HKDSE Chemistry 2014 Paper1A
Diunggah oleh
BILLYNG330Deskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
HKDSE Chemistry 2014 Paper1A
Diunggah oleh
BILLYNG330Hak Cipta:
Format Tersedia
This section consists of two parts. There are 24 questions in PART I and 12 questions in PART II.
Choose the best answer for each question. Candidates may refer to the Periodic Table printed on page 20 of Question-Answer Book B.
PART I 1. Which of the following substance is the main constituent of town gas? A. B. C. D. 2. hydrogen methane carbon monoxide gaseous naphtha
Element Q forms a stable Q2+ ion. What may the atomic number of Q be? . A. B. C. D. 6 11 14 20
3.
The formula for ozone is O3. If one mole of ozone contains x atoms, how many atoms will atoms will one mole of oxygen gas contain? A. B. C. D. x/3. 2x/3. 3x/2. 3x.
4.
The atomic number of element X is 15. X reacts with chlorine to form a chloride. Which of the following can represent the electronic diagram of the chloride? (Only electrons in the outermost shells are shown.)
2014-DSE-CHEM-1A-2
5.
Which of the following environmental problems is NOT caused by excessive burning of fossil fuels? A. B. C. D. the corrosion of marble statues. the formation of smog. a higher incidence of liver diseases. global warning.
6.
A detergent has the following structure:
Which of the following statements concerning the detergent is correct? A. B. C. D. 7. Its hydrocarbon chain is hydrophilic. It can be manufactured from vegetable. It is readily degraded by micro-organisms. It acts as an emulsifier in the cleaning process.
The formula of a solid dibasic acid is H2X. 2.88 g of the acid is dissolved in some distilled water and the solution is then diluted to 250.0 cm3 with distilled water . 25.0 cm3 of the diluted solution requires 16.0 cm3 of 0.40 M sodium hydroxide solution for complete neutralization. What is the molar mass of H2X.? A. B. C. D. 22.5 g 45.0 g. 90.0 g. 180.0 g.
8.
Compound X is a white solid. When X is warmed with sodium hydroxide solution, a gas is evolved which turns moist red litmus paper blue. When chloride water is added to an aqueous solution of X, a brown solution is formed. X is probably A. ammonium bromide. B. ammonium chloride. C. sodium bromide. D. sodium chloride.
2014-DSE-CHEM-1A-3
9.
When a glass of wine is left overnight, it becomes sour. Which of the following reactions is responsible for this change? A. B. C. D. Fermentation. Oxidation. Dehydration. Esterification.
10.
A zinc-carbon cell is connected to a voltmeter as shown in the below diagram.
Which of the following statements concerning the set-up is INCORRECT ? A. B. C. D. 11. electrons flow from the zinc case to the carbon rod in the external circuit. The zinc case gradually becomes thinner as the cell discharges. Manganese () oxide acts as an oxidizing agent. Ammonium chloride acts as a reducing agent.
Which of the following statements concerning one mole of nitrogen gas is/are correct? (1) It has a mass of 14.0 g. (2) It occupies the same volume as 4.0 g of helium gas at room temperature and pressure. (3) It contains 6.02 1023 atoms of nitrogen. (Relative atomic masses : He=4.0, N = 14.0 Avogadro constant=6.02 1023 mol-1) A. B. C. D. (1) only. (2) only. (1) and (3) only (2) and (3) only
2014-DSE-CHEM-1A-4
12.
A mixture contains only copper (II) oxide and anhydrous copper (II) sulphate. Which of the following methods can be used to separate copper (II) oxide from the mixture? (1) (2) (3) Add water to the mixture and then filter. Add dilute nitric acid to the mixture and then filter. Add concentrated hydrochloric acid to the mixture and then filter. A. B. C. D. (1) only. (2) only. (1) and (3) only (2) and (3) only
13.
Directions : Q.13 and Q.14 refer to the following experiment.
Which of the following combinations is correct? A. B. C. D. 14. Gas X Chlorine Chlorine Hydrogen Oxygen Gas Y Hydrogen Oxygen Chlorine Hydrogen
Which of the following statements concerning the above experiment is/are correct? (1) (2) (3) Platinum electrodes should be used. The concentration of Na+ (aq) ions around the cathode increase . The solution changes from colorless to pink. A. B. C. D. (1) only. (2) only. (1) and (3) only (2) and (3) only
2014-DSE-CHEM-1A-5
15.
In Which of the following equations does the underlined substances become reduced? A. B. C. D. CuSO4 + Zn 2 FeCl2 + Cl2 Pb(OH)2 + 2HNO3 MgCO3 + 2HCl ZnSO4 + Cu 2FeCl3 Pb(NO3)2 + 2H2O MgCl2 + CO2 + H2O
16.
A student performed a titration in which he added an acid from a burette to an alkali contained in a conical flask. The following diagrams show the initial and finial readings of the burette.
What was the volume of the acid added form the burette to the conical flask? A. B. C. D, 17. 24.5 cm3. 24.6 cm3. 24.7 cm3. 32.3 cm3.
Which of the following statements concerning the reaction of aqueous ammonia with with hydrochloric acid is correct? A. B. C. D. The reaction is exothermic. A white precipitate is formed. Ammonium chloride and chloride are produced. The product ammonium chloride is a covalent compound.
18.
Metal X reacts with dilute hydrochloric acid to liberate hydrogen. But metal Y and metal Z have no reaction with the dilute acid. The oxide of metal Y decomposes on heating but the oxide of metal Z does not. Which of the following arrangements represents the order of increasing reactivity of the three metals? A. B. C. D. X<Y<Z Y<Z<X X<Z<Y Z<Y<X
2014-DSE-CHEM-1A-6
19.
Which of the following methods is NOT used to preserve food ? A. B. C. D. Adding salt. Adding vinegar Adding aqueous ammonia. Storing at a low temperature.
20.
What volume of water is required to dilute 100 cm3 of 8 M hydrochloric acid to a concentration of 2 M? A. B. C. D. 200 cm3 300 cm3 400 cm3 700 cm3
21.
Which of the following statements concerning a silver oxide cell is/are correct? (1) (2) (3) The cell is rechargeable. The cell can maintain a steady voltage during discharge. The positive electrode of the cell is silver oxide. A. B. C. D. (1) only. (2) only. (1) and (3) only (2) and (3) only process is/are correct?
22.
Which of the following statements concerning the contact (1) (2) (3)
The sulphur dioxide used in the process can be produced by roasting sulphide ores. A gaseous mixture of sulphur dioxide and oxygen in the mole ratio of 1 : 2 is passed into the catalytic conversion chamber. The sulphur trioxide produced is absorbed by water in the absorption tower. A. B. C. D. (1) only. (2) only. (1) and (3) only (2) and (3) only
2014-DSE-CHEM-1A-7
Directions: Each question below (Question Nos. 23 to 24) consists of two separate statements . Decide whether each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of first statement. Then select one option from A to D according to the following table : A. Both statements are true and the 2 nd statement is a correct explanation of the 1 st statement. B. Both statements are true but the 2 nd statement is NOT a correct explanation of the 1 st statement. C. The 1 st statement is false but the 2 nd statement is true. D. Both statements are false. 1 st statement 23. The basicity of ethanoic acid is four 2 nd statement One molecule of ethanoic acid contains four atoms of hydrogen Carbon dioxide can trap the solar energy which is re-radiated from the earths surface to the atmosphere.
24.
Huge quantities of carbon dioxide produced by the burning of fossil fuels cau
2014-DSE-CHEM-1A-8
PART II 25. The formula below can be used to represent the structure of some polymers.
(X represents an atom or a group of atoms.) Which of the following combinations is INCORRECT ? X A. B. C. D. 26. H Cl CH3 C6H5 Name of polymers Polyethene Polyvinyl chloride Perspex Polystyrene
A certain amount of silver oxide is heated in a tested tube. Which of the following graphs represents the correct plot of the mass of the contents of the test tube against time.
2014-DSE-CHEM-1A-9
27.
Which of the following statements concerning the recycling of plastics are correct? (1) (2) (3) The recycling of plastic can help to solve the disposal problem of plastic. The recycling of plastic can save raw materials derived from petroleum. It is difficult to separate different types of plastic in the recycling process. A. B. C. D. (1) and (2) only (1) and (3) only (2) and (3) only (1),(2) and (3)
28.
Which of the following products may be formed from the cracking of heavy oil fraction? (1) (2) (3) CO. C2H4. C8H18. A. B. C. D. (1) and (2) only (1) and (3) only (2) and (3) only (1),(2) and (3)
29.
Which of the following are characteristics of a homologous series? (1) (2) (3) Members of the series can be represented by the same general formula. Members of the series have same physical properties. Members of the series have similar chemical properties. A. B. C. D. (1) and (2) only (1) and (3) only (2) and (3) only (1),(2) and (3)
30.
Which concentrated sulphuric acid is added dropwise to a solution containing urea and methanal in a test tube with shaking, a white solid can be formed. Which of the following statements about this experiment is/ are correct? (1) (2) (3) The reaction involved is condensation polymerization. Concentration sulphuric acid acts as an oxidizing agent. The white solid is a thermoplastic substance. A. B. C. D. (1) only (2) only (1) and (3) only (2) and (3) only
2014-DSE-CHEM-1A-10
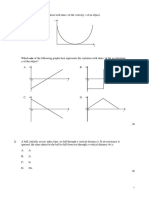
31. .
A mix xture of am mmonia and oxygen is passed p over red hot plat tinum for a few minute es. the fo ollowing gra aphs represents a plot of o the mass of platinum m against tim me?
Which of
32. .
The reaction r betw ween X(aq) ) and Y(aq) is represen nted by the following f eq quation: X(aq) ) + Y(aq) Z(aq) Whic ch of the fol llowing grap phs represen nts the chan nge of conce entration of f Y(aq) with h time when w fixed quantities of o X(aq) an nd Y(aq) are e introduced d into a closed containe er?
33. .
On he eating, blue e copper(II) sulphate cr rystals gradu ually chang ge to a white e powder. Which of th he follow wing statem ments are correct? (1) (2) (3) Heat wou uld be liber rated if wate er is added to t the white e powder. On further heating, the t white po owder woul ld turn redd dish-brown. A chemic cal change occurs o durin ng the heati ing of the bl lue crystals. A. B. C. D. (1) and (2) only (1) and (3) only (2) and (3) only (1), (2) and d (3)
201 14-DSE-CHE EM-1A-11
34.
The following experiment on the study of the rate of reaction between hydrochloric acid and sodium thiosulphate solution: 10 cm3 portions of 2.0M hydrochloric acid were added to four separate conical flasks each containing sodium thiosulphate solution which was prepared respectively as follows:
sodium thiosulphate solution conical flask concentration volume W X Y Z 1.0M 1.5M 2.5M 3.0M 80 cm3 60 cm3 30 cm3 20 cm3
volume of water 10 cm3 30 cm3 60 cm3 70 cm3
In which of the above conical flasks does the reaction proceed at the fastest rate? A. B. C. D. W X Y Z
Directions: Each question below (Question Nos. 23 to 24) consists of two separate statements . Decide whether each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of first statement. Then select one option from A to D according to the following table : A. Both statements are true and the 2 nd statement is a correct explanation of the 1 st statement. B. Both statements are true but the 2 nd statement is NOT a correct explanation of the 1 st statement. C. The 1 st statement is false but the 2 nd statement is true. D. Both statements are false. 1 st statement 35. 36. Concentrated sulphuric acid canbe used to prepare nitric acid. When car exhaust gases are bubbled into citrated blood, the colour of the blood gradually becomes cherry red. 2 nd statement Sulphuric acid is more volatile than nitric acid Nitrogen dioxide is present in car exhaust gases.
END OF SECTION A
2014-DSE-CHEM-1A-12
Anda mungkin juga menyukai
- Good Hope 1718 Paper 1 PDFDokumen22 halamanGood Hope 1718 Paper 1 PDFVincent haBelum ada peringkat
- Test 21kinematics20151002Dokumen26 halamanTest 21kinematics20151002laguiiniBelum ada peringkat
- Po Leung Kuk No.1 W.H.Cheung College Yearly Examination (2021-2022) FORM 6 CHEMISTRY PAPER 2 Suggested AnswersDokumen4 halamanPo Leung Kuk No.1 W.H.Cheung College Yearly Examination (2021-2022) FORM 6 CHEMISTRY PAPER 2 Suggested AnswersChun Kit LauBelum ada peringkat
- PTSD Checklist (PCL)Dokumen1 halamanPTSD Checklist (PCL)Manikanta Sai KumarBelum ada peringkat
- F3 Maths 1314 1stexam Paper 1Dokumen3 halamanF3 Maths 1314 1stexam Paper 1伊貝P-0% (1)
- Redox Reactions, Chemical Cells and Electrolysis (Less than 40 chars: 35 charsDokumen38 halamanRedox Reactions, Chemical Cells and Electrolysis (Less than 40 chars: 35 charsミーチェルBelum ada peringkat
- Review Worksheet - KeyDokumen4 halamanReview Worksheet - KeySAMMARBelum ada peringkat
- Herbal Abortifacient Drugs A ReviewDokumen6 halamanHerbal Abortifacient Drugs A Reviewyogesh ushirBelum ada peringkat
- Queens 2020-2021 Mock Exam F6 Math Paper 2Dokumen15 halamanQueens 2020-2021 Mock Exam F6 Math Paper 2Gabriel Fung100% (1)
- Infants and ToddlersDokumen14 halamanInfants and ToddlersJosias Smith100% (1)
- New Senior Secondary MASTERING BIOLOGY OXFORD Mock - Bio - Set6 - e - AnsDokumen7 halamanNew Senior Secondary MASTERING BIOLOGY OXFORD Mock - Bio - Set6 - e - AnsChun Kit LauBelum ada peringkat
- English Language Paper 1 (Sample Paper) : Question-Answer BookDokumen21 halamanEnglish Language Paper 1 (Sample Paper) : Question-Answer BookPinky Chu100% (1)
- Assignment AC U2 Final eDokumen28 halamanAssignment AC U2 Final eYuenHei KwokBelum ada peringkat
- CH 3Dokumen15 halamanCH 3Choi MingBelum ada peringkat
- Analysis of Grape and WineDokumen116 halamanAnalysis of Grape and WineElenaTrofimBelum ada peringkat
- Chemsheets GCSE 1282 Revision 18 ANSDokumen2 halamanChemsheets GCSE 1282 Revision 18 ANSchinkey lolBelum ada peringkat
- HKDSE Chem FX Mock Exam Paper 1 2012 Set 1 Eng AnsDokumen10 halamanHKDSE Chem FX Mock Exam Paper 1 2012 Set 1 Eng Ansleung_ting_2Belum ada peringkat
- Measure pH Using IndicatorsDokumen20 halamanMeasure pH Using IndicatorsRyanBelum ada peringkat
- HKDSE Chem FX ExamS5 2011 Set1 EngDokumen27 halamanHKDSE Chem FX ExamS5 2011 Set1 Eng12376590Belum ada peringkat
- Tank Cleaning ConsiderationsDokumen1 halamanTank Cleaning ConsiderationsAdele PollardBelum ada peringkat
- 1.4 Market FailureDokumen42 halaman1.4 Market FailureRuban PaulBelum ada peringkat
- Reacting Masses WorksheetDokumen1 halamanReacting Masses WorksheetMazanda YalinduaBelum ada peringkat
- F.4 Acids and Exercise)Dokumen69 halamanF.4 Acids and Exercise)arielshy100% (5)
- HKDSE Chemistry MC Chapter 13Dokumen8 halamanHKDSE Chemistry MC Chapter 13ScribdBelum ada peringkat
- Spxflow PDFDokumen317 halamanSpxflow PDFAnonymous q2iHVf100% (3)
- PP Chem EDokumen43 halamanPP Chem ETacky HongBelum ada peringkat
- Mole Concept ExerciseDokumen2 halamanMole Concept Exercisechong56100% (1)
- Calculation Exercise Answer Key Provides Detailed SolutionsDokumen6 halamanCalculation Exercise Answer Key Provides Detailed SolutionsBelladonna Lee100% (1)
- Past Paper - Microscopic WorldDokumen7 halamanPast Paper - Microscopic Worldapi-3739994100% (1)
- Hkdse Chemistry - A Modern View (Chemistry) : Coursebook 3 Suggested AnswersDokumen71 halamanHkdse Chemistry - A Modern View (Chemistry) : Coursebook 3 Suggested AnswersDennis Tik Hei FungBelum ada peringkat
- Afterschool Mole Calculation Exercise Ans.Dokumen32 halamanAfterschool Mole Calculation Exercise Ans.J TBelum ada peringkat
- HKDSE Physics Formulae List (English Version)Dokumen5 halamanHKDSE Physics Formulae List (English Version)flowerinsnowBelum ada peringkat
- NSS Chemistry Part 2 Structural Questions and AnswersDokumen22 halamanNSS Chemistry Part 2 Structural Questions and AnswersFelix YueBelum ada peringkat
- Structured Questions: HKDSE Chemistry A Modern View Part VIII Chemical Reactions and EnergyDokumen21 halamanStructured Questions: HKDSE Chemistry A Modern View Part VIII Chemical Reactions and EnergyNg Swee Loong StevenBelum ada peringkat
- SSGS 17-18 F.6 Final Exam 1 and 2 CHEMDokumen37 halamanSSGS 17-18 F.6 Final Exam 1 and 2 CHEMKelvin ChowBelum ada peringkat
- MSS 1718MockPaper2Dokumen8 halamanMSS 1718MockPaper2Kelvin ChowBelum ada peringkat
- 0910 F4 M1+M2 Half Yearly ExamDokumen4 halaman0910 F4 M1+M2 Half Yearly ExamAaron KwongBelum ada peringkat
- MATHEMATICS Extended Part Module 1 (Calculus and Statistics) Question-Answer BookDokumen20 halamanMATHEMATICS Extended Part Module 1 (Calculus and Statistics) Question-Answer BookliBelum ada peringkat
- MSS 1718MockPaper1ADokumen11 halamanMSS 1718MockPaper1AKelvin Chow100% (1)
- Exam Paper - M1 (By Topic)Dokumen19 halamanExam Paper - M1 (By Topic)Henry Leung100% (1)
- F3 Challenging Exercise 11Dokumen3 halamanF3 Challenging Exercise 11pgkbgjrh5pBelum ada peringkat
- Laws of IndicesDokumen52 halamanLaws of IndicesAnonymous PPYjNttBelum ada peringkat
- The Ultimate Question Bank: Dse Chem MasteryDokumen48 halamanThe Ultimate Question Bank: Dse Chem MasteryYip AvaBelum ada peringkat
- Topic 2 Microscopic World IDokumen15 halamanTopic 2 Microscopic World IBelladonna LeeBelum ada peringkat
- F.3 Heat NoteDokumen12 halamanF.3 Heat Noteskywalker_handsomeBelum ada peringkat
- Chapter 3 Change of State: Multiple-Choice QuestionsDokumen63 halamanChapter 3 Change of State: Multiple-Choice Questionssuperpooh-1Belum ada peringkat
- Nutrition in humans: Modes and processesDokumen9 halamanNutrition in humans: Modes and processesCHIU KEUNG OFFICIAL PROBelum ada peringkat
- Reacting Masses: Calculating Amounts in Chemical ReactionsDokumen36 halamanReacting Masses: Calculating Amounts in Chemical ReactionsRyan100% (1)
- Gas Exchange in Humans 1 QP PDFDokumen9 halamanGas Exchange in Humans 1 QP PDFSyakir FahmieBelum ada peringkat
- New Senior Secondary Physics at Work (Second Edition)Dokumen10 halamanNew Senior Secondary Physics at Work (Second Edition)Jeffrey YuetBelum ada peringkat
- Answers To 2017-2018 F3-CHEM Final Examination: Section A: Multiple ChoicesDokumen13 halamanAnswers To 2017-2018 F3-CHEM Final Examination: Section A: Multiple Choicesjonas hoBelum ada peringkat
- NSS Chemistry Part 2 The Microscopic World HKCEE Past Paper Question The Microscopic World I Ns - Multiple Choice QuestionsDokumen32 halamanNSS Chemistry Part 2 The Microscopic World HKCEE Past Paper Question The Microscopic World I Ns - Multiple Choice QuestionsミーチェルBelum ada peringkat
- 2019 Chem Dse Paper IB MSDokumen10 halaman2019 Chem Dse Paper IB MSYuet Ki SoBelum ada peringkat
- Year 8 Science Examination Semester 2 2016: Multiple Choice Questions - Book 1Dokumen19 halamanYear 8 Science Examination Semester 2 2016: Multiple Choice Questions - Book 1Preeti GillBelum ada peringkat
- Hkdse Chemistry - A Modern View: (Second Edition) (Reprinted With Minor Amendments 2019)Dokumen25 halamanHkdse Chemistry - A Modern View: (Second Edition) (Reprinted With Minor Amendments 2019)Tat LBelum ada peringkat
- 4ACh01 (Quadratic Equations in One Unknown)Dokumen32 halaman4ACh01 (Quadratic Equations in One Unknown)api-3826695Belum ada peringkat
- Chapter 2 Multiple-Choice QuestionsDokumen8 halamanChapter 2 Multiple-Choice QuestionsDavid LouBelum ada peringkat
- Biology CH.41Dokumen56 halamanBiology CH.41yanaaBelum ada peringkat
- HKDSE Past Paper 3D Trigonometry (2 Files Merged) PDFDokumen10 halamanHKDSE Past Paper 3D Trigonometry (2 Files Merged) PDFJackNgBelum ada peringkat
- MATHEMATICS Compulsory Part Paper 1: Question-Answer BookDokumen35 halamanMATHEMATICS Compulsory Part Paper 1: Question-Answer Book20/21-5B-(05) HoMeiYi/何美誼Belum ada peringkat
- Concentration of SolutionsDokumen20 halamanConcentration of SolutionsRyanBelum ada peringkat
- Supplementary Notes 補充筆記 (Centers of Triangle 三角形的心)Dokumen3 halamanSupplementary Notes 補充筆記 (Centers of Triangle 三角形的心)Henry Leung0% (1)
- Chemistry 1995 Paper 2Dokumen14 halamanChemistry 1995 Paper 2api-3824003Belum ada peringkat
- HKDSE Chem FX Mock Exam Paper 1 2012 Set 1 EngDokumen28 halamanHKDSE Chem FX Mock Exam Paper 1 2012 Set 1 Engleung_ting_2100% (1)
- Sec 4 Chem Prelim Paper 1Dokumen16 halamanSec 4 Chem Prelim Paper 1TeckluckyBelum ada peringkat
- 7 (N) - 1Dokumen24 halaman7 (N) - 1Vinaigrette HeBelum ada peringkat
- 2017-18 F5 CHE Yearly Exam Paper 1A (Multiple Choice)Dokumen12 halaman2017-18 F5 CHE Yearly Exam Paper 1A (Multiple Choice)夜紫薇Belum ada peringkat
- 4th QUARTER EXAMINATION IN TLE 8Dokumen3 halaman4th QUARTER EXAMINATION IN TLE 8judy ann sottoBelum ada peringkat
- Deck Damage and Penetrations: Prepared by Richard B. Heagler, P.EDokumen9 halamanDeck Damage and Penetrations: Prepared by Richard B. Heagler, P.ENelzon MamaniBelum ada peringkat
- Magnum 3416 SC: Product InformationDokumen2 halamanMagnum 3416 SC: Product InformationDridi BadredineBelum ada peringkat
- Reglas para Añadir Al Verbo Principal: Am Is Are ReadDokumen8 halamanReglas para Añadir Al Verbo Principal: Am Is Are ReadBrandon Sneider Garcia AriasBelum ada peringkat
- Final ESIA On Construction Materials - Tamiru BultoDokumen110 halamanFinal ESIA On Construction Materials - Tamiru BultoKayo Shankulie100% (1)
- Amazon To Unionize or NotDokumen4 halamanAmazon To Unionize or NotPatrick MutetiBelum ada peringkat
- F6003 5W40 Synthetic Oil Technical Data SheetDokumen1 halamanF6003 5W40 Synthetic Oil Technical Data SheetValeriy ValkovetsBelum ada peringkat
- INTUSSUSCEPTIONDokumen1 halamanINTUSSUSCEPTIONMaecy PasionBelum ada peringkat
- Optimize soil preparation with a versatile mini tillerDokumen2 halamanOptimize soil preparation with a versatile mini tillerRickson Viahul Rayan C100% (1)
- The Human Excretory System: A 40-Character GuideDokumen3 halamanThe Human Excretory System: A 40-Character GuideMelvel John Nobleza AmarilloBelum ada peringkat
- Cognitive Development of Infants and ToddlersDokumen1 halamanCognitive Development of Infants and ToddlersCyrell Rondina100% (2)
- QIMA - Ethical Audit Preparation Document - ENDokumen3 halamanQIMA - Ethical Audit Preparation Document - ENMD Nurul HudaBelum ada peringkat
- Kuratif RacunDokumen18 halamanKuratif RacunYsrwncyBelum ada peringkat
- Lecture 3 FertilityDokumen30 halamanLecture 3 Fertilityანთეა აბრამიშვილიBelum ada peringkat
- 1644 CV Europass Daniel MatosDokumen2 halaman1644 CV Europass Daniel MatosDaniel MatosBelum ada peringkat
- Sugar Crisis in Pakistan Research PaperDokumen10 halamanSugar Crisis in Pakistan Research Paperrehan9891Belum ada peringkat
- Shavuot 5774Dokumen4 halamanShavuot 5774Andrea KingBelum ada peringkat
- The Cricket War .TextDokumen2 halamanThe Cricket War .TextNikita SyrotiukBelum ada peringkat
- Time ManagementDokumen30 halamanTime ManagementVaibhav Vithoba NaikBelum ada peringkat
- GS I: Factors Driving Development of Global Textile IndustriesDokumen54 halamanGS I: Factors Driving Development of Global Textile IndustriesAMIT RAJBelum ada peringkat
- Uia Teaching Hospital BriefDokumen631 halamanUia Teaching Hospital Briefmelikeorgbraces100% (1)
- GSR (ROAD) 2015Dokumen74 halamanGSR (ROAD) 2015Gautam RaiBelum ada peringkat
- LENZE E84AVxCx - 8400 StateLine-HighLine-TopLine 0.25-45kW - v9-0 - ENDokumen291 halamanLENZE E84AVxCx - 8400 StateLine-HighLine-TopLine 0.25-45kW - v9-0 - ENClaudioBelum ada peringkat