Che 91164 Enthalpy
Diunggah oleh
api-218511741Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Che 91164 Enthalpy
Diunggah oleh
api-218511741Hak Cipta:
Format Tersedia
No Brain Too Small CHEMISTRY
Progress of reaction
Progress of reaction
AS 91164
Thermochemical principles
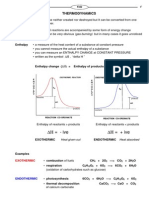
Classification of reactions as exothermic and endothermic
Chemical reactions are accompanied by energy changes. Enthalpy is the heat content of a system, or the
amount of energy within a substance, both kinetic and potential. It has the symbol H. It is not possible to
actually measure the heat content of a substance just "sitting there" but it is possible to measure how
much the enthalpy changes during a reaction. The symbol is used to represent change. Therefore we
refer to the change in enthalpy, or H.
Bond breaking is endothermic energy has to be put IN to break a bond. Imagine the energy you will need to supply
to separate your fingers that you have inadvertently and somewhat carelessly super glued together! Bond making
is exothermic releases energy. This is a little harder to imagine, but as you glued those fingers together you also
noticed they felt a bit warmer!
Enthalpy changes are classified as either:
Exothermic
reactants lose chemical potential energy which is converted to heat energy
reaction mixture warms up (as energy is
released to the surroundings)
H is negative
Enthalpy of products < enthalpy of reactants
Examples: NaOH dissolving in water, Mg
reacting with acid, all combustion reactions,
rusting iron, mixing water with an anhydrous
salt, respiration, steam condensing, water
freezing.
Endothermic
reactants absorb heat energy which is
converted to chemical potential energy
reaction mixture cools down (as energy us
absorbed from surroundings)
H is positive
Enthalpy of reactants < enthalpy of
products
Examples: NH
4
Cl dissolving in water,
photosynthesis, ice melting, water boiling,
making an anhydrous salt from a hydrate
e.g. CuSO
4
from CuSO
4
.5H
2
O
We also draw energy level diagrams for phase changes such as ice melting to water at 0 C. These diagrams do not
have any activation energy because in phase changes only bond breaking or bond making occurs, not both.
No Brain Too Small CHEMISTRY
Thermochemical equations
This is a balanced chemical equation that includes an enthalpy term.
State symbols are important in these equations. (s) solid, (l) liquid, (g) gas and (aq) aqueous.
E.g.
H
2
SO
4
(aq) + 2NaOH(aq) Na
2
SO
4
(aq) + 2H
2
O(l); H = -114 kJ mol
-1
1 mol 2 mol
This means that when one mol of dilute sulfuric acid reacts with two mol of aqueous sodium hydroxide,
then 114 kJ of heat energy are released (since the sign of H is negative).
NH
4
NO
3
(s) + aq NH
4
+
(aq) + NO
3
-
(aq); H = +19 kJ mol
-1
1 mol
This means that when one mol of ammonium nitrate dissolves in water 19 kJ of heat energy are absorbed
(since the sign of H is positive).
But what do the equations mean?
There is a direct relationship between the amount of substances that reacts and forms in an equation. The
factor that determines the exact relationship is the mole ratio (the numbers in front of the substances in
the balanced equation).
E.g.
2H
2
(g) + O
2
(g) 2H
2
O(l)
2 mol 1 mol 2 mol
BUT if one mole of hydrogen reacts with 0.5 moles of oxygen, one mole of water forms.
If two moles of oxygen reacts with excess* hydrogen, 4 moles of water forms. *excess = more than enough
What relationship exists between amount of reactant or product substance and change in enthalpy
(H)?
The heat absorbed and produced in a chemical reaction also varies directly as the amount of substance
that reacts. The exact amount is determined by the heat change for the reaction (H).
If you double the amount of substance reacted then you will double the heat change.
Examine the following reaction between hydrogen and oxygen to form water
2H
2
(g) + O
2
(g) 2H
2
O (l) : H = - 572 kJ
2 mol 1 mol 2 mol
Remind yourself what this means! When 2 moles of hydrogen gas reacts with 1 mole of oxygen gas (to
form 2 moles of liquid water), 572 kJ of energy are released
OR
The enthalpy change when 2 mol of hydrogen reacts with 1 mol of oxygen is - 572 kJ, H = - 572 kJ
(NEVER SAY 572 kJ of energy are released since the bit already says released AND itll be marked
wrong.)
No Brain Too Small CHEMISTRY
But back to this.
2H
2
(g) + O
2
(g) 2H
2
O (l) : H = - 572 kJ
2 mol 1 mol 2 mol
When 2 mol of hydrogen reacts with 1 mol of oxygen, 572 kJ of energy are released
When 1 mol of hydrogen reacts with mol of oxygen, 286 kJ of energy are released
When 4 mol of hydrogen reacts with 2 mol of oxygen, 1046 kJ of energy are released
Another example
2C
4
H
10
(l) + 13O
2
(g) 8CO
2
(g) + 10H
2
O(g) H = -5315 kJ
The equation tells us that when 2 moles of C
4
H
10
(g) burn in oxygen, H = -5317 kJ
Since the relationship is a direct one then when 1 mole of C
4
H
10
(g) is burnt, it would release half as much
energy: H = -2657.5 kJ
Extra! Here we could also write H = -2657.5 kJ mol
-1
because ONE mole of C
4
H
10
(l) is being completely
burned in oxygen.
If 4 moles of C
4
H
10
were burnt excess oxygen they would release 10630 kJ OR H = -10630 kJ. (Note: BUT
NOT release 10630 kJ, remember the sign tells us it is released/exothermic).
Calculations involving masses of stuff?
Since the heat released or absorbed in change is directly proportional to the H in the equation, ratios can
be used to solve problems involving heat and the amount of substance.
Procedure - perform the following steps
calculate the number of moles of substance reacted or formed.
create a proportion using the mole ratio and heat in the chemical equation.
solve for missing quantity.
E.g.
Calculate the amount of heat released when 25 grams of C
4
H
10
(l) is burned in oxygen using the equation
provided.
2C
4
H
10
(l) + 13O
2
(g) 8CO
2
(g) + 10H
2
O (g); H = -5315 kJ
2 moles -5315 kJ
116* g -5315 kJ
25g 25 x -5315
116
= -1145.5 kJ
1145.5 kJ of heat would be released when 25 g is burned OR H = -1145.5 kJ*
*kJ and not kJ mol
-1
since we werent burning a mole of butane, just 25 g.
Okay but the ones in the exams always look more complicated!! Well yes and NO! Same basic principle!!
Write equation & write number of moles underneath.
Work out the masses this would represent e.g. H
2
O = 1 + 1 + 16 = 18 g BUT if the equation says
3H
2
O then its 54 g.
Write the masses you have been given underneath. Work out how much would have reacted with /
been made by ratios.
M(C
4
H
10
) = (4 x 12) + (10 x 1)
= 58 g mol
-1
.
So 2 mol has a mass of 116 g
No Brain Too Small CHEMISTRY
There is NO NEED to work out the mass of everything in the equation if you have been asked how much
energy is released when x g of butane is burned. Dont need to work out MASS of oxygen, carbon dioxide
and water! If it says what mass of CO
2
is produced when H = - 5000 kJ, then dont work out the mass of
butane, oxygen and water!
E.g. What mass of CO
2
is produced when H = - 5000 kJ
2C
4
H
10
(l) + 13O
2
(g) 8CO
2
(g) + 10H
2
O (g); H = -5315 kJ
2 mol 13 mol 8 mol 10 mol
8 x (12+16+16)
352 g
8 mol ------------ -5315 kJ
352 g ----------- -5315 kJ
x g ----------- -5000 kJ
so x = (-5000 x 352) / -5315 = 331 g
Calculating H using bond energies
Bond energy is a measure of the intramolecular bond strength in a covalent bond:
AB(g) A(g) + B(g)
Notice that the reactants and products are all gases. Also notice that the products are atoms.
Bond energies are given in data tables and show the average energy required to break one mole of that
bond, the value being calculated from many different molecules.
Bond energies are positive, because bond breaking is an endothermic process. Bond making has the same
value but the negative sign.
i.e. Br
2
(g) Br(g) + Br(g) +192 kJ mol
1
Br(g) + Br(g) Br
2
(g) -192 kJ mol
1
Bond energy calculations
Calculate the heat of reaction for the following: C
2
H
6
(g) + Cl
2
(g) C
2
H
5
Cl(g) + HCl(g), given the following
bond energies:
CH 413 kJ mol
1
ClCl 242 kJ mol
1
CCl 339 kJ mol
1
HCl 431 kJ mol
1
Write out the equation using structural formulae, with every bond shown. Work out which bonds are
broken and which will be made.
C C
H
H
H H
H
H
C C
H
H
H H
H
H
Cl
Cl
Cl
Cl
C
H
H
H C
H
H
Cl
C
H
H
H C
H
H
Cl
H Cl
H Cl
Bond breaking (+H) Bond making (H)
CH +413 kJ mol
1
CCl -339 kJ mol
1
ClCl +242 kJ mol
1
HCl -431 kJ mol
1
+655 -770
H = bond breaking + bond making
H = +655 + (770) = 115 kJ mol
1
+ +
No Brain Too Small CHEMISTRY
Note: multiple bonds have their own bond energies. For example, it takes 598 kJ mol
1
to break the C=C
double bond, but 346 kJ mol
1
to break the CC.
Use the bond energy data given to predict the enthalpy of reaction for the equation below.
CH
2
=CH
2
(g) + Br
2
(g) CH
2
BrCH
2
Br(g)
BrBr 192 kJ mol
1
CBr 276 kJ mol
1
C = C 598 kJ mol
1
CC 346 kJ mol
1
C
H
H
C
H
H
C
H
H
C
H
H
Br
Br
Br
Br
C
H
H
Br C
H
H
Br
C
H
H
Br C
H
H
Br
Bond breaking (+H) Bond making (H)
C = C +598 kJ mol
1
CC -346 kJ mol
1
BrBr +192 kJ mol
1
2 x CBr 2 x -276 kJ mol
1
+790 kJ mol
1
-898 kJ mol
1
H = bond breaking + bond making
H = +790 + (898) = 108 kJ mol
1
Notes.
+
Anda mungkin juga menyukai
- Power of Attorney in FactDokumen4 halamanPower of Attorney in FactRinku Kokiri100% (21)
- Surety Bond Basics PresentationDokumen23 halamanSurety Bond Basics PresentationGrant W DavisBelum ada peringkat
- Promissory Note Vs Bill PF ExchangeDokumen2 halamanPromissory Note Vs Bill PF ExchangelexscribisBelum ada peringkat
- Construction Basics TrainingDokumen12 halamanConstruction Basics TrainingHadrien Faryala100% (1)
- Optimal Capital StructureDokumen4 halamanOptimal Capital StructurevinyspBelum ada peringkat
- What Is A Coupon BondDokumen3 halamanWhat Is A Coupon BondNazrul IslamBelum ada peringkat
- Bond Enthalpy WorksheetDokumen6 halamanBond Enthalpy WorksheetTanisha DamleBelum ada peringkat
- ThermochemistryDokumen31 halamanThermochemistryDavidson ChanBelum ada peringkat
- Determinants of Interest RatesDokumen27 halamanDeterminants of Interest RatesraviBelum ada peringkat
- Heat Energy Is Absorbed And: Energy Cannot Be Created or Destroyed But Can Be Converted From One Form To AnotherDokumen12 halamanHeat Energy Is Absorbed And: Energy Cannot Be Created or Destroyed But Can Be Converted From One Form To AnotherAeyyjayyBelum ada peringkat
- Chem 17 LE 1 Answers1Dokumen11 halamanChem 17 LE 1 Answers1alyssa100% (1)
- Practice Makes Perfect in Chemistry: Oxidation-ReductionDari EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionPenilaian: 5 dari 5 bintang5/5 (1)
- ICFAI MIFA Course Group C Financial Markets Exam - Paper1Dokumen27 halamanICFAI MIFA Course Group C Financial Markets Exam - Paper1Ameya RanadiveBelum ada peringkat
- CHEM1612 Worksheet 2 - Answers To Critical Thinking QuestionsDokumen4 halamanCHEM1612 Worksheet 2 - Answers To Critical Thinking QuestionsKeBelum ada peringkat
- Enthalpy of Chemical ReactionsDokumen4 halamanEnthalpy of Chemical ReactionsMuhammad Rafif Zarrar SenaBelum ada peringkat
- A Energetics Notes Chem Unit 1 - (New)Dokumen8 halamanA Energetics Notes Chem Unit 1 - (New)Khaila SimmondBelum ada peringkat
- Thermochemistry BookletDokumen29 halamanThermochemistry Bookletdurmic_suadaBelum ada peringkat
- Thermochemistry 4 (Born-Haber Cycle and Bond Energy) - 2022Dokumen13 halamanThermochemistry 4 (Born-Haber Cycle and Bond Energy) - 2022Glory100% (1)
- 1 - 1 - 1 - 1 F I Cal - 1 - 1 - 1 Cal Cal - 1 - 1 - 1 - 1 - 1Dokumen3 halaman1 - 1 - 1 - 1 F I Cal - 1 - 1 - 1 Cal Cal - 1 - 1 - 1 - 1 - 1Andrea LeopandoBelum ada peringkat
- Bond Dissociation EnergiesDokumen5 halamanBond Dissociation Energiesjean ruBelum ada peringkat
- PhaseIII Chem L2 EnthalpyDokumen27 halamanPhaseIII Chem L2 EnthalpyPrisha JainBelum ada peringkat
- Thermochemistry: Chem B Jan 2010 - Ms. Jessvin Sidhu 1Dokumen33 halamanThermochemistry: Chem B Jan 2010 - Ms. Jessvin Sidhu 1blackmoneygrabberBelum ada peringkat
- Final Initial: Products ReactantsDokumen14 halamanFinal Initial: Products ReactantsAlisa DowningBelum ada peringkat
- Enthalpy of Reaction and Hess' LawDokumen4 halamanEnthalpy of Reaction and Hess' LawJbreBelum ada peringkat
- WRITING THERMOCHEMICAL EQUATION OfficialDokumen17 halamanWRITING THERMOCHEMICAL EQUATION OfficialJohn Paul FerrerasBelum ada peringkat
- Topic 15Dokumen41 halamanTopic 15api-485190482Belum ada peringkat
- Energy 2Dokumen27 halamanEnergy 2Diana ToroBelum ada peringkat
- Thermochemistry Enthalpy H: Investice Do Rozvoje VzděláváníDokumen7 halamanThermochemistry Enthalpy H: Investice Do Rozvoje VzděláváníEva IndriyaniBelum ada peringkat
- Class 11 Chemistry Chapter 6 Chemical Thermodynamics Important Questions With AnswersDokumen15 halamanClass 11 Chemistry Chapter 6 Chemical Thermodynamics Important Questions With AnswersMinato NamikazeBelum ada peringkat
- Topic10 AnswersDokumen8 halamanTopic10 AnswersBiblee ChasBelum ada peringkat
- Experiment 2 Enthalpy of Chemical Reactions and Hess's LawDokumen15 halamanExperiment 2 Enthalpy of Chemical Reactions and Hess's LawUzo Paul NwabuisiBelum ada peringkat
- Hesss Law Awesome Ib Packet Questions OnlyDokumen6 halamanHesss Law Awesome Ib Packet Questions OnlyEmmanuel JoyBelum ada peringkat
- Topic18 AnswersDokumen40 halamanTopic18 AnswersEduardoBelum ada peringkat
- Energetics by Abhishek JaguessarDokumen10 halamanEnergetics by Abhishek Jaguessarreedoye21Belum ada peringkat
- Chapter 5a (AS-Level) : Chemical EnergeticsDokumen12 halamanChapter 5a (AS-Level) : Chemical EnergeticsMohamed AkkashBelum ada peringkat
- 1.4 EnergeticsDokumen12 halaman1.4 EnergeticschwalidBelum ada peringkat
- Homework - EnthalpyDokumen5 halamanHomework - EnthalpyUmar PatelBelum ada peringkat
- Energy TutorialDokumen5 halamanEnergy TutorialIdil WarsameBelum ada peringkat
- Chapter 9 - Termochemistry 55Dokumen55 halamanChapter 9 - Termochemistry 55ABC_Ais Batu CampurBelum ada peringkat
- Chapter 11: Thermochemistry - Heat and Chemical Changes Part 1 - Notes: Enthalpy and Bond EnergiesDokumen11 halamanChapter 11: Thermochemistry - Heat and Chemical Changes Part 1 - Notes: Enthalpy and Bond EnergiesSarthakBelum ada peringkat
- CH 5 Part 2 Bond Enthalpies and Hess LawDokumen50 halamanCH 5 Part 2 Bond Enthalpies and Hess LawSafiye TerBelum ada peringkat
- Respuestas Termoquimica ChangDokumen8 halamanRespuestas Termoquimica ChangIsabelBelum ada peringkat
- Tutorial-1-With AnswerDokumen4 halamanTutorial-1-With AnswerHayicBelum ada peringkat
- CM150-2 - Exercise 3 - Progress 1Dokumen5 halamanCM150-2 - Exercise 3 - Progress 1owl lawletBelum ada peringkat
- Energy Change in Chemical Reaction Alauddin Sir A & O Level Chemistry TeacherDokumen8 halamanEnergy Change in Chemical Reaction Alauddin Sir A & O Level Chemistry TeacherMunshi LazimuzzamanBelum ada peringkat
- Chemical Reactions and HeatDokumen37 halamanChemical Reactions and HeatDamir BalmassovBelum ada peringkat
- Physics-Third Law of ThermodynamicsDokumen17 halamanPhysics-Third Law of ThermodynamicsShubham JainBelum ada peringkat
- Ib Enthalpy KHDokumen39 halamanIb Enthalpy KHSamer EhabBelum ada peringkat
- U15 S4 HW Packet 13-20Dokumen27 halamanU15 S4 HW Packet 13-20Rohith GudatiBelum ada peringkat
- Lecture-Unit 7 Chemical EnergeticsDokumen5 halamanLecture-Unit 7 Chemical EnergeticsKemoy FrancisBelum ada peringkat
- Week 3: Thermochemical Equations: Laboratory OperationsDokumen16 halamanWeek 3: Thermochemical Equations: Laboratory OperationsColleen CastueraBelum ada peringkat
- Energetics - Summary: Exothermic EndothermicDokumen7 halamanEnergetics - Summary: Exothermic EndothermicKiara LimBelum ada peringkat
- Enthalpy Changes ChemDokumen4 halamanEnthalpy Changes Chemp01084240882Belum ada peringkat
- Lesson 04: Thermochemistry Unit 02: Thermochemical Equations Learning ObjectivesDokumen7 halamanLesson 04: Thermochemistry Unit 02: Thermochemical Equations Learning ObjectivesLelouchBelum ada peringkat
- HW02 - Thermo 2-SolutionsDokumen9 halamanHW02 - Thermo 2-SolutionsKeerthana KumarBelum ada peringkat
- Chapter 13 - ThermochemistryDokumen22 halamanChapter 13 - ThermochemistryvaogerBelum ada peringkat
- Enthalpy 093302Dokumen22 halamanEnthalpy 093302Mikhoy RiveralBelum ada peringkat
- Final Revision WorksheetDokumen26 halamanFinal Revision Worksheetawash0takuBelum ada peringkat
- 8 - Thermodynamics - Lecture 8Dokumen19 halaman8 - Thermodynamics - Lecture 8Ramy MaamounBelum ada peringkat
- 08-Bond Energies and Enthalpy ChangesDokumen3 halaman08-Bond Energies and Enthalpy ChangesNkemzi Elias NzetengenleBelum ada peringkat
- Consider The Reaction H2 (G) + 12 O2 (G) H2O (L) Delta H - 285.84 KJmol. How Many Grams of Hydrogen Gas Are Needed To ProduceDokumen1 halamanConsider The Reaction H2 (G) + 12 O2 (G) H2O (L) Delta H - 285.84 KJmol. How Many Grams of Hydrogen Gas Are Needed To ProduceAntonio Hernando MañeruBelum ada peringkat
- Enthalpy S&G 06Dokumen13 halamanEnthalpy S&G 06OnSolomonBelum ada peringkat
- Topic 17 - Equilibrium HL - AnswersDokumen7 halamanTopic 17 - Equilibrium HL - Answers赵倞Belum ada peringkat
- Hchemskillspart2Dokumen2 halamanHchemskillspart2api-340005475Belum ada peringkat
- q m C ΔT: SolutionDokumen7 halamanq m C ΔT: SolutionMjhay Tanchiatco DavidBelum ada peringkat
- Thermo SoalanDokumen10 halamanThermo SoalanMuhammad Nazif AzmiBelum ada peringkat
- Ive + Ive: ThermodynamicsDokumen10 halamanIve + Ive: ThermodynamicsAbbas HaiderBelum ada peringkat
- 2 5 Marking ScheduleDokumen6 halaman2 5 Marking Scheduleapi-218511741Belum ada peringkat
- IUPAC HandoutDokumen9 halamanIUPAC HandoutjanellamaikaBelum ada peringkat
- Chemistry Research TaskDokumen4 halamanChemistry Research Taskapi-218511741Belum ada peringkat
- Eslwriting Video Worksheet CosmeticsDokumen5 halamanEslwriting Video Worksheet Cosmeticsapi-2185117410% (1)
- First Spontaneous Reactions WorksheetDokumen2 halamanFirst Spontaneous Reactions Worksheetapi-2185117410% (1)
- Quantitative Chem Notes Titrations OnlyDokumen18 halamanQuantitative Chem Notes Titrations Onlyapi-218511741Belum ada peringkat
- Entropy Notes and Exam QuestionsDokumen3 halamanEntropy Notes and Exam Questionsapi-218511741100% (1)
- Aldehydes and Ketones ExperimentDokumen2 halamanAldehydes and Ketones Experimentapi-218511741Belum ada peringkat
- On WorksheetDokumen2 halamanOn Worksheetapi-218511741Belum ada peringkat
- Level 2 Basic Facts Worksheet AnswersDokumen9 halamanLevel 2 Basic Facts Worksheet Answersapi-218511741Belum ada peringkat
- Iron - Thiocyanate EquilibriumDokumen7 halamanIron - Thiocyanate Equilibriumapi-218511741Belum ada peringkat
- Fats and Oils NotesDokumen1 halamanFats and Oils Notesapi-218511741Belum ada peringkat
- Esterification ExperimentDokumen2 halamanEsterification Experimentapi-218511741Belum ada peringkat
- Substitution Notes For StudentsDokumen2 halamanSubstitution Notes For Studentsapi-218511741Belum ada peringkat
- Oxidation of Organic Compounds WorksheetDokumen3 halamanOxidation of Organic Compounds Worksheetapi-218511741Belum ada peringkat
- Names and Structures Small Test 2Dokumen1 halamanNames and Structures Small Test 2api-218511741Belum ada peringkat
- Opticalisomerism 09Dokumen2 halamanOpticalisomerism 09api-218511741Belum ada peringkat
- Organic Names and Formula QuestionsDokumen1 halamanOrganic Names and Formula Questionsapi-218511741Belum ada peringkat
- Organic Names and Formula Answers OnlyDokumen1 halamanOrganic Names and Formula Answers Onlyapi-218511741Belum ada peringkat
- Security List Details PDFDokumen1.795 halamanSecurity List Details PDFsantoshBelum ada peringkat
- ACCRINT FunctionDokumen3 halamanACCRINT FunctionfrazbuttBelum ada peringkat
- 11 - TransitionMetalDokumen37 halaman11 - TransitionMetaltuyenvip441999Belum ada peringkat
- CM1 Mock Paper B 2021 Answers 12345Dokumen44 halamanCM1 Mock Paper B 2021 Answers 12345vanessa8pangestuBelum ada peringkat
- Nomenclature of Inorganic CompoundsDokumen4 halamanNomenclature of Inorganic CompoundsBeverly PeBelum ada peringkat
- Unit 4 Rate of Reaction AnswersDokumen38 halamanUnit 4 Rate of Reaction Answersareyouthere92Belum ada peringkat
- HJM ModelsDokumen12 halamanHJM ModelsPrateek SabharwalBelum ada peringkat
- Corbonyl CompOUND AND Corboxilic AcidDokumen12 halamanCorbonyl CompOUND AND Corboxilic AcidApex InstituteBelum ada peringkat
- Duration Gap Analysis Session 6Dokumen33 halamanDuration Gap Analysis Session 6Tharindu PereraBelum ada peringkat
- Chapter 12 QDokumen4 halamanChapter 12 QRebecca Lau100% (1)
- Technical Bulletin: 3M VHB DurabilityDokumen6 halamanTechnical Bulletin: 3M VHB DurabilityAlvin Dela CruzBelum ada peringkat
- Half Yearly ProblemDokumen16 halamanHalf Yearly Problemaleena'Belum ada peringkat
- Security Valuation G3 730AMDokumen3 halamanSecurity Valuation G3 730AMKearn CercadoBelum ada peringkat
- P Block 17-18Dokumen23 halamanP Block 17-18Aditya BansalBelum ada peringkat
- Rearreglos SigmatrópicosDokumen31 halamanRearreglos SigmatrópicoskmiloBelum ada peringkat
- CHM12 3lu6 PDFDokumen97 halamanCHM12 3lu6 PDFLin Xian XingBelum ada peringkat
- MCQ 464Dokumen10 halamanMCQ 464Tonmoy MajumderBelum ada peringkat
- Datascope Reference DataDokumen3 halamanDatascope Reference DataGurupraBelum ada peringkat
- Solutions To Preparatory Problems: Problem 1. Graphite OxideDokumen25 halamanSolutions To Preparatory Problems: Problem 1. Graphite OxideNebojsaZecBelum ada peringkat
- Money MarketDokumen36 halamanMoney MarketbkrsanerBelum ada peringkat
- Fixed Income ValuationDokumen54 halamanFixed Income ValuationAnurag MishraBelum ada peringkat
- Credit RatingDokumen90 halamanCredit Ratingraj sharma100% (1)