Sheet 'H+ Estimation' of Biomass Hydrolysis
Diunggah oleh
nizar ahmad0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
56 tayangan1 halamanThis is sheet of 'H+ Estimation' which is part of my spreadsheet/workbook 'Biomass Hydrolysis'. Available as online spreadsheet at http://calcol.ucoz.com.
Hak Cipta
© Attribution Non-Commercial (BY-NC)
Format Tersedia
PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniThis is sheet of 'H+ Estimation' which is part of my spreadsheet/workbook 'Biomass Hydrolysis'. Available as online spreadsheet at http://calcol.ucoz.com.
Hak Cipta:
Attribution Non-Commercial (BY-NC)
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
56 tayangan1 halamanSheet 'H+ Estimation' of Biomass Hydrolysis
Diunggah oleh
nizar ahmadThis is sheet of 'H+ Estimation' which is part of my spreadsheet/workbook 'Biomass Hydrolysis'. Available as online spreadsheet at http://calcol.ucoz.com.
Hak Cipta:
Attribution Non-Commercial (BY-NC)
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 1
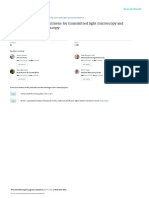
1.
Input data in yellow cells
2. Observe whether Q_trial ~ Q_actual
3. If Q_trial is not ~ Q_actual, set Q_trial = Q_actual
manually
4. Do no. 3 untill Q_trial ~ Q_actual
1. Calculation of concentration of acid solution added to fresh wood
Specify weight percent of acid for hydrolysis* 1.00
* weight of pure acid divided by water from wood (moisture) plus from acid solution
Specify weight of dry wood to be hydrolysed 100 kg
Specify moisture of wood to be hydrolysed 50 %w of fresh wood
Water from wood to be hydrolysed 100 kg
Specify weight of pure acid (H2SO4) to be added 2 kg
Water from acid solution to be added 100 kg
Then:
Concentration of acid solution added to fresh wood 2 %w
2. Calculation of Corrected Hydrogen Ion Concentration (Neutralizing Capacity of Wood)
Specify temperature of hydrolysis 140 oC 413.15 K
EQW (neutralizing capacity of wood) 131 meq/kg OD wood
EQR (neutralizing capacity of residue) 49 meq/kg OD wood
A (an empirical constant) 0.4
LS (Liquid-to-solid ratio) 2 kg/kg
EQ (equivalents of cations released to solution) 8.2 meq/kg OD wood
Concentrations of sulfate plus bisulfate ions 0.103072 mol/ 1 kg of water
Concentration of cations in solution 0.041414 eq/ 1 kg of water
D (dielectric constant for water) 37.38198
K (bisulfate dissociation equilibrium constant) 0.000265
S (Debye-Hückel limiting slope at temperature) 0.950495
Q_trial (bisulfate dissociation quotient) 0.000797
Target Q_trial ~ Q_actual
Q_actual (bisulfate dissociation quotient) 0.000792
Then
[H+] (Corrected Hydrogen Ion Concentration) = 0.062946
Concentration of sulfate ions in solution [HSO4-] 0.101783
Concentrations of bisulfate ions in solution [SO42-] 0.001289
IS (ionic strength) 0.105649
Create PDF with GO2PDF for free, if you wish to remove this line, click here to buy Virtual PDF Printer
Anda mungkin juga menyukai
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (345)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- REHS2346 - Weld Repair Procedure For The Rear Axle A-Frame and Rear Axle Housing On 785, 789, and 793 Off-Highway TrucksDokumen34 halamanREHS2346 - Weld Repair Procedure For The Rear Axle A-Frame and Rear Axle Housing On 785, 789, and 793 Off-Highway TrucksrubenBelum ada peringkat
- Spectrometric Identification of Organic CompoundsDokumen466 halamanSpectrometric Identification of Organic CompoundsMarie L100% (5)
- Plastics PDFDokumen18 halamanPlastics PDFV Phanindra BoguBelum ada peringkat
- Heat Balance of Rotary DryerDokumen2 halamanHeat Balance of Rotary Dryernizar ahmadBelum ada peringkat
- Analysis of Cement by Banana FiberDokumen48 halamanAnalysis of Cement by Banana FiberKyaw KhineBelum ada peringkat
- Design of Rotary DryerDokumen2 halamanDesign of Rotary Dryernizar ahmad57% (7)
- Muhamad+Nizar CV 20120504Dokumen3 halamanMuhamad+Nizar CV 20120504nizar ahmadBelum ada peringkat
- Muhamad Nizar - CVDokumen12 halamanMuhamad Nizar - CVnizar ahmadBelum ada peringkat
- Vvsizing: Prepared By: Muhamad NizarDokumen4 halamanVvsizing: Prepared By: Muhamad Nizarnizar ahmadBelum ada peringkat
- Sounds of EquipmentsDokumen3 halamanSounds of Equipmentsnizar ahmadBelum ada peringkat
- Biodiesel KineticsDokumen4 halamanBiodiesel Kineticsnizar ahmad0% (1)
- EFs For MiningDokumen5 halamanEFs For Miningnizar ahmadBelum ada peringkat
- Sheet 'Intro' of Biomass HydrolysisDokumen1 halamanSheet 'Intro' of Biomass Hydrolysisnizar ahmadBelum ada peringkat
- Sheet 'Main Calculations' of Biomass HydrolysisDokumen2 halamanSheet 'Main Calculations' of Biomass Hydrolysisnizar ahmadBelum ada peringkat
- LalruDokumen98 halamanLalruAbhinav KumarBelum ada peringkat
- Comprehensive Chemical Kinetics BamfordDokumen633 halamanComprehensive Chemical Kinetics BamfordDiana Montagut50% (2)
- A Report On Fire Extinguisher OperationDokumen3 halamanA Report On Fire Extinguisher OperationrajkotbpBelum ada peringkat
- Estimate For Water SupplyDokumen2 halamanEstimate For Water SupplyAbdulsamad RacmanBelum ada peringkat
- NDT-SA-ARAMCO-MCCL-PT-57 Rev 00-Date-26-June-2023Dokumen20 halamanNDT-SA-ARAMCO-MCCL-PT-57 Rev 00-Date-26-June-2023SANJEEV YADAVBelum ada peringkat
- Eucomex - Eucomex - EnglishDokumen2 halamanEucomex - Eucomex - EnglishNelson IbarraBelum ada peringkat
- Royal Ultra 865MDokumen9 halamanRoyal Ultra 865Mjuanfdez42368Belum ada peringkat
- BelgJBotJansenetal1998 PDFDokumen10 halamanBelgJBotJansenetal1998 PDFalexBelum ada peringkat
- Module 1 DOMESTIC REFRIGERATION AND AIR CONDITIONINGDokumen6 halamanModule 1 DOMESTIC REFRIGERATION AND AIR CONDITIONINGKaiRae AsakuraBelum ada peringkat
- Introductions To Water and Wastewater Treatment Basics of Water Supply Networks Forecasting Methods On Site and Centralized Treatment SystemsDokumen397 halamanIntroductions To Water and Wastewater Treatment Basics of Water Supply Networks Forecasting Methods On Site and Centralized Treatment SystemsSrivvass ReddyBelum ada peringkat
- A Case Study On Solar Vapour Absorption Refrigeration SystemDokumen7 halamanA Case Study On Solar Vapour Absorption Refrigeration SystemShivam MaheraBelum ada peringkat
- Astm D1208 - 1 (En)Dokumen3 halamanAstm D1208 - 1 (En)rezokaBelum ada peringkat
- Protego 2020 - 21Dokumen429 halamanProtego 2020 - 21jleonclau1Belum ada peringkat
- Stainless Steel Putty (ST) : Technical Data SheetDokumen2 halamanStainless Steel Putty (ST) : Technical Data SheetSreenivasBelum ada peringkat
- Metallic BiomaterialsDokumen26 halamanMetallic BiomaterialspufarinaaBelum ada peringkat
- University of Cambridge International Examinations International General Certifi Cate of Secondary EducationDokumen16 halamanUniversity of Cambridge International Examinations International General Certifi Cate of Secondary Educations_rashidaBelum ada peringkat
- PE6705 Water Flooding and Enhanced Oil Recovery L T P C 3 0 0 3 Objective: Unit I 9Dokumen6 halamanPE6705 Water Flooding and Enhanced Oil Recovery L T P C 3 0 0 3 Objective: Unit I 9Prince ImmanuelBelum ada peringkat
- Year 10 Revision Paper QPDokumen15 halamanYear 10 Revision Paper QPketamineBelum ada peringkat
- Ge 2009Dokumen3 halamanGe 2009RICHARD MACIENTE SILVINO DA SILVABelum ada peringkat
- Melane Zintle Prac 1Dokumen3 halamanMelane Zintle Prac 1Zintle MelaneBelum ada peringkat
- Air Pollution - A Review: October 2020Dokumen29 halamanAir Pollution - A Review: October 2020NasrinTalebpourBelum ada peringkat
- Mitsubishi CBN PCDDokumen70 halamanMitsubishi CBN PCDtoms4Belum ada peringkat
- Simulation of Wax Deposition Model For Various FieldDokumen53 halamanSimulation of Wax Deposition Model For Various FieldAyauwu LovedayBelum ada peringkat
- Chemistry Sample Syllabus 2 Id 1029717v1Dokumen13 halamanChemistry Sample Syllabus 2 Id 1029717v1Wong Weng SiongBelum ada peringkat
- PROTEINSDokumen5 halamanPROTEINSYLADE, ERICCA ANDREABelum ada peringkat
- 207 HD 52505 Uv Jam PetrochemicalDokumen2 halaman207 HD 52505 Uv Jam Petrochemicalriza abdollahyBelum ada peringkat