take place in the United States. Prostate cancer is now the second leading cause of cancer death in men, exceeded only by lung cancer. It accounts for 29% of all male cancers and 9% of male cancer-related deaths.2 The annual detection rate of prostate cancer increased then declined corresponding with the greater than before use of PSA to screen for prostate cancer. For example, the estimated incidences were 99,000; 165,000; 334,500; and 179,300 in 1988, 1993, 1997 and 1999 respectively.2 Age-adjusted mortality rates have recently paralleled incidence rates, with an increase followed by a decrease in the early 1990s. It has been proposed that declines in mortality rates reveal the benefit of PSA screening, but others have suggested that these observations may be explained by independent phenomena such as improved treatment effects.2 The prostate is a gland in the male reproductive system, therefore prostate cancer is a disease which only impacts men. The word "prostate" derives from Medieval Latin prostate and Medieval French prostate. The ancient Greek word prostates means "one is standing in front", from proistanai meaning "set before". Therefore, the prostate is named because of its position - it lies at the base of the bladder.1 The prostate, approximate size of a walnut, is an exocrine gland of the male reproductive system, and it is located directly under the bladder, in front of the rectum.3 An exocrine gland is considered one whose secretions pass into a system of ducts that end up ultimately to the exterior of the body (like sweat glands or salivary glands). It is approximately the size of a walnut. The urethra - a tube that extends from the bladder to the end of the penis and transports urine and semen out of the body - passes through the prostate.2 There are thousands of tiny glands in the prostate creating a fluid that generates part of the semen. This fluid also protects and nurtures the sperm. When a male has an orgasm, the seminal vesicles
discharge a milky liquid in which the semen travels. The liquid is made in the prostate gland, while the sperm is produced and stored in the testicles. When a male climax, contractions force the prostate to secrete this fluid into the urethra and leave the body through the penis.2 The prostate gland is also engaged in urine control (continence) by the application of prostate muscle fibers. By contract and release, these muscle fibers control the flow of urine passing through the urethra.2 Figure 1 shows the location of the prostate gland and nearby organs. The epithelial cells in the prostate gland manufacture a protein called PSA. The role of the PSA is to keep the semen in its liquid state.4 Some of the PSA leaks into the bloodstream and it can be measured by blood test. High levels of PSA can indicate either prostate cancer or some kind of prostate condition (e.g. Benign Prostatic Hyperplasia). In the past, most experts regarded PSA levels less than 4 ng/ml as acceptable.4 Due to the outcomes of more recent studies, some suggested lowering the cutoff levels that defines if a PSA value is normal or elevated. Some researchers inspire using less than 2.5 or 3 ng/mL as a cutoff for normal values, especially for younger patients (<50 yrs old). Younger patients are likely to have smaller prostates and lower PSA values, so any elevation of the PSA in younger men above 2.5 ng/ml is a cause for concern.4 In the vast majority of cases, the prostate cancer originates in the gland cells - this is called adenocarcinoma.5 Prostate cancer is generally a very slow progressing disease. In fact, many men die of old age without ever knowing they had prostate cancer; it is only when an autopsy is done that doctors know it was there. Several studies have specified that about 80% of all men in their eighties had prostate cancer when they died but due to lack of symptoms, nobody knew about their disease.5 Experts declare that prostate cancer starts with tiny alterations in the shape and size of the prostate gland cells - Prostatic Intraepithelial Neoplasia (PIN). Prostatic Intraepithelial Neoplasia means dysplastic changes involving glands and ducts of the prostate that may be a precursor of adenocarcinoma.5 Low grade (PIN 1) is characterized by mild dysplasia with cell crowding, variation in nuclear size and shape, and irregular cell spacing. High grade (PIN 2 and 3) is described by moderate to severe dysplasia with cell crowding, nucleomegaly, and irregular cell spacing. Any man who scored high-grade PIN after a prostate biopsy is considered at a considerably greater risk of having cancer cells in his prostate.5 It is important to recognize the stage of the cancer, or how far it has spread. Staging benefits the doctor in describing prognosis and assists in the selection of proper therapies. To
date, the most common system for determining cancers stage is the TNM (Tumor/Nodes/Metastases) System. It includes defining the size of the tumor, how many lymph nodes are involved, and whether there is any other metastases.6 There are many ways to observe whether the cancer has spread. Computed Tomography (CT) can show disease spread inside the pelvis, bone scan can display whether the cancer has spread to the bones, and endorectal coil magnetic resonance imaging will assess the prostatic capsule and the seminal vesicles.6 At present, there is no standard imaging modality that is reliable in demonstration of stage of prostate cancer. PSA, digital rectal examination (DRE), transrectal ultrasound (TRUS) and TRUS-directed sextant biopsies remain currently the diagnostic procedures of choice for the clinical staging of patients with potentially organ-confined cancer of the prostate.6 Prostate cancer treatments are determined based on PSA levels and the tumor grade. It has been suggested that prostate tumor grade is one of the most important prognosticators in treatment outcome.7 The most commonly used grading system is the Gleason score system. A pathologist will examine the biopsy samples under a microscope. If cancer tissue is identified, the pathologist then grades the tumor. The Gleason System of grading goes from 2 to 10. The higher the number, the more abnormal the tissues are compared to normal prostate tissue.6 Two numbers are added up to get a Gleason score: a number from 1 to 5 for the most common pattern observed under the microscope (this is the predominant grade and must be more than 51% of the sample), and another number from 1 to 5 for the second most common pattern (this is the secondary grade and must make up more than 5% but less than 50% of the sample).6 Unfortunately, there is no standard single best treatment for localized prostate cancer, as each patient characteristic remains unique and diverse. The current treatment options for localized prostate cancer consist of surgery, radiation therapy, hormonal manipulation, and observation as well as various combinations thereof. The most common RT treatment for prostate can be achieved by external beam or brachytherapy by radioactive seed implant.6 By far, external beam radiation therapy remained the prevailing form of radiation treatment for adenocarcinoma of the prostate in the past 30 to 40 years.7 Recently, a rising number of patients have been treated with 3D-CRT techniques due to advancements in technology and treatment planning systems. While the relative ratio of patients treated with radical prostatectomy has expanded over the past 10 years, the total number treated with radiation therapy also continues to rise. Possible causes for the persistent use of this modality are numerous but include medically
non-surgical candidates, relatively low morbidity, cost, preservation of normal sexual function in some patients, less time lost from work, and patient preference.7 There are two broad categories of radiation therapy; both are intended to target the tumor while minimizing exposure to the healthy surrounding tissue. In the first category, the radiation is administered much like an x-raya procedure called external beam radiation therapy (EBRT). This type of radiation therapy is generally administered by a machine called a linear accelerator, or linac. The second category is brachytherapy, or internal radiation therapy. Brachytherapy fights cancer by placing radioactive sources directly into or next to the treatment area. Three-dimensional conformal radiation therapy, or 3D-CRT, is a type of external beam radiation therapy that utilizes computers and special imaging techniques to accurately shape the radiation beams so that nearby normal tissue receives less radiation and is capable to repair more quickly.8 Depending on computerized three-dimensional images of the prostate, bladder and rectum, the x-ray radiation beam is shaped to conform to the prostate gland. In this way, less radiation reaches the surrounding normal tissues. Intensity modulated radiation therapy, or IMRT, is a newer, specific form of 3D-CRT that permits radiation to be more exactly designed to fit the tumor and further limits the amount of radiation received by healthy tissue near the tumor. IMRT utilizes non-uniform beam intensity patterns with computer-aided optimization to achieve superior dose distribution. Because of this new ability of manipulating the intensities of individual rays within each beam, IMRT allows greater control of dose distribution that, when integrated with different image guided techniques to precisely delineate target volumes and deliver the planned treatments, may improve tumor control and reduce normal tissue toxicity. This can also safely permit a higher dose of radiation to be deposited in the tumor, and potentially enhance the chance of a cure.8 IMRT permits for the radiation dose to conform more accurately to the three-dimensional shape of the tumor by modulatingor controllingthe intensity of the radiation beam in multiple small volumes. In particular, IMRT is able to create concave-shaped isodose distribution that can more closely conform the shapes or boundaries of the target and critical structures in three dimensions. In contrast, the isodose distribution intended for 3D-CRT plans are convex, which can be suboptimal in treating certain disease sites. IMRT also allows higher radiation doses to be absorbed to regions within the tumor while minimizing the dose to surrounding normal critical structures. Treatment is carefully planned by using CT or MRI images of the patient in conjunction with computerized dose calculations to define the dose
intensity pattern that will best conform to the tumor shape. Typically, combinations of multiple intensity-modulated fields coming from different beam directions produce a custom tailored radiation dose that maximizes tumor dose while also minimizing the dose to adjacent normal tissues.8 The continuing improvements in computer hardware, software and radiotherapy equipment have enabled the employment of sophisticated 3D-CRT techniques permitting accurate delivery of higher doses of external beam radiation to the prostate with steep drop-off of the dose to adjacent organs. Thus, the incidence of treatment related side effects stands lower with 3D-CRT techniques than with conventional techniques for the same given therapeutic doses of radiation to the tumor. To minimize some of the limitations of 3D-CRT, the more complex conformal therapy utilizes modulated beam intensity in addition to beam shaping to attain preferred dose distribution. This intensity modulated radiation therapy (IMRT) technique includes the use of non-uniform dynamic radiation beams of various intensities to reach conformal dose distributions. Another common form of RT treatment of prostate cancer is brachytherapy. During brachytherapy radiation treatment is delivered to the prostate via the location of radioactive materials inside the prostate. There are two forms of brachytherapy; including low-dose rate (LDR) and high-dose rate (HDR).3 LDR brachytherapy or permanent seed implant treatment uses up to one hundred small radioactive seeds that are inserted into the prostate gland through hollow needles under ultrasound guidance. These radioactive seeds distribute radiation continuously over a period of several weeks to months then decay to be inactive. These seeds remain in the prostate forever. HDR brachytherapy technique was developed to supplement external beam therapy to treat patients with high risk prostate cancer. Patients receive about five weeks of external beam radiation therapy, followed by one to three HDR brachytherapy sessions. In this treatment, the radiation is delivered into the prostate via radioactive isotopes (often, Iridium-192) temporarily. When the patient undergoes treatment, 12 to 18 previously inserted catheters are connected to the HDR machine, which regulates the delivery of the Iridium-192 radioactive source to the specific areas in each of these catheters. The treatment usually lasts about 10 to 20 minutes per session, and the patient typically receives three to four sessions over a two-day period.3
Treatment and screening studies mostly pay attention to their reports on survival outcomes, but to advise our patients, we must also acquire high-quality data regarding treatment related toxicities. Unwanted side-effects from radiation therapy to the prostate might be irreversible and reduce quality of life. Evaluating toxicity is therefore essential in evaluating the therapeutic benefit of radiation therapy and planning treatment for future patients.9 Common acute radiation related GI toxicity includes bowel urgency, frequency, diarrhea, and presence of rectal blood. Late RT-generated GI toxicity consists of proctitis (manifested by rectal frequency, urgency, and bleeding), obstruction, and necrosis.9 It is acknowledged that radiotherapy dosimetric factors, such as total dose, dose per fraction, volume irradiated, irradiation site and dose inhomogeneity, effect the development of late radiation toxicity.9 Other factors, either environmental or genetic, can also influence the development of late toxicity. Examples of such factors involve additional treatment (e.g. the use of systemic treatment or surgery) and patient characteristics (age, smoking history, body mass index, hemoglobin level and co-morbid conditions, such as diabetes, hypertension, vascular and connective tissue diseases).6 It is significant to account for the effect of such factors in the analysis of all trials of radiotherapy. Initially it might help in the choice of treatment modality, e.g. radical prostatectomy, external beam radiotherapy, brachytherapy or active surveillance. For patients who receive radiation therapy, the likelihood of late toxicity may help in presenting planning corrections to better individualize RT treatments.6 Statement of the Problem There is no standard single best treatment for localized prostate cancer, as each patient characteristic remains unique and diverse. The current treatment options for localized prostate cancer consist of surgery, radiation therapy, hormonal manipulation, and observation as well as various combinations thereof. External beam radiation therapy remained the prevailing form of radiation treatment for adenocarcinoma of the prostate in the past 30 to 40 years. However, unwanted side-effects from radiation therapy to the prostate might be irreversible and reduce quality of life. Evaluating toxicity is therefore essential in evaluating the therapeutic benefit of radiation therapy and planning treatment for future patients. Acute and late GI toxicity rates after radiation therapy for prostate cancer are substantial and have been the topic of many studies. It is suggested that the dose conformity perceived with IMRT is considerably enhanced with better sparing of critical uninvolved surrounding structures contrasted with that seen with the 3D-CRT
technique. IMRT may potentially be superior to 3D-CRT in permitting dose escalation without higher rates of toxicity and morbidity. Purpose of the Study The purpose of this study is to compare acute and late toxicities of high dose radiation therapy delivered either by 3D-CRT or IMRT. The outcomes of this study may potentially help patients in the choice of treatment modality. Additionally, understanding the likelihood of late toxicity can help in presenting planning corrections to better individualize RT treatments. Assumptions of the Study From the literature review, the dose conformity perceived with IMRT is substantially better because of improved sparing of critical uninvolved surrounding structures compared with that established with the 3D-CRT technique. IMRT may potentially be superior to 3D-CRT in permitting dose escalation without higher rates of morbidity. Definition of Terms Analytical Anisotropic Algorithm (AAA). AAA is the dose calculation algorithm. The AAA algorithm calculates how the radiation will behave as it interacts with different kinds of tissues, and accounts for the electron scatter and resulting dose effects.3 Biopsy. A medical test commonly performed by a surgeon or an interventional radiologist involving sampling of cells or tissues for examination. 4 Benign Prostatic Hyperplasia (BHP). Benign Prostatic Hyperplasia is an increase in size of the prostate. BHP is not a cancer but must be treated. 4 Brachytherapy. It is a form of radiotherapy where a radioactive source is positioned inside or next to the area requiring treatment. 6 Computed Tomography (CT). Computed tomography is a radiologic imaging technique that uses computer processing to generate an image of tissue density in slices through the patient's body. CT scans use x-rays aimed from multiple, different points around the patient to obtain their images. CT simulation refers to the process process by which the radiation treatment fields are defined, filmed and marked out on patients skin. CT simulator is competent of scanning in the treatment position and linked to a radiotherapy treatment planning system. The final plan would incorporate all the 3-dimensional information obtained by the scan. 6 Cone Beam CT (CBCT). CBCT is a tomographic scanning technology that can scan and acquire a specified volume of the patient and generate a 3D data set. CBCT technology found on
linear accelerators has enabled three dimensional imaging of the patient in the treatment position.3 Digital Rectal Examination (DRE). A digital (finger) rectal examination is done for men as part of a complete physical examination to check the prostate gland.6 Doppler Ultrasonography. Doppler ultrasonography is a non-invasive diagnostic procedure that changes sound waves into an image that can be viewed on a monitor. Sonography can be enhanced with Doppler measurements, which employ the Doppler effect to assess whether structures (usually blood) are moving towards or away from the probe, and its relative velocity.10 Dose Volume Histogram (DVH). The purpose of a DVH is to summarize 3D dose distributions in a graphical 2D format. Bin doses are along the horizontal axis, and structure volumes (either percent or absolute volumes) are on the vertical. DVH allows the clinician to evaluate the uniformity of the dose to the tumor and sparing of healthy structures.11 Elastography. Elastography is a non-invasive method in which stiffness or strain images of soft tissue are used to detect or classify tumors. A tumor or a suspicious cancerous growth is normally 5-28 times stiffer than the background of normal soft tissue.10 Endorectal coil magnetic resonance imaging. Endorectal coil magnetic resonance imaging (MRI) is a type of medical imaging in which MRI is used in conjunction with a coil placed into the rectum in order to acquire high quality images of the area surrounding the rectum. The technique has revealed higher accuracy than other modalities in assessing seminal vesicle invasion and extra-capsular extension (ECE) of prostate cancer.6 Exocrine glands. Exocrine glands are glands whose secretions pass into a system of ducts that lead ultimately to the exterior of the body.5 External Beam Radiation Therapy (EBRT). EBRT is a method for delivering a beam or several beams of high-energy x-rays to a patient's tumor site. Beams are produced outside the patient (usually by a linear accelerator) and are targeted at the tumor site.8 Gleason Score. Most pathologists grade prostate cancer according to the Gleason score, which assigns a grade from 1 to 5 based on how the cancerous cells look compared to normal prostate cells. The grade refers to the cancer's appearance and indicates how quickly a cancer is growing.7
Gray (Gy). Gray is an SI (International System of Units) unit of radiation that refers to the dose of ionizing radiation. One gray is equal approximately to the absorbed dose delivered when the energy per unit mass imparted to matter by ionizing radiation is one joule per kilogram.15 Harmonic Ultrasound Imaging. The basic theory of harmonic imaging is that body tissue reflects ultrasound signals at twice the scanning frequency - the second harmonic of the scanning frequency.10 Histology. The study of the form of structures seen under the microscope.2 Heterogeneity. In radiation therapy, heterogeneity is referred as non-uniform tissue density. The heterogeneous composition of the human body presents numerous tissues types and cavities with widely differing radiologic properties. These include air cavities, lungs, bones, and fat, which attenuate and scatter the beam differently.3 Inflammatory bowel disease (IBD). IBD is a group of chronic intestinal diseases characterized by inflammation of the bowel -- the large or small intestine. The most common types of inflammatory bowel disease are ulcerative colitis and Crohn's disease.8 Intensity-modulated radiation therapy (IMRT). IMRT is an advanced type of highprecision radiation therapy that uses computer-controlled linear accelerators to deliver highly conformal radiation doses to a malignant tumor or specific areas within the tumor. IMRT permits for the radiation dose to conform more precisely to the three-dimensional (3-D) shape of the tumor by modulatingor controllingthe intensity of the radiation beam in multiple small volumes. IMRT also lets higher radiation doses to be aimed to regions within the tumor while minimizing the dose to surrounding normal critical structures.8 Linear accelerator. The linear accelerator utilizes microwave technology (similar to that used for radar) to accelerate electrons in a part of the accelerator called the "wave guide," then allows these electrons to collide with a heavy metal target. As a result of the collisions, highenergy x-rays are produced from the target. These high energy x-rays are shaped as they exit the machine to conform to the shape of the patient's tumor and the customized beam is directed to the patient's tumor.3 MRI Spectroscopy (MRS). MRS is a special technique used for characterization of the biochemistry of tumors, infarcts, and other pathology. MRS is useful for demonstrating aspects of physiology such as tumor aggressiveness and anaerobic metabolism.10
Multileaf Collimator (MLC). MLCs are located in the head of the treatment machine in which they replace the simple rectangular jaw system on the treatment machine with a set of thin blades that can be individually positioned, under computer control.3 Necrosis. Necrosis is death of body tissue. It occurs when there is not enough blood supplied to the tissue, whether from injury, radiation, or chemotherapy. Necrosis is not reversible.9 On Board Imager (OBI). A linear accelerator fitted with an on board imager (OBI), allows imaging of the treatment field before and during the delivery of radiotherapy. This allows radiotherapy to be adjusted before each treatment session starts, using the images obtained from the machine, and hence deliver optimal treatment to the patient.3 Prostatic Intraepithelial Neoplasia (PIN). PIN is a noncancerous growth of the cells lining the internal and external surfaces of the prostate gland. Having high-grade prostatic intraepithelial neoplasia may increase the risk of developing prostate cancer.5 Proctisis. Proctitis is inflammation of the lining of the rectum, the lower end of the large intestine leading to the anus.9 Prostate-specific antigen (PSA). PSA is a protein manufactured by the prostate gland that may be found in elevated levels in the blood when a person develops certain diseases of the prostate, notably prostate cancer. PSA is specific, because it is present only in prostate tissue. It is not specific for prostate cancer, however, as it may also be elevated in men with benign enlargement of this organ.4 Prostatectomy. Prostatectomy is a surgical removal of the prostate.7 Radioimmunoscintigraphy. One of the most promising approaches for imaging cancer is the increasing use of radioimmunoscintigraphy. Distinctly different from anatomic imaging, with its size criteria, radioimmunoscintigraphy detects signal from a radiolabeled antibody that recognizes prostate tissue.10 Radiation Therapy Oncology Group (RTOG). The Radiation Therapy Oncology Group (RTOG) is a national clinical cooperative group funded by the National Cancer Institute (NCI) since 1968 to increase the survival and improve the quality of life of patients diagnosed with cancer.8 Three-dimensional conformal radiation therapy (3D-CRT). Three-dimensional conformal radiation therapy, or 3D-CRT, is a type of external beam radiation therapy that utilizes
computers and special imaging techniques to accurately shape the radiation beams so that nearby normal tissue receives less radiation and is capable to repair more quickly.8 TNM . TNM is a cancer staging system that describes the extent of cancer in a patients body. T describes the size of the tumor and whether it has invaded nearby tissue, N describes regional lymph nodes that are involved, and M describes distant metastasis (spread of cancer from one body part to another).6 Transrectal ultrasound (TRUS). TRUS, also called prostate ultrasound, provides images of the prostate and surrounding tissue and allows the physician to examine the gland for abnormalities.6 Treatment Planning System (TPS). A computerized treatment planning system, TPS, is an essential tool in the design of a RT treatment of cancer patients. A typical installation of a TPS is in a RT network, virtual or real, in which it functions together with other systems required for patient treatment.3 Limitations of the Study A limitation of this study includes the same planned target margins around the prostate for the 3D versus IMRT techniques, and limited DVH data. For all plans the PTV was defined by adding a margin of 10 mm from the CTV. A 10 mm margin takes into account possible movements of the CTV, systematic and random set-up errors. It has been considered as sufficiently safe, given the incidence of systematic and random set-up errors. Another limitation of this study, since severity of the majority of GI symptoms (e.g. rectal bleeding or rectal mucous) is difficult to quantify, the standards of reporting results without adequate assessment of baseline characteristics (patient reported vs. physician reported) were uneven. Methodology External beam radiation therapy remains the prevailing form of radiation treatment for adenocarcinoma of the prostate. This study analyzed extends to gastrointestinal toxicities of patients treated with 3D-CRT or IMRT. This retrospective study will be based on studying previous results of treatments of patients between 65 and 85 year of age, treated with conformal fields. The Radiation Therapy Oncology Group (RTOG) acute and late toxicity scales will be used to score GI morbidity. Data from previous studies published in various radiotherapy journals will be analyzed. Moreover, treatment data of 10 anonymous patients treated between 2009 and 2011 at NorthShore University Heath Systems will be evaluated.
Chapter II: Literature Review Prostate cancer is the most common cancer diagnosed in North American men, apart from skin cancers.2 It is projected that in 2012, approximately 241,740 new cases and 28,170 prostate cancer-related deaths will take place in the United States. Although the introduction of PSA testing has led to a stage migration toward less-advanced disease at presentation, men with locally advanced or high-risk prostate cancer still institute approximately 17% of all newly diagnosed cases.4 There is no standard single best treatment for localized prostate cancer, as each patient characteristic remains unique and diverse. The current treatment options for localized prostate cancer consist of surgery, RT, hormonal manipulation, and observation as well as various combinations thereof.6 Recently, a rising number of patients have been treated with 3DCRT techniques due to advancements in technology and treatment planning systems.7 However, unwanted side-effects from radiation therapy to the prostate might be irreversible and reduce quality of life. This study will compare acute and late toxicities of high dose radiation therapy delivered either by 3D-CRT or IMRT. To better understand these techniques and their outcome, it is important to become familiar with the fundamental principles and techniques that are presently being employed in treatment planning for patient diagnosed with a prostate cancer. Moreover, understanding the likelihood of late toxicity associated with radiation therapy treatment can potentially help patients in the choice of treatment modality and in presenting planning corrections to better individualize RT treatments.9 This chapter will include discussion on cancers stage, imaging techniques, application of EBRT, target volume and normal tissue definition, and gastrointestinal toxicity following radiation therapy. Imaging techniques Because researchers have analyzed cancer staging based on imaging techniques, it is important to recognize the stage of the cancer, or how far it has spread. Knowledge of the cancers stage benefits the doctor in describing prognosis and assists in the selection of proper therapies. To date, the most common system for determining cancers stage is the TNM (Tumor/Nodes/Metastases) System. It includes defining the size of the tumor, how many lymph nodes are involved, and whether there is any other metastases.6 The current staging system is based on the American Joint Committee on Cancer (AJCC) Staging System.6 Tables 1, 2, and 3 illustrates the TNM staging system.
According to Tony Eng et al7 imaging techniques, like TRUS, CT, and MRI, have enhanced progressively and supplied useful information. However, as authors noted, each has specific restrictions by itself and precise diagnosis and staging of prostate cancer with any single imaging modality are still incomplete. TRUS is a low-cost simple procedure and continues to be one of the vital parts of prostate cancer evaluation. CT is less operator-dependent and provides size, density and symmetry information and is fairly cost effective in staging prostate cancer whereas MRI provides better specific detailed architectural information of the prostate gland, its border, in particular the prostatic apex, and adjacent organs.7 Some of the new emerging modalities, including color and power Doppler ultrasonography, ultrasound contrast agents, intermittent and harmonic ultrasound imaging, MR contrast imaging, MRI with fat suppression, MRI spectroscopy, three-dimensional MRI spectroscopy imaging (MRSI), elastography, and radioimmunoscintigraphy has been lately studied by el-Gabry and associates.10 Although most of these newer imaging techniques are restricted in availability and necessitate further confirmation, el-Gabry has confirmed that combining conventional MRI findings with metabolic abnormalities provided by MRSI is able to considerably enrich cancer localization and assessment of its spread outside the prostate, thus upgrade the chance diagnosis, staging, and treatment planning for patients with prostate cancer.10 At present, there is no standard imaging modality that is reliable in demonstration of stage of prostate cancer. PSA, DRE, TRUS and TRUS-directed sextant biopsies remain currently the diagnostic procedures of choice for the clinical staging of patients with potentially organconfined cancer of the prostate.6 External Beam Radiation Therapy As stated earlier, EBRT remained the prevailing form of radiation treatment for adenocarcinoma of the prostate in the past 30 to 40 years. EBRT includes immobilization, simulation, treatment planning, and sometimes verification before actual treatment. During a conventional simulation the patient lays down in a supine (or prone to push the small bowels upward out of the pelvic fields) position that is easily to reproduce. Sometimes, custom immobilization is necessary, especially if a small field is treated. Contrast media for urethra (retrograde urethrogram), bladder and rectum may be necessary to pinpoint the prostate radiographically. 3D-CRT is used to treat tumors that in the past might have been measured too close to vital organs and structures for radiation therapy. For example, 3D-CRT lets radiation to
be delivered to head and neck tumors in a way that minimizes exposure of the spinal cord, optic nerve, salivary glands and other important structures. In prostate cancer treatments the rectum, bladder, and femoral heads can be spared. Typically, a 4-field box technique with two opposing lateral (LAT), anterior-posterior (AP) and posterior-anterior (PA) fields was used most often.11 Figures 2 and 3 shows standard 4 field technique treatment field borders. Other 3D-CRT techniques for prostate were generated using a conventional three-field (AP, RAO, LAO), sixfield (LAT-LAT, RAO, LAO, RPO, LPO), and eight-field (LAT-LAT, AP, PA, RAO, LAO, RPO, LPO) coplanar approach.11 In the 1970s, medical linear accelerators were invented and developed.2 Linear accelerators could deliver high doses of radiation therapy to deep parts of the body, while sparing the superficial tissues. Even though the high energy X-ray beams that save superficial normal tissues, organs adjoining to the cancer often obtained large volumes of high radiation dose. This large treatment volume was necessary to guarantee that the dose to a tumor was always delivered. If too small radiation fields were employed to avoid treating the healthy tissues, there was a risk that the radiation would not adequately cover the tumor.3 Many radiation oncologists, and their patients, would agree to the extra radiation dose to the normal adjacent organs in order to secure adequate coverage of the prostate target volume. This extra radiation is mostly accountable for the side effects and complications that go together with high dose curative radiation therapy. In order to maintain the complication rates at tolerable level, most physicians hold the total radiation dose to a moderate and safe range. There is evidence that higher radiation doses may cure more prostate cancers but this would be at too great a risk with conventionally fractionated and planned radiation therapy.12 However, the ongoing improvements in computer hardware and software and radiotherapy equipment have enabled the application of sophisticated 3-D conformal treatment techniques permitting precise delivery of higher doses of external beam radiation to the prostate with steep drop-off of the dose to adjacent organs. As Heemsbergen, Peeters, and Koper et al 13 discussed, the possibility of treatment related side effects are lower with 3-D conformal techniques than with conventional techniques for the same given therapeutic doses of radiation to the tumor.13 The common techniques of 3D-CRT could include the use of multiple static fields, custom blocks, sophisticated patient immobilization devices, and CT-based simulation and 3Dtreatment planning systems with beam's eye view. The use of beam's eye view permits discrete
selection of beam directions, field sizes and shapes to conform to the shape of the target and minimize radiation dose to surrounding critical normal structures.13 Figures 4a and 4b illustrates beams-eye-view of 3D conformal treatment plan of prostate. To minimize some of the limitations of 3D-CRT, the more complex conformal therapy employs modulated beam intensity in addition to beam shaping to accomplish desired dose distribution. This modulated RT was studied by Iwamoto and Maher 14, who described that IMRT involves the use of non-uniform dynamic radiation beams of various intensities to reach conformal dose distributions. Most commonly IMRT to the prostate used either a 5-field coplanar plan consisting of an anteriorposterior (AP) and four oblique fields (e.g. 45, 135, 225, and 315), or seven-field coplanar plan consisting of an AP and six oblique fields (40, 80, 120, 240, 280, and 320) Figure 5 shows IMRT beam arrangement for prostate irradiation. The dose conformity perceived with IMRT is significantly improved with better sparing of critical uninvolved surrounding structures compared with that witnessed with the 3DCRT technique. IMRT may potentially be superior to 3D-CRT in allowing dose escalation without increased morbidity.14 Target volume and normal tissue definition Three-dimensional treatment planning usually starts with the patient obtaining an imaging scan to generate a detailed 3 dimensional illustration of the tumor and surrounding organs.3 A treatment planning computer will collect these CT images into a virtual patient. The role of radiation oncologist is to delineate the tumor volume as well as the other organs whose dose tolerance will put limitations on the treatment planning process. The latter process was discussed by the International Commission on Radiation Units & Measurements (ICRU), who released the documents Report no. 50 and 62. The ICRU recommends certain volumes be identified in an external photon beam treatment plan.15 First, the gross tumor volume (GTV) must be identified. Because of the risk of multi-focal disease, GTV usually contains the whole prostate. Then the radiation oncologist will add a safety margin in relation for microscopic spread of disease. It is documented from surgical data that cancer cells can spread outside the prostate to the seminal vesicles even when the tumor is restricted to the gland.2 The safety margin for cancer spread is called the clinical target volume (CTV). CTV helps the radiation oncologist to be certain that the areas of local spread will not be underdosed.15 Another margin needs to be added to the clinical target volume to account for setup variation and internal organ
motion. Because patients are living and breathing human beings, it is impossible to control their movements down to the exact millimeter. Also, even with the application of an immobilization device, there is still some dissimilarity with daily treatment setup. A margin of 5-10 mm is generally added to the CTV to adjust for the deviation in positioning of the target volume and patient. This is called Planned Target Volume (PTV).15 These margins provide the radiation oncologist with a superb view of patients anatomy in three dimensions and can choose the best way to tailor the radiation beam to the size and shape of the tumor while, at the same time, spare as much of the normal tissue as possible. The field shaping is prepared with multi-leaf collimators (MLCs) or custom fabricated field shaping blocks. With such complex and detailed treatment planning the dose to the normal tissues surrounding the tumors is minimized which reduces the risks of complications.3 Gastrointestinal toxicity following radiation therapy Several studies have demonstrated that higher doses of RT improve local tumor control; however, dose escalation is ultimately limited by the occurrence of side-effects. According to Lars Budaus et al8, GI complications are the most frequently considered end points in the published analyses, with rectal bleeding accounting for the majority of late GI toxicity. Because of its objectivity, rectal bleeding is sometimes the singular end point of the treatment, but the Radiation Therapy Oncology Group (RTOG) toxicity scale is also frequently used. Table 4 and 5 illustrates acute and late GI toxicity caused by radiation therapy treatment. Fatigue is a common side effect for several months following radiation therapy. Short-term effects include nausea and loss of appetite, increased frequency or change in quality of bowel habits not requiring medication, diarrhea, severe mucous or blood discharge, abdominal distension, obstruction, fistula, or perforation. Common late toxicity include cramping, bowel movements 25 per day, rectal discharge or bleeding, necrosis, fistula, abdominal pain or tenesmus requiring tube decompression or bowel diversion.8 As stated earlier the most commonly measured functional end points in the published analyses are GI complications and rectal bleeding. Recognized risk factors for acute or late toxicities after RT include advanced age, larger rectal volume, and history of prior abdominal surgery, the concomitant use of androgen deprivation, preexisting diabetes mellitus, hemorrhoids, and inflammatory bowel disease (IBD).8 In general, according to Bentel11, the
tolerance of an organ to the radiation is inversely proportional to the volume of the organ irradiated. Dose Escalation Similarly, Dearnaley et al16 testified that frequency and severity of GI complications are raised with increased dose and volume treated. The researchers tested the effects on tumor control and side effects of escalating radiotherapy dose and investigated the appropriate target volume margin. The initial tumor target volume encompassed the prostate and base of seminal vesicles. Treatments were randomized to deliver a dose of 64 Gy with either 1.0 or 1.5 cm margin around tumor volume and also to treat either with or without a 10 Gy boost to the prostate alone with no additional margin. The results of this randomized study propose that dose escalation enhances biochemical control of disease and that both radiation dose and technique effects radiation induced side effects. The authors concluded that dose escalating from 64 to 74 Gy using 3D-CRT may improve long term PSA control, but a treatment margin of 1.5 cm is unnecessary and is associated with increased acute bowel and bladder reactions and more late rectal side effects.16 To provide an example of the relative frequency of GI toxicities for a large series in the literature, Zelefsky et al17 demonstrated that maximal treatment doses have been limited by the relatively low radiation tolerance of the rectum and bladder, commonly included within the safety margins of treatment elds. Three-dimensional conformal radiation therapy treatment plans deals with some of these problems by conforming with high precision the spatial distribution of the prescribed dose to the prostate, while reducing dose the rectum and bladder. In that study on 743 patients the tumor target dose was increased from 64.8 to 81 Gy in increments of 5.4 Gy. Tumor response was assessed by post-treatment decrease of PSA to levels of <1.0 ng/ml. The researchers provided evidence for a significant effect of dose escalation on the response of human prostate cancer. However, results also found 14% with grade 1; 8%, grade 2; 0.8%, grade 3; and only one patient with grade 4 GI toxicity.17 In similar study Michalski, Winter, and Roach et al18 reported clinical cancer control outcomes on Radiation Therapy Oncology Group (RTOG) 9406, a 3D-CRT dose escalation trial for localized adenocarcinoma of the prostate. In this multi-institutional dose escalation study 1,051 patients were registered on five sequential dose levels. Three dose levels were initially planned: 68.4 Gy, 73.8 Gy, and 79.2 Gy. Because dose-limiting toxicity was not identified in the
first three dose levels, the study remained open to accrue patients to two additional dose levels: 74 Gy and 78 Gy. Treatment was administered to the PTVs using 3D conformal fields shaped to exclude as much of the bladder and rectum as possible. Elective pelvic nodal irradiation was not allowed. Results from this study demonstrated that dose escalated 3D-CRT yields favorable outcomes for localized prostate cancer. Moreover, acute tolerance to 79.2 Gy was excellent with no patients experiencing Grade 3 acute toxicity. The acute toxicity rate was comparable to that reported for previous lower dose levels. There was no Grade 4 or 5 late complications noted during the period of observation.18 Woel, Beard, and Chen19 et al discussed the acute GI toxicity during dose-escalated 3DCRT. A modified intrarectal balloon (Medrad) was used for prostate gland localization and immobilization. The study analyzed dose escalation beyond conventional doses of 70 Gy for localized prostate cancer and consequential toxicity levels. Toxicity to the surrounding normal tissue, particularly radiation proctopathy, has been the critical dose-limiting factor precluding maximal dose escalation. The percent of patients experiencing any increase in GI symptoms during treatment and those requiring intervention (dietary or medical) were enumerated. Compared to a baseline, about 70% of patients experienced during treatment an increase in bowel movements (BM), loose BM, tenesmus, and hemorrhoidal symptoms. Woel concluded that dose escalation using 3D-CRT approach with an intrarectal balloon for prostate localization and immobilization was well tolerated. Acute GI symptoms were noted to resolve with standard dietary and/or medical interventions shortly after the treatments.19 Furthermore, Huang, Pollack, and Leavy et al20 led study to identify dosimetric, anatomic, and clinical factors that correlate with late rectal toxicity after 3D-CRT for prostate cancer. Results from this showed that the rate of developing Grade 2 or higher late rectal toxicity was 25%. A significant volume effect was observed at rectal doses of 60, 70, 75.6, and 78 Gy, and the risk of developing rectal complications increased exponentially as greater volumes were irradiated. Although the percentage of rectal volume treated correlated significantly with the incidence of rectal complications at all dose levels, the absolute rectal volume appeared to be a factor only at the higher doses of 70, 75.6, and 78 Gy. The study concludes that the DVH analyses clearly indicated a volume effect on the probability of developing late rectal complications. Therefore, dose escalation may be safely achieved by adherence to dose-volume
histogram constraints during treatment planning and organ localization at the time of treatment to ensure consistent patient setup.20 Another study was performed to find predictors for rectal and intestinal acute toxicity in patients with prostate cancer treated with 70 Gy 3D-CRT. Vavassori, Fiorino, and Rancati et al21 evaluated results of RT treatments for prostate cancer of 1,132 patients treated at 22 different institutions. The dose was specified at the ICRU reference point (the isocenter) and was delivered with 1.82-Gy fractions once daily with a linear accelerator (6 MV photons). The findings regarding volumes and the mean rectal dose have established the existence of a dose volume effect for acute toxicity, proposing that the mean rectal dose is more predictive for rectal bleeding. The researchers concluded that larger irradiated volumes were associated with a greater risk of toxicity for a number of symptoms. Seminal vesicle irradiation, which involves irradiation of the most cranial part of the rectum, was connected with increased tenesmus; PTV receiving the largest dose was highly predictive for mucous discharge, and pelvic irradiation was linked with rectal pain.21 There are various studies that compare 3D-CRT treatments to IMRT treatments for prostate cancer. Pollack, Hanlon, and Horovitz et al22 debated the response of prostate cancer to radiation in the pre-PSA era. Large palpable tumors resolved within months of treatment with relatively modest radiation doses of 64-70 Gy. The use of PSA-based failure as an endpoint, however, has made it clear that cure rates were much lower than appreciated. Authors agreed that, while doses in this range are still widely used today, doses above 70 Gy are associated with a significant reduction in biochemical failure. The use of 3D-CRT to escalate radiation dose has resulted in modest increases in rectal and bladder toxicity. The application of IMRT allows for greater sparing of the surrounding normal tissues and, hence, the potential to further escalate dose. The study describes results of dose escalation, the ability of IMRT to reduce rectal and bladder exposure to high radiation doses and the use of new imaging methods to more accurately target the prostate.22 Similarly, Zalefsky, Fuks, and Happersett et al23 conducted study aimed to compare acute and late toxicities of high-dose radiation for prostate cancer delivered by either 3D-CRT or IMRT. 61 patients with clinical stage T1c- T3 prostate cancer were treated with 3D-CRT and 171 with IMRT to a prescribed dose of 81 Gy. To quantitatively evaluate the differences between conventional 3D-CRT and IMRT, 20 randomly selected patients were planned concomitantly by
both techniques and the resulting treatment plans were compared. Results of the study showed that, compared with conventional 3D-CRT, IMRT enhanced the coverage of the CTV by the prescription dose and reduced the volumes of the rectal and bladder walls carried to high dose levels, indicating better conformity with IMRT. Acute and late urinary toxicities were not significantly different for the two methods. However, the combined rates of acute grade 1 and 2 rectal toxicities and the risk of late grade 2 rectal bleeding were significantly lower in the IMRT patients. The 2-year actuarial risk of grade 2 bleeding was 2% for IMRT and 10% for conventional 3D-CRT. According to the authors of the study this data demonstrates the feasibility and safety of high-dose IMRT for patients with localized prostate cancer. Moreover, it provides a proof-of-principle that this method improves dose conformity relative to tumor coverage and exposure to normal tissues.23 De Meerleer, Fonteyene, and Vakaet et al24 analyzed late toxicity and biochemical relapse-free survival (bRFS) after IMRT for prostate cancer. In this study patients received a median PTV dose of 74 Gy with a hard constraint on maximum rectum dose of 72 Gy. Later in the study, median PTV and maximum rectum dose were increased to 76 and 74 Gy, respectively. Results showed that most patients had no or grade 1 late toxicity. The researchers observed grade 2 late toxicity (IBD, abdominal cramps, incontinence, anal pain and diarrhea) in less than 20% of the patients and all grade 2 GI complaints resolved after 6 months. There was no late grade 3 or 4 toxicity level reported in this study. The data presented in this study demonstrated that IMRT as primary therapy for localized or locally advanced prostate cancer offers excellent biochemical outcome. Late morbidity is low and disappears after a median duration time of 6 months.24 Deville, Both, and Hwang et al25 carried out a study to assess whether whole-pelvis (WP) IMRT is associated with increased toxicity compared with prostate-only (PO) IMRT. By conducting this study authors continued a debate regarding the therapeutic ratio of RT for suspected microscopic lymph node involvement. The WP patients received initial WP IMRT to 45 Gy in 1.8-Gy fractions; all patients received a total dose of 79.2 Gy to the prostate. As the loco-regional benefit of pelvic nodal irradiation continues to be clarified, this study provides the longest reported follow-up to date in the largest cohort of prostate cancer patients treated with WP IMRT in the non-adjuvant setting. Findings of this study suggest that dosimetric differences in bowel, bladder, and rectum are evident in the low-dose and median-dose regions. IMRT allows for an improved toxicity profile with no differences in late GI and GU clinical toxicities
between WP and PO IMRT and a significant but apparently transient difference only in lowgrade acute GI toxicity.25 Another study conducted by Goenka, Magsanoc, and Pei et al26 compared acute and late toxicities in patients treated with IMRT and 3D-CRT in the postprostatectomy salvage setting. With salvage radiation therapy (SRT) in the postprostatectomy setting, the need to deliver sufficient radiation doses to achieve a high probability of tumor control is balanced with the risk of increased toxicity. The authors found that IMRT results in a significant reduction in late GI side effects. Despite the fact that patients treated with IMRT were more likely to be treated with a higher dose than patients treated with 3D-CRT, researchers did not see an increase in late GU toxicity, urinary incontinence, or erectile dysfunction. The data support the idea that IMRT significantly improves the therapeutic ratio associated with SRT in the postprostatectomy setting and the risk of developing late GI toxicity with postprostatectomy salvage RT is significantly reduced compared with 3D-CRT.26 Shu, Lee, and Xia et al27 conducted study to report the toxicity profile of patients treated with 3D-CRT or IMRT receiving doses of 82 Gy or more to portions of their prostate. Despite the higher doses, the incidence of late morbidity compared favorably with those seen with conventional techniques or 3D-CRT. Advances in imaging with MRSI allowed mapping of the distribution of tumor within the prostate gland. By using IMRT, as described in this study, higher doses are achieved in the area at highest risk (DIL), whereas doses elsewhere are limited to respect normal tissue tolerances. Results of this study show that most patients can tolerate hot spots within the prostate of 82 Gy or more. When treated as described, toxicity profiles should be similar to, if not better than, what is observed using conventional doses of radiation. The results of this study supported the continued use of IMRT in the treatment of prostate cancer and proved the hypothesis that higher doses of radiation can be given safely as long as normal tissue tolerances are respected.27 Volume of the Irradiated Organ Data from several studies available in various professional publications suggests that GI complications and rectal bleeding are related to the volume of the organ irradiated. The aim of study conducted by Luo, Yang, and Narayan et al28 was to develop and validate benchmark dosevolume histograms (DVHs) of bladder and rectum for both 3D-CRT and IMRT, and to evaluate quantitatively the benefits of using IMRT vs. 3D-CRT in treating localized prostate
cancer. In their study 3D-CRT rectal doses were: mean 39.3 Gy, V60 21.8%, V70 13.6%. IMRT plans resulted in similar mean dose values to rectum 34.9 Gy, but lower values of V70 (9.3%). By showing both 3D-CRT and IMRT planning on a selected number of patients DVH benchmarks were created to guide the planning process without the need to double-plan treatment for each patient. From the DVHs comparisons, it was presented that the IMRT technique offers more benefit in sparing rectum.28 The average mean dose, V60 and V70 to bladder and rectum were significantly reduced with IMRT compared with 3D-CRT. Similarly, Fiorino, Alongi, Perna et al29 studied relationship between DVHs of the intestinal cavity (IC) and moderate-severe acute bowel toxicity in men with prostate cancer treated with pelvic nodal irradiation. Their study group involved of 191 patients with localized prostate cancer who underwent whole-pelvis radiotherapy, 91 of whom were treated with a 3DCRT (50.4 Gy, 1.8 Gy/fraction) and 84 of whom were treated with IMRT. The researcher found out that the incidence of acute and late GI toxicity was increased in patients treated with conventional whole-pelvis irradiation (WPRT) because of the inclusion of a large volume of the bowel in the treatment field. Overall, 22 of the 191 patients experienced acute Grade 23 GI toxicity; of these 22 patients, 19 were in the 3D-CRT group, whereas only 3 were in the IMRT group. When V40 was < 170 cc, V45 was < 100 cc, and V50 was < 33 cc, the incidence of acute GI toxicity dropped from 21% to 3.6%. Moreover, treatment was interrupted in 12 patients because of toxicity; 11 of the 12 patients were in the 3D-CRT group. These findings confirmed superiority of IMRT over 3D-CRT in minimizing GI toxicities.29 In different study Fiorino, Sanguineti and Cozzarini30 again investigated the role of IMRT in reducing the risk of upper gastrointestinal (uGI) and lower gastrointestinal (lGI) toxicity following WPRT after radical prostatectomy. For 3D-CRT patients RT was carried by means of a box technique with 18 MV X-ray beams shaped around the PTV obtained as the union of all PTVs (nodes + prostatic bed) by cerrobend blocks or MLCs (4550.4 Gy, 1.8 Gy/fr). The goal of IMRT was to deliver more than 98%/95% of the prescribed dose to more than 95% of PTV while keeping dose homogeneity as high as possible. Concerning the sparing of IC, the researchers reduced the dose without compromising PTV coverage, starting from the fraction of IC receiving more than 4050 Gy, and then gradually reducing the fraction of IC receiving more than 20 30 Gy. In the 3D-CRT series of study, the irradiation of the pelvic LN resulted in an increased dose to rectum, bladder and small bowel and an increased risk of late grade 3 GI toxicity was
found. The main result of this study is the evidence of a dramatic reduction of acute upper GI toxicity when IMRT is applied. DVH data confirmed that IMRT severely reduces the portions of IC receiving doses between 20 and 50 Gy, while increasing the fraction of IC receiving more than 1015 Gy as well as that receiving more than 5560 Gy. A careful dosevolume analysis of bowel toxicity performed by the researchers showed that the high-dose (i.e. 4050 Gy) region of IC is strongly associated with the probability of acute uGI toxicity. Anderson, Yu, and Peschel31 et al investigated changes in rectal volume (RV) and rectal diameter (RD) of patients with prostate adenocarcinoma during radiotherapy, which could potentially affect treatment toxicity and tumor control. The researchers studied if the development of IMRT utilizing inverse treatment planning software and dynamic multileaf collimators allowed for a more conformal dose distribution with strict constraints on nearby structures. In this study IMRT has been compared with 3D-CRT and results have shown superior outcomes of IMRT in regards to dosimetry. A significant decrease in RV and RD occurs during prostate IMRT delivery. More than half of patients of this study had decreased RV and over a third had decreased RD. This observation is pertinent to prostate localization, planning margins, and implies that DVH analysis of rectal irradiation based on pre-treatment CT scanning may inaccurately estimate the risk of rectal toxicity when the initial RV is larger than 70 cm3.31 Another study conducted by Finoglietto, Laliberte, and Allaw et al32 compared the dose coverage of planning and clinical target volume (PTV, CTV), and organs-at-risk (OAR) between IMRT and 3D-CRT before and after internal organ variation in prostate cancer. The researchers wondered if the advantage of IMRT over 3D-CRT during planning continued throughout the treatment when interfraction volume changes are important. To reply to this demand, they assessed the CTV/PTV and OAR coverage for 10 localized prostate cancer patients. All patients were treated with IMRT according to strict guidelines regarding maximum dose to OAR and PTV, and were selected for this study because of significant internal volume changes that arise routinely in clinical practice. A fictional 3D-CRT plan was generated for each patient, following the same guidelines of maximum dose to OAR as the IMRT plan. The two plans were then matched on each patient at two points in time, and comparative assessment of both plans before and after actual volume changes was presented. The study concluded that, despite significant internal motion (developing from prostate shrinkage or extreme bladder/rectal filling) during
treatment course, IMRT plans persisted to be better than 3D-CRT with respect to PTV coverage and OAR avoidance.32 Malone, Croke, and Roustan-Delatour et al33 explored four consensus guidelines (European Organization for Research and Treatment of Cancer, Faculty of Radiation Oncology Genito-Urinary Group, Princess Margaret Hospital, Radiation Therapy Oncology Group) of standardizing the CTV delineation and the dosimetric benefits of IMRT. A total of 20 patients treated with postoperative RT were included in this study. The 3D-CRT plans were applied to cover the guideline-generated planning target volumes (66 Gy in 33 fractions). DVHs were analyzed for CTV/PTV coverage and to evaluate OAR irradiation. These 3D-CRT plans were compared with the RTOG planning target volume to evaluate the advantages of IMRT. Results showed that using 3D-CRT the DVHs rarely met the rectal constraints, independent of the guideline used. The IMRT plans rose to significant OAR sparing compared with the 3D-CRT plans. However, the authors concluded that the risk of local recurrence after radical prostatectomy remains high, despite adjuvant RT. Treatment volumes determined by using current consensus guidelines vary significantly, which can affect the clinical outcomes and treatment-related toxicity. The outcome of this study indicates that IMRT results in significant OAR sparing in the postoperative setting. The toxicity associated with postoperative RT remains poorly defined, and the role of dosimetric and volumetric parameters of postoperative RT is unclear.33 Pederson, Fricano, and Correa et al34 tried to characterize the late GI toxicity for prostate cancer patients treated with IMRT and propose DVH guidelines to limit late treatment-related toxicity. In this study 296 consecutive men were treated with IMRT for adenocarcinoma of the prostate. Four groupings of DVH parameters were outlined based on the percentage of rectal tissue receiving 70 Gy (V70), 65 Gy (V65), and 40 Gy (V40). These DVH groupings, as well as clinical and treatment characteristics, were correlated to maximal Grade 2+ GI toxicity. Results showed that one potential clinical benefit of IMRT in prostate cancer is its capability to reduce rectal toxicity. The frequency of late Grade 2 and Grade 3 GI toxicity in this study was lower than the rates of late toxicity seen with 3D-CRT. The researchers concluded that DVH constraints to the rectum can help to reduce the morbidity of treatment, especially for men who are older or receiving anticoagulant therapy. They recommend the use of rectal constraint of V70 10%, V65 20%, and V40 40% for patients treated to the prostate (with or without seminal
vesicles) and rectal V70 20%, V65 40%, and V40 80% for men treated to an initial wholepelvic field.34 Another study was performed to report the toxicity after IMRT for patients with localized prostate cancer, as a sole treatment or after radical prostatectomy. Chen, Weltman, and Hanriot et al35 presented a retrospective evaluation of the initial toxicity following the technical implementation of IMRT for treatment of localized prostate cancer patients. Clinical and treatment related factors, including normal tissue DVH constraints, were analyzed as possible risk factors for GI toxicity. From the 125 patients, 73 (58.4%) presented acute Grade 1 or Grade 2 GI toxicity. Grade 3 GI acute toxicity occurred in only 2 patients (1.6%). Authors concluded that IMRT is a well tolerable technique for routine treatment of localized prostate cancer, with short and medium-term satisfactory toxicity profiles. According to the data presented in this study, rigid compliance to DHV constraints may avoid higher incidences of normal tissue complication.35 The objective of the study conducted by Cheung, Sisxel, and Morton et al36 was to access toxicities after delivering a hypofractionated IMRT boost with individualized intrafraction PTV margins and daily online correction for prostate position. Phase I of this study concerned delivering 42 Gy in 21 fractions using 3D-CRT, followed by a Phase II IMRT boost of 30 Gy in 10 fractions. A uniform 10-mm PTV margin was used for the first phase of treatment. PTV margins for Phase II were patient-specific and were calculated from the respiratory and intrafraction motion data obtained from Phase I. In 33 patients who had completed this research, the average PTV margin used during the hypofractionated IMRT boost was 3 mm in the lateral direction, 3 mm in the superior-inferior direction, and 4 mm in the anteroposterior direction. No patients developed acute Grade 3 rectal toxicity. The authors concluded that PTV margins can be reduced considerably with daily online correction of prostate position. Delivering a hypofractionated boost with this high-precision IMRT technique resulted in acceptable acute GI toxicity.36 Three dimensional conformal treatment techniques utilize smaller treatment fields with more complicated field arrangements to permit for higher dose delivery to the target volumes while limiting the dose to the surrounding normal tissues and potentially reducing treatmentrelated side effects. However, the use of smaller fields involves more accurate patient setup procedures, with a higher possibility for missing part of the target volume. The study undertaken
by Alang O, Jamgade A, and Ali I et al37 evaluated the effect of daily setup error and interfraction organ motion on the overall dosimetric radiation treatment plans. Twelve patients undergoing definitive IMRT treatments for prostate cancer were evaluated in this study. Each patient was treated to a dose of 8100 cGy given in 45 fractions. The results of this study suggested significant underdosing with inaccurate target localization and emphasize the importance of accurate patient setup and target localization. At an individual patient level, the difference in the D95 value for the prostate volume could be >1200 cGy and for the PTV8100 could approach almost 2000 cGy when comparing corrected against uncorrected plans. It was presented that the IMRT technique offers more benefit in limiting toxicity levels; however, higher precision in target localization and setup accuracy is required.37
Chapter III: Methodology There is no standard single best treatment for localized prostate cancer, as each patient characteristic remains unique and diverse. To date, external-beam radiation therapy remained the prevailing form of radiation treatment for adenocarcinoma of the prostate for the past 30 to 40 years. However, unwanted side-effects from radiation therapy to the prostate might be irreversible and reduce quality of life. Evaluating toxicity is therefore essential in evaluating the therapeutic benefits of radiation therapy and planning treatments for future patients. The purpose of this study is to compare acute and late toxicities of high dose radiation therapy delivered either by conventional 3D-CRT or IMRT. This section provides information of how patients were selected, a brief description on available patient diagnosis, instruments to conduct the study, and how data was collected and analyzed. Subject Selection and Description A random selection of 10 patients treated between 2009 and 2011 at NorthShore University Heath Systems in Evanston, Illinois. The sample population included male patients between 65 and 85 year of age that were treated for prostate cancer with EBRT. For the purpose of this study only patients who underwent prostatectomy will be evaluated. All study subjects were treated with EBRT, either 3D-CRT or IMRT. Only patients who obtained the full course of prescribed treatments with indication of complete documentation of gastrointestinal side effects were included in the study group. All patients received a dose between 68.4 Gy to 77.4 Gy in 38 to 42 fractions. Patient 1 The patient is a 76 year old man with T1cN0M0 biochemical recurrence of prostate cancer. He was found to have an elevated PSA of 4.1 in 2002 and underwent TRUS biopsies with the finding of Gleason grade 3+4=7 adenocarcinoma with pattern 5 involving 5/6 left core biopsies, right cores were negative. Patient 2 The patient is a 65 year old man diagnosed with a T2N0M0 stage II adenocarcinoma of the prostate, pre-op PSA in 2 range, Gleason score of 4+4=8, predominant right lobe disease with focal left lobe involvement, 50% gland involvement, PNI, 8 negative LNs, and positive surgical margins. Patient3
The patient is a 72 year old gentleman with T1cN0M0, Gleasons 4+3 adenocarcinoma of the prostate. The patient reports being in his usual state of health until June of 2009, when routine PSA returned 6.6. He was subsequently referred to outside Urology service. Work-up culminated in TRUS/prostate biopsy, which revealed multiple cores (3 of 12) positive for Gleason's 4+3=7 adenocarcinoma of the prostate. Patient4 The patient is a 70 year old gentleman with past medical history significant for pT2cNoMo Gleason's 4+3=7 adenocarcinoma of the prostate. He underwent margin-negative robotic-assisted laparoscopic prostatectomy in July of 2007. Pathology was notable for negative surgical margins. His most recent PSA=0.52. Patient 5 The patient is a 66 year old man found to have stage T1cN0M0 adenocarcinoma of the prostate. He underwent biopsies showing Gleason grade 6 adenocarcinoma in 1/6 right and 2/6 left cores. He chose to enter the institutional active surveillance program and repeated biopsies few months later, which showed 3/6 positive right cores and 6 negative cores. One of the 3 positive cores was >50% involved with Gleason grade 6 cancer and he was thus advised to consider radiation therapy treatment. Patient 6 The patient is an 82 year old man who found that his PSA was an elevated to 20. He underwent TRUS biopsies with the finding of Gleason grade 4+4=8 adenocarcinoma stage T2N0M0, involving 2/6 left core biopsies and 6/6 right cores without perineural invasion. CT showed a large prostate with asymmetrical right seminal vesicle enlargement. Patient 7 The patient is a 71 year old man who found to have an elevated PSA of 6.43. He underwent TRUS biopsies with the finding of Gleason grade 3+3=6 adenocarcinoma, stage T1cN0M0, involving 1/6 right lobe core biopsies; left cores were negative. Patient 8 The patient is a 68 year old man with a T2N0M0 stage II adenocarcinoma of the prostate, PSA of 6.2. He underwent radical retropubic prostatectomy. Pathology revealed adenocarcinoma, Gleason grade 4+4=8 with tertiary pattern 5 disease, involving the apex and left lobe w/ 10% prostate involvement.
Patient 9 The patient is an 84 year old man who was alarmed by elevated screening PSA of 15.49. The patient underwent prostate biopsy that revealed adenocarcinoma, T2cN0M0 stage IIB. Gleason grade was reported 4+5=9, involving 6/6 cores in the right lobe, and 3/6 cores in the left lobe. There was also PNI present at the time of diagnosis. Patient 10 The patient is an 80 year old man with elevated PSA level of 12. Repeat biopsies showed Gleason grade 4+3=7 adenocarcinoma in 6/6 right cores and 1/6 left cores, stage T3BN0M0. His PSA apparently continued to rise to 18 before surgery. Surgery showed 5 negative pelvic nodes, Gleason grade 4+3=7 adenocarcinoma involving both lobes with extracapsular extension, extensive perineural invasion and involvement of both seminal vesicles. Instrumentation Several instruments were significant to conduct this research. All patients were simulated on a Phillips Brilliance Big Bore 16 slice CT scanner. The Brilliance CT Big Bore configuration integrates the 85 cm large bore and 60 cm true scan field-of-view. 512 by 512 centimeter (cm) matrix, 3 mm slice thickness was used for acquisition of all the planning scans. Patients were simulated according to standard protocol of the department in a supine position, hands on the chest holding a blue ring. To increase set up accuracy preceding CT scans all patient were placed in a Vak-Loc bag. A CT image of the pelvis was taken from the top of lumbar vertebrae (L1) to the bottom of the ischial tuberosities. The acquired images were imported from the CT scanner to the treatment planning computer. The treatment planning system (TPS) used was Eclipse, version 8.9, integrating the analytical anisotropic algorithm (AAA). All patients of this study had daily cone beam computed tomography (CBCT) scans that were taken on either Varian Trilogy or Varian 23 iX linear accelerators. Both of these machines are equipped with on board imaging (OBI) which is capable of performing CBCT. Data Collection Procedures The frequency and severity of GI complications are raised with increased dose and volume treated. Therefore, in this section, data will be divided into two different categories. The first category will concern GI complications caused by increased dose of the treatment, while the second one will concentrate on GI complications caused by increased volume of irradiated
organs. Moreover, each of these categories will be subdivided into two sections. The first one will concentrate on 3D-CRT treatment technique and the second one will be dedicated to the IMRT approach. Data analysis The comparison of patient plans using 3D-CRT or IMRT combined with the evaluation of GI side effects and patients conditions at follow up will be analyzed. Results from the plans will be evaluated using the dose volume histograms (DVH). Data analyzed from DVH includes: dose, dose coverage to the whole prostate, seminal vesicles, and rectum. In terms of severity of GI complications, the Radiation Therapy Oncology Group (RTOG) toxicity scales will be used. The data gathered will be assessed to conclude the best treatment method for EBRT for prostate cancer. Limitations A limitation of this study includes the same planned target margins around the prostate for the 3D versus IMRT techniques, and limited DVH data. For all plans the PTV was defined by adding a margin of 10 mm from the CTV. A 10 mm margin takes into account possible movements of the CTV, systematic and random set-up errors. It has been considered as sufficiently safe, given the incidence of systematic and random set-up errors. Another limitation of this study, since severity of the majority of GI symptoms (e.g. rectal bleeding or rectal mucous) is difficult to quantify, the standards of reporting results without adequate assessment of baseline characteristics (patient reported vs. physician reported) were uneven. Summary Evaluating toxicity is essential in evaluating the therapeutic benefits of radiation therapy and planning treatments for future patients. This study was undertaken to compare gastrointestinal toxicities following EBRT for prostate cancer. The study focused on 10 randomly selected patients treated between 2009 and 2011 at NorthShore University Heath Systems in Evanston, Illinois. For the purpose of this study only patients who underwent prostatectomy will be evaluated. All study subjects were treated with EBRT, either 3D-CRT or IMRT. Only patients who obtained the full course of prescribed treatments with indication of complete documentation of gastrointestinal side effects were included in the study group. All patients received dose between 68.4 Gy to 77.4 Gy in 38 to 42 fractions.
Figures Figure 1. The location of the prostate gland and nearby organs
Figure 2. AP view of treatment field borders
Figure 3. Lateral view of treatment field borders
Figure 4. 3D conformal treatment plan of prostate.
Figure 4a: beams-eye-view of an anterior prostate and pelvic nodal field conformally shaped Figure 4b: beams-eye-view of a lateral prostate and pelvic nodal field conformally shaped. The prostate is in red, pelvic nodes in translucent purple, rectum in blue, bladder in solid purple and bowel in yellow.
Figure 5. IMRT beam arrangements used for prostate irradiation: (a) 7-field technique; (b) conventional 5-field IMRT technique; (c) the opposed matched field technique.
Tables
Table 1. Primary tumor (T) Tx T0 T1 T1a T1b T1c T2 T2a T2b T2c T3 T3a T3b T4 Primary tumor cannot be assessed. No evidence of primary tumor. Clinically unapparent tumor neither palpable nor visible by imaging. Tumor incidental histologic finding in 5% of tissue resected. Tumor incidental histologic finding in >5% of tissue resected. Tumor identified by needle biopsy (e.g., because of elevated PSA). Tumor confined within prostate. Tumor involves less than one-half of one lobe. Tumor involves more than one-half of one lobe but not both lobes. Tumor involves both lobes. Tumor extends through the prostate capsule. Extracapsular extension (unilateral or bilateral). Tumor invades seminal vesicle(s). Tumor is fixed or invades adjacent structures other than seminal vesicles such as external sphincter, rectum, bladder, levator muscles, and/or pelvic wall.
Table 2. Regional lymph nodes (N).
NX NO N1
Primary tumor cannot be assessed. No evidence of primary tumor. Clinically unapparent tumor neither palpable nor visible by imaging. Pathologic
pNX Regional nodes not sampled. pN0 pN1 No positive regional nodes. Metastases in regional node(s).
Table 3. Distant metastasis (M). M0 M1 No distant metastasis. Distant metastasis.
M1a Non-regional lymph node(s). M1b Bone(s). M1c Other site(s) with or without bone disease.
Table 4. Acute gastrointestinal complications according to the Radiation Therapy
Oncology Group (RTOG). Grade 1 Increased frequency or change in GI quality of bowel habits not requiring medication Rectal discomfort not requiring analgesics. Grade 2 Diarrhea requiring parasympatholytic drugs. Mucous discharge not necessitating sanitary pads Rectal or abdominal pain requiring analgesics. Grade 3 Diarrhea requiring parenteral support Severe mucous or blood discharge necessitating sanitary pads Abdominal distension (flat plate radiograph demonstrates distended bowel loops). Grade 4 Obstruction, fistula, or perforation GI bleeding requiring transfusion; Abdominal pain or tenesmus requiring tube decompression or bowel diversion.
Table 5. Late gastrointestinal complications according to the Radiation Therapy Oncology Group (RTOG). Grade 1 Mild diarrhea. Mild GI cramping. Bowel Grade 2 Moderate diarrhea. Intermittent, severe cramping. Grade 3 Watery diarrhea. Obstruction requiring surgery. Grade 4 Necrosis. Perforation. Fistula. Abdominal pain or tenesmus requiring tube decompression or bowel diversion.
Bowel movements (5 Bleeding requiring surgery.
movements 2 per day). 5 per day. Slight rectal discharge or bleeding. Moderate excessive, rectal discharge. Intermittent, frequent bleeding.
References
1. American Cancer Society. Cancer Statistics, 2012. Available at: http://onlinelibrary.wiley.com/doi/10.3322/caac.20138/full. Accessed: June 9, 2012. 2. National Cancer Institute. Prostate Cancer Screening. Available at: http://www.cancer.gov/cancertopics/pdq/screening/prostate/HealthProfessional/page2. Accessed June 9, 2012. 3. Washington C, Leaver D. Principles and Practice of Radiation Therapy. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2010. 4. Filella X, Alcover J, et al. Clinical evaluation of free PSA/total PSA (prostate-specific antigen) ratio in the diagnosis of prostate cancer. Eu J Cancer. 1997;33(8):1226-1229. 5. Bostwick D, Eble J. Urological Surgical Pathology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2007. 6. Chao KSC, Perez CA, Brady LW. Radiation Oncology Management Decisions. 2nd ed. Philadelphia PA: Lippincott Williams & Wilkins; 2002. 7. Eng T, Herman T. Primary radiation therapy for localized prostate cancer. Urol Onc. 2002;20(2):239-257. 8. Badaus L, Bolla M, et al. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of literature. Eu Urol. 2012;61(1):112-117. 9. Epstain B, Hanks G. Radiation therapy techniques and dose selection in the treatment of prostate cancer. Sem Rad Oncol. 1993;3(3):179-186. 10. el-Gabry E, Halpern E, Strup S, Gomella L. Imaging prostate cancer current and future applications. Oncol. 2001;15(3):325336. 11. Bentel G. Radiation Therapy Planning. 2nd ed. St. Louis, MO: McGraw-Hill; 1996. 12. Perez CA, Pilepich MV, Zivnuska F. Tumor control in definitive irradiation of localized carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1986;2(4):523-531. 13. Heemsbergen W, Peeters S, Koper P. et al. Acute and late gastrointestinal toxicity after radiotherapy in prostate cancer patients: consequential late damage. Int J Radiat Oncol Biol Phys. 2006;66(1):3-10. 14. Iwamoto R, Maher K. Radiation therapy for prostate cancer. Sem Oncol Nur. 2001; 17(2):90-100. 15. Khan FM. The Physics of Radiation Therapy. 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2010.
16. Dearnaley D, Hall E. Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br J Cancer. 2005;92(3): 488-498. 17. Zelefsky M, Leibel S, Gaudin P, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41(3):491500. 18. Michalski J, Winter K, Roach M, et al. Clinical outcome of patients treated with 3D conformal radiation therapy (3D-CRT) for prostate cancer on RTOG 9406. Int J Radiat Oncol Biol Phys. 2012;83(3):363-370. 19. Woel R, Beard C, Chen M, et al. Acute gastrointestinal, genitourinary, and dermatological toxicity during dose-escalated 3D-conformal radiation therapy (3D-CRT) using an intrarectal balloon for prostate gland localization and immobilization. Int J Radiat Oncol Biol Phys. 2005;62(2):392-396. 20. Huang C, Pollack A, Leavy E, et al. Late rectal toxicity: dose-volume effects of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002; 54(5):1314-1321. 21. Vavassori V, Fiorino C, Rancati T. Predictors for rectal and intestinal acute toxicities during prostate cancer high-dose 3D-CRT: results of a prospective multicenter study. Int J Radiat Oncol Biol Phys. 2007;67(5):1401-1410. 22. Pollack A, Hanlon A, Horovitz E, et al. Radiation therapy dose escalation for prostate cancer: a rationale for IMRT. World J Urol. 2003;21(4):200208. 23. Zalefsky M, Fuks Z, et al. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol. 2000;55(3):241-249. 24. De Meerleer G, Fonteyene V, Vakaet L, et al. Intensity-modulated radiation therapy for prostate cancer: late morbidity and results on biochemical control. Radiother Oncol. 2007;82(2):160-166. 25. Deville C, Boths, Hwang W, et al. Clinical toxicities and dosimetric parameters after whole-pelvis versus prostate-only intensity-modulated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;55(3):763-772.
26. Goenka A, Magsanoc J, Pei X, et al. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eu Urol. 2011;60(6):1150-1151. 27. Shu H, Lee T, Xia P, et al. Toxicity following high-dose three-dimensional conformal and intensity-modulated radiation therapy for clinically localized prostate cancer. Urology. 2001;57(1):102-107. 28. Luo C, Yang C, Narayan S, et al. Use of benchmark dose-volume histograms for selection of the optimal technique between three-dimensional conformal radiation therapy and intensity-modulated radiation therapy in prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66(4):1253-1262. 29. Fiorino C, Alongi F, Perna L, et al. Dose-volume relationships for acute bowel toxicity in patients treated with pelvic nodal irradiation for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75(1):29-35. 30. Fiorino C, Sanguineti G, Cozzarini C. Rectal dose-volume constraints in high-dose radiotherapy of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2003;57(4):953962. 31. Anderson N, Yu J, Peschel R, et al. A significant decrease in rectal volume and diameter during prostate IMRT. Radiother Oncol. 2011;98(2):187-191. 32. Finoglietto P, Laliberte B, Allaw A, et al. Persistently better treatment planning results of intensity-modulated (IMRT) over conformal radiotherapy (3D-CRT) in prostate cancer patients with significant variation of clinical target volume and/or organs-at-risk. Radiother Oncol. 2008;88(1):77-87. 33. Malone S, Croke J, Roustan-Delatour N, et al. Postoperative radiotherapy for prostate cancer: a comparison of four consensus guidelines and dosimetric evaluation of 3D-CRT versus tomotherapy IMRT. Int J Radiat Oncol Biol Phys. 2012;83(3):112-119. 34. Pederson A, Fricano J, Correa D, et al. Late toxicity after Intensity-Modulated Radiation Therapy for localized prostate cancer: an exploration of DoseVolume Histogram parameters to limit genitourinary and gastrointestinal toxicity. Int J Radiat Oncol Biol Phys. 2012;82(8):235-241.
35. Chen M, Weltman E, Hanriot R, et al. Intensity modulated radiotherapy for localized prostate cancer: rigid compliance to dose-volume constraints as a warranty of acceptable toxicity? Radiother Oncol. 2007;2(6):169-176. 36. Cheung P, Sisxel K, Morton G, et al. Individualized planning target volumes for intrafraction motion during hypofractionated intensity-modulated radiotherapy boost for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;62(2):418-425. 37. Alang O, Jamgade A, Ali I, et al. The dosimetric impact of daily setup error on target volumes and surrounding normal tissue in the treatment of prostate cancer with intensitymodulated radiation therapy. Med Dosim. 2012;In Press.
Anda mungkin juga menyukai
- Prostate Cancer ThesisDokumen5 halamanProstate Cancer Thesisaflozmfxxranis100% (2)
- 2520 4931 1 SMDokumen20 halaman2520 4931 1 SMhh1790Belum ada peringkat
- Asdafas AllDokumen2.719 halamanAsdafas AllAlexandr TrotskyBelum ada peringkat
- Swog 8794Dokumen7 halamanSwog 8794yingming zhuBelum ada peringkat
- JournalDokumen6 halamanJournalIka RachmadantBelum ada peringkat
- (Recent Results in Cancer Research 203) Florian Otto, Manfred P. Lutz (Eds.) - Early Gastrointestinal CancerDokumen244 halaman(Recent Results in Cancer Research 203) Florian Otto, Manfred P. Lutz (Eds.) - Early Gastrointestinal CancerTolga ŞanlıBelum ada peringkat
- Earlytoxicitiesofultrahypofractionatedstereotacticbodyradiotherapyforintermediaterisklocalizedprostatecancerusingcone Beamcomputedtomographyandreal Timethree DimensionaltransperinealultrasoDokumen8 halamanEarlytoxicitiesofultrahypofractionatedstereotacticbodyradiotherapyforintermediaterisklocalizedprostatecancerusingcone Beamcomputedtomographyandreal Timethree DimensionaltransperinealultrasoHollis LukBelum ada peringkat
- Quantitative Ultrasound Characterization of Responses To Radiotherapy in Cancer Mouse ModelsDokumen10 halamanQuantitative Ultrasound Characterization of Responses To Radiotherapy in Cancer Mouse Modelstaufiqur776Belum ada peringkat
- Modern Advancement in Diagnosis and Treatment of Clinical CancerDokumen4 halamanModern Advancement in Diagnosis and Treatment of Clinical CancerBIJCRBelum ada peringkat
- Bilateral Breast Metastases of Rectal Adenocarcinoma: Case Report and Review of The LiteratureDokumen5 halamanBilateral Breast Metastases of Rectal Adenocarcinoma: Case Report and Review of The LiteratureIJAR JOURNALBelum ada peringkat
- Radioisotopes in MedicineDokumen5 halamanRadioisotopes in MedicineEnya BautistaBelum ada peringkat
- Down-Staging of Early Stage Prostate Can PDFDokumen9 halamanDown-Staging of Early Stage Prostate Can PDFNelma TuiranBelum ada peringkat
- Ecr2014 C-2270Dokumen27 halamanEcr2014 C-2270salahzotoBelum ada peringkat
- Impact of Cold Somatostatin Analog Administration On Somatostatin Receptor ImagingDokumen7 halamanImpact of Cold Somatostatin Analog Administration On Somatostatin Receptor ImagingUvi Cancino RamosBelum ada peringkat
- 35Dokumen174 halaman35sggdgdBelum ada peringkat
- The Dilemmas of Prostate CancerDokumen8 halamanThe Dilemmas of Prostate CancerAugusto MilanezBelum ada peringkat
- Three-Dimensional Conformal Radiation Therapy (3DCRT) For Prostate CancerDokumen34 halamanThree-Dimensional Conformal Radiation Therapy (3DCRT) For Prostate CancerMuhammad Safwan Ahmad FadzilBelum ada peringkat
- Mucoepidermoidcarcinoma of The Pancreas: A Case ReportDokumen3 halamanMucoepidermoidcarcinoma of The Pancreas: A Case ReportIJAR JOURNALBelum ada peringkat
- 2019 Article 6402Dokumen8 halaman2019 Article 6402shahrasyiddBelum ada peringkat
- Narang 2020Dokumen14 halamanNarang 2020Johnny CshBelum ada peringkat
- Towards Personalized Treatment For Prostate CancerDokumen202 halamanTowards Personalized Treatment For Prostate CancerEduar Antonio Landazuri IdroboBelum ada peringkat
- PHD Thesis On Prostate CancerDokumen5 halamanPHD Thesis On Prostate Cancermandyfroemmingfargo100% (2)
- Rodriguez Canales2016Dokumen22 halamanRodriguez Canales2016Triaprasetya HadiBelum ada peringkat
- Klopp 2013Dokumen8 halamanKlopp 2013Insighte ChildcareBelum ada peringkat
- Correlation With Biochemical FailureDokumen11 halamanCorrelation With Biochemical FailureStirBelum ada peringkat
- Efficacy of Trans-Arterial Radio Embolization With Yattrium-90 For Hepatic MalignanciesDokumen8 halamanEfficacy of Trans-Arterial Radio Embolization With Yattrium-90 For Hepatic MalignanciesIJAR JOURNALBelum ada peringkat
- Colorectal Cancer: What Is Colorectal Carcinoma?Dokumen4 halamanColorectal Cancer: What Is Colorectal Carcinoma?Ryana Fitriana IIBelum ada peringkat
- Thesis ProformaDokumen18 halamanThesis ProformaDanish Ahmad KhanBelum ada peringkat
- Prostate MRI and Image Quality The Radiologist'sDokumen4 halamanProstate MRI and Image Quality The Radiologist'sdeaBelum ada peringkat
- bfcr163 Pet-Ct PDFDokumen34 halamanbfcr163 Pet-Ct PDFMirza BaigBelum ada peringkat
- Papaer Prueba 2Dokumen9 halamanPapaer Prueba 2Joselyn CastroBelum ada peringkat
- Jurnal Tugas Minggu Ke 3-AuNP Prostate Cancer MiceDokumen25 halamanJurnal Tugas Minggu Ke 3-AuNP Prostate Cancer Micepath gamingBelum ada peringkat
- Kyalo Staging-TNM, Dukes Focused Investigations of CRCDokumen6 halamanKyalo Staging-TNM, Dukes Focused Investigations of CRCanon_619577898Belum ada peringkat
- Lung Cancer Diagnosis Using Deep Learning VGG-19Dokumen8 halamanLung Cancer Diagnosis Using Deep Learning VGG-19IJRASETPublicationsBelum ada peringkat
- Chapter TwoDokumen7 halamanChapter TwoPrometheus OkoyeBelum ada peringkat
- 2023 APESTNet With Mask R-CNN For Liver Tumor Segmentation and ClassificationDokumen19 halaman2023 APESTNet With Mask R-CNN For Liver Tumor Segmentation and ClassificationgraythomasinBelum ada peringkat
- A Phase 1 Trial of Focal Salvage Stereotactic BodyDokumen11 halamanA Phase 1 Trial of Focal Salvage Stereotactic BodyAhmedBelum ada peringkat
- September 2013 - Radiotherapy For Localised Prostate Cancer - DR V KhooDokumen4 halamanSeptember 2013 - Radiotherapy For Localised Prostate Cancer - DR V KhooHyago FrançaBelum ada peringkat
- Published VersionDokumen13 halamanPublished Versionvinubharath.testerBelum ada peringkat
- Clinical Significance of TC-MDP Imaging & Molecular Biology in The Diagnosis of Bone MetastasesDokumen6 halamanClinical Significance of TC-MDP Imaging & Molecular Biology in The Diagnosis of Bone MetastasesIOSR Journal of PharmacyBelum ada peringkat
- Prostate Cancer PHD ThesisDokumen7 halamanProstate Cancer PHD Thesistonichristensenaurora100% (2)
- Annals Case Reports PDF Final Final.25.05.l22.Dokumen13 halamanAnnals Case Reports PDF Final Final.25.05.l22.rossbar13Belum ada peringkat
- Baumann 2016 IJROBP Development Validation of Bladder Contouring AtlasDokumen9 halamanBaumann 2016 IJROBP Development Validation of Bladder Contouring AtlaszanBelum ada peringkat
- Clinical Oncology: C.W. Williamson, H.C. Liu, J. Mayadev, L.K. MellDokumen12 halamanClinical Oncology: C.W. Williamson, H.C. Liu, J. Mayadev, L.K. MellSebastian GarciaBelum ada peringkat
- Case Study On Prostatic CancerDokumen21 halamanCase Study On Prostatic CancerJai - Ho100% (3)
- Natural History of Asymptomatic Papillary Thyroid Microcarcinoma: Time-Dependent Changes in Calcification and Vasc...Dokumen10 halamanNatural History of Asymptomatic Papillary Thyroid Microcarcinoma: Time-Dependent Changes in Calcification and Vasc...Bob KernsBelum ada peringkat
- Proton Radiotherapy For Solid Tumors of ChildhoodDokumen12 halamanProton Radiotherapy For Solid Tumors of ChildhoodInayah Mumpuni BudiatiBelum ada peringkat
- Vertebral HemangiomaDokumen6 halamanVertebral HemangiomanotallowedBelum ada peringkat
- Development of Prognostic Signatures For Intermediate Risk Papillary Thyroid CancerDokumen12 halamanDevelopment of Prognostic Signatures For Intermediate Risk Papillary Thyroid Cancernfp.panjaitanBelum ada peringkat
- Prostate Cancer Therapy - What You Must KnowDari EverandProstate Cancer Therapy - What You Must KnowPenilaian: 4 dari 5 bintang4/5 (1)
- Prostate Cancer Thesis PDFDokumen4 halamanProstate Cancer Thesis PDFanashahwashington100% (2)
- Fonc 13 1162683Dokumen2 halamanFonc 13 1162683vinadata01Belum ada peringkat
- Man Gad Lao 2018Dokumen41 halamanMan Gad Lao 2018Dinar Dewinta MaharaniBelum ada peringkat
- An Introduction To Radiation Protection in MedicineDokumen287 halamanAn Introduction To Radiation Protection in MedicineYoussef MhamdiBelum ada peringkat
- The PACE Trial: Radiotherapy Planning and Delivery Guidelines (Pace-A and Pace-C)Dokumen33 halamanThe PACE Trial: Radiotherapy Planning and Delivery Guidelines (Pace-A and Pace-C)Анастасия АнохинаBelum ada peringkat
- UK Prostate Trials For 10th Uro-Onc Meeting 2013Dokumen30 halamanUK Prostate Trials For 10th Uro-Onc Meeting 2013Prof_Nick_JamesBelum ada peringkat
- Cesium 131 Prostate BrachytherapyDokumen7 halamanCesium 131 Prostate BrachytherapyPablo Del PozoBelum ada peringkat
- Dos 514 Practice ProblemsDokumen3 halamanDos 514 Practice Problemsapi-210258673Belum ada peringkat
- Diversity 2Dokumen3 halamanDiversity 2api-210258673Belum ada peringkat
- Fall Comp SummaryDokumen2 halamanFall Comp Summaryapi-210258673Belum ada peringkat
- Fall Comp SummaryDokumen2 halamanFall Comp Summaryapi-210258673Belum ada peringkat
- Dos 514 Practice Problems 2Dokumen8 halamanDos 514 Practice Problems 2api-210258673Belum ada peringkat
- DiversityDokumen3 halamanDiversityapi-210258673Belum ada peringkat
- Research Mini ProposalDokumen34 halamanResearch Mini Proposalapi-210258673Belum ada peringkat
- KkresumeDokumen2 halamanKkresumeapi-210258673Belum ada peringkat
- Dos 416 Week 2 DiscussionDokumen3 halamanDos 416 Week 2 Discussionapi-210258673Belum ada peringkat
- Flow ChartDokumen2 halamanFlow Chartapi-210258673Belum ada peringkat
- Budget Activity 1Dokumen1 halamanBudget Activity 1api-210258673Belum ada peringkat
- June Case StudyDokumen9 halamanJune Case Studyapi-210258673Belum ada peringkat
- Dos 711 Certificate of CompletionDokumen1 halamanDos 711 Certificate of Completionapi-210258673Belum ada peringkat
- Case Log TotalsDokumen2 halamanCase Log Totalsapi-210258673Belum ada peringkat
- October CaseDokumen11 halamanOctober Caseapi-210258673Belum ada peringkat
- Citations Week VIIDokumen2 halamanCitations Week VIIapi-210258673Belum ada peringkat
- Citations Week IxDokumen3 halamanCitations Week Ixapi-210258673Belum ada peringkat
- Competency EvaluationDokumen2 halamanCompetency Evaluationapi-210258673Belum ada peringkat
- Transmission Factor CalculationDokumen9 halamanTransmission Factor Calculationapi-2102586730% (1)
- HeterogeneityDokumen8 halamanHeterogeneityapi-210258673Belum ada peringkat
- September Case StudyDokumen9 halamanSeptember Case Studyapi-210258673Belum ada peringkat
- August Case StudyDokumen8 halamanAugust Case Studyapi-210258673Belum ada peringkat
- November Case StudyDokumen7 halamanNovember Case Studyapi-210258673Belum ada peringkat
- Dos 425 Week IIIDokumen2 halamanDos 425 Week IIIapi-210258673Belum ada peringkat
- April Case StudyDokumen7 halamanApril Case Studyapi-210258673Belum ada peringkat
- March Case StudyDokumen7 halamanMarch Case Studyapi-210258673Belum ada peringkat
- Summer EvaluationsDokumen4 halamanSummer Evaluationsapi-210258673Belum ada peringkat
- February Case StudyDokumen6 halamanFebruary Case Studyapi-210258673Belum ada peringkat
- Spring EvaluationDokumen4 halamanSpring Evaluationapi-210258673Belum ada peringkat
- Case Presentation - Iort Breast CancerDokumen25 halamanCase Presentation - Iort Breast Cancerapi-498552797Belum ada peringkat
- Brfo193 Radiotherapy Dose Fractionation Third-Edition PDFDokumen137 halamanBrfo193 Radiotherapy Dose Fractionation Third-Edition PDFMed MedBelum ada peringkat
- Telemedicine Test 10ºDokumen5 halamanTelemedicine Test 10ºCarlosBelum ada peringkat
- Cancer Colon and Rectum (Wardah)Dokumen23 halamanCancer Colon and Rectum (Wardah)WardahAliBelum ada peringkat
- Abdomen & Pelvis Pathology - CT-scanDokumen53 halamanAbdomen & Pelvis Pathology - CT-scanKeithKam100% (1)
- Dissertation On Cervical Cancer ScreeningDokumen7 halamanDissertation On Cervical Cancer ScreeningSomeToWriteMyPaperUK100% (1)
- Pa Tho Physiology and NCP Lung CancerDokumen2 halamanPa Tho Physiology and NCP Lung CancerAlleizarg EuqorBelum ada peringkat
- Breast Cancer and Pregnancy: How To Proceed?Dokumen3 halamanBreast Cancer and Pregnancy: How To Proceed?IJAR JOURNALBelum ada peringkat
- Radiology Pediatric Bone TumorsDokumen69 halamanRadiology Pediatric Bone Tumorsdrqazi777Belum ada peringkat
- Sead Ferizovic BrochureDokumen2 halamanSead Ferizovic BrochureNedim VrtagicBelum ada peringkat
- Small Round Cell Tumors of Soft Tissue and BoneDokumen13 halamanSmall Round Cell Tumors of Soft Tissue and BoneAdriana Gabriela Ugarte MacíasBelum ada peringkat
- Dilemma A Case of Ovarian Sertoliform Endometrioid CarcinomaDokumen8 halamanDilemma A Case of Ovarian Sertoliform Endometrioid CarcinomaArasse Dino Gonzales MartinezBelum ada peringkat
- GP 96Dokumen24 halamanGP 96Study MaterialBelum ada peringkat
- Proton TherapyDokumen5 halamanProton TherapySONY MANDAPBelum ada peringkat
- Veterinary OncologyDokumen311 halamanVeterinary Oncologyzoran gacevski100% (3)
- Soft Tissue Tumors: AdipocyticDokumen29 halamanSoft Tissue Tumors: AdipocyticDaffa ArkanantaBelum ada peringkat
- Pathophysiology For Colorectal CancerDokumen12 halamanPathophysiology For Colorectal CancerRosemarie Carpio100% (6)
- Jurnal Double Kontras FIXDokumen33 halamanJurnal Double Kontras FIXWisnuHeriPurwantoBelum ada peringkat
- Exam 3Dokumen8 halamanExam 3pauchanmnlBelum ada peringkat
- Biology Project: Lung CancerDokumen12 halamanBiology Project: Lung CancerKarm ParmarBelum ada peringkat
- Ojsadmin, 1340Dokumen4 halamanOjsadmin, 1340Dr NisrinBelum ada peringkat
- CancerDokumen55 halamanCancerDana KaremBelum ada peringkat
- Case Study Intramedullary Spinal Cord TumorDokumen13 halamanCase Study Intramedullary Spinal Cord TumorCitra KristiBelum ada peringkat
- Chapter 7 - NeoplasiaDokumen23 halamanChapter 7 - NeoplasiaAgnieszka WisniewskaBelum ada peringkat
- Colorectal Cancer: By:-Vaibhav SwarupDokumen18 halamanColorectal Cancer: By:-Vaibhav SwarupOlga GoryachevaBelum ada peringkat
- Gyne Book 20122Dokumen194 halamanGyne Book 20122თამარ ჩალაურიBelum ada peringkat
- Oncology and Palliative Care 2023 FinalDokumen99 halamanOncology and Palliative Care 2023 FinalBelinda ELISHABelum ada peringkat
- Benign and Malignant Lesions in Respiratory CytologyDokumen43 halamanBenign and Malignant Lesions in Respiratory CytologyfadoBelum ada peringkat
- Breast Cancer Metastasis and Drug Resistance Progress and ProspectsDokumen416 halamanBreast Cancer Metastasis and Drug Resistance Progress and ProspectsRichard Balili100% (1)
- Types of Ovarian CancerDokumen5 halamanTypes of Ovarian Cancerوسام وميض عبدالله CBelum ada peringkat