Objective
Diunggah oleh
naim rashidHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Objective
Diunggah oleh
naim rashidHak Cipta:
Format Tersedia
Experiment 4 : The aldol condensation reaction : Preparation of Benzalacetophenones (Chalcones )
OBJECTIVE: To synthesis benzalacetophenones (chalcones) by aldol condensation reaction. To determine the percentage yield of the crude product. To identify the melting point of the purified product. To characterize benzalacetophenones (chalcones) from the H1 NMR spectrum. . INTRODUCTION: An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a -hydroxyaldehyde or -hydroxyketone, followed by a dehydration to give a conjugated enone. Benzaldehyde reacts with a ketone in the presence of base to give ,-unsaturated ketones. This reaction is an example of a crossed aldol condensation where the intermediate dehydrates to produce the resonance-stabilized unsaturated ketone.
As illustrated for the reaction of benzaldehyde with a general ketone, the ketone looses a proton
from an -carbon to form an enolate ion, which attacks the carbonyl carbon of the aldehyde to yield, after protonation, a -hydroxy ketone. This intermediate is, for aromatic aldehydes at least, instable and undergoes base-catalyzed dehydration to yield the unsaturated product.
PROCEDURES: To run the reaction, 0.75g of 3-nitrobelzaldehyde is placed in a 50mL Erlenmeyer flask. Then, 0.60mL of acetophenone and 4.0mL of 95% ethanol are added to the flask. The flask is swirled to mix the reagent and dissolved any solids present. The flask is warmed on the hot plate to dissolve the solids. Then, it is cooled at room temperature. 0.5mL of sodium hydroxide is added into the mixture and swirled until it solidified or the mixture become very cloudy. For isolation of the crude product, 10mL of ice water is added into the flask. The mixture is stirred if solids or oil is present to break up the solid mass. The mixture is transferred to a beaker with 15mL ice water. The precipitate is stirred to break it up and the solid is collected on B chner funnel. The product is washed with cold water. The solid is allowed to dry for 30minutes. The solid is weighed and the percentage yield is determined. For the crystallization, 0.50g of sample is crystallized from 20mL of hot methanol. The flask is gently scratched to induce crystallization while cooling. The literature melting point is 146oC.
RESULT: Mass of 3-nitrobenzaldehyde = 0.7677 g Mass of crude product Melting point = 0.4195 g =140 oC
Calculation: Mass of 3-nitrobenzaldehyde = 0.7677 g No. mol of 3-nitrobenzaldehyde = Mass of 3-nitrobenzaldehyde / MW = 0.7677g / 151.12 g/mol = 0.005 mol
From the above reaction equation, 1 mol 3-nitrobenzaldehyde 1 mol 3-nitrochalcone Therefore, no.mol of 3-nitrochalcone is 0.005 mol. Mass of 3-nitrochalcone (theoretical) = No.mol of 3-nitrochalcone x MW = 0.005 mol x 253.26 g/mol = 1.2663 g (theoretical)
Mass of 3-nitrochalcone (actual) = 0.5136 g Percentage yield = (Actual yield / Theoretical yield )x 100% = (0.5136 / 1.2663 )x 100% = 40.56%
Percentage error = (Theoretical yield Actual yield) / Theoretical yield x 100% = (1.2663 0.5136) / 1.2663 x 100% = 59.44 %
DISCUSSION: In this experiment, benzalacetophenones (chalcones) are prepared by the reaction of a substituted benzaldehyde with acetophenone in aqueous base. Based on the chemical equation, the limiting reactant for the reaction is 3-nitrobenzaldehyde. By comparing the number of mol of both compound, the theoretical mass of 3nitrochalcone can be calculated. From the result, quite small percent of crude product have been obtained. The product seems to be not pure enough since the percentage yield obtained is only 40.56%. The melting point of the purified product is 140oC that is quite close to the literature melting point which is 146oC. We did not get result closes to the theoretical, due to several errors that occur while doing this experiment. Firstly, error while collecting the solids by using Buchner funnels. The pressure that we use to collect the solids is too high and causes the filter paper full of holes. That will cause the amount of crude product become less and give effect to their weight. The temperature of the oven should be below 50oC to avoid the bond between molecule break and give bad effect for our result. We have to make sure that the crude product is dry enough before being weighed to avoid error while weighing. Based on our H1 NMR spectrum result, there are 4 basic types of H present in the ratio of 4:1:1:1:5. Using the chemical shift charts, the H can be assigned to the peaks approximately as below: 7.4ppm (4H) : ArH 5.3ppm (1H) : CH=CH 3.4ppm (1H) : CHC=OAr
2.5ppm (3H) : ArH We observed that there are 4 distinct signals, with chemical shift of approximately 7.4, 5.3, 3.4 and 2.5ppm. One of the signal (7.4ppm) is noticeably downfield of the others, indicates hydrogen atom that are likely to be near an electronegative group that deshield the ArH group. The presences of 4 distinct signals suggest that there are three distinct proton environments in the molecule. The signal at 7.4ppm is multiplet. Since the signal is more downfield and has 4 hydrogen on a benzene ring with nitrogen oxide substituent, we conclude that this signal is from ArH group and next to CH group. The signal at 5.3 and 3.4ppm is to be a duplet since the adjacent carbon has 1 hydrogen atom. At signal 3.4ppm, we conclude that it is ketone (CHC=OAr) and 5.3ppm as carbon double bond since there is only 1 hydrogen attach to each carbon. Lastly, at signal 2.5ppm, it seems to be a quartet since there is 3 hydrogen at the aromatic compound (ArH). The ArH group is said to occur upfield since the C=O is less electronegative than NO. Electronegative groups attached to the C-H system decrease the electron density around the protons, and there is deshield so the chemical shift increases.
CONCLUSION: From this experiment, 3-nitrochalcone is prepared by aldol condensation reaction between substituted benzaldehyde (3-nitrobenzaldehyde) and acetophenone in aqueous base. The percentage yield of the crude product obtained is 40.56% and the melting point of the purified product is 140oC. The 3-nitrochalcone has been successfully characterized from the H1 NMR spectrum. . REFERENCES: T.W. Graham Solomons & Craig B. Fryhle Organic Chemistry, 9th Edition (2007)
Donald L. Pavia, Gary M. Lampman, George S. Kriz, & Randall G. Enge Introduction of Organic Laboratory Techniques, 2nd Edition (2005) http://en.wikipedia.org/wiki/Aldol_condensation http://www.cis.rit.edu/htbooks/nmr/inside.htm http://en.wikipedia.org/wiki/Chalcone
NAME : MUHAMMAD NAIM BIN MOHD RASHID
ID NUMBER : 2012216634
INSTRUCTOR S NAME : DR. NORIZAN AHMAT
PARTNERS NAME : MUHAMMAD FAID BIN A. KADIR
DATE OF EXPERIMENT : 11. 11 .2013
DATE OF SUBMISSION : 25. 11. 2013
Anda mungkin juga menyukai
- Organic Chemistry II Experiment Sodium Borohydride ReductionDokumen10 halamanOrganic Chemistry II Experiment Sodium Borohydride ReductionAlohaaSwezzBelum ada peringkat
- The Aldol Condensation ReactionDokumen3 halamanThe Aldol Condensation ReactionJoshua CastilloBelum ada peringkat
- Aldol Condensation to Synthesize ChalconeDokumen8 halamanAldol Condensation to Synthesize ChalconeMuhammad Amirul AfifiBelum ada peringkat
- PH CH 126.1 Fischer Esterification of Methyl Benzoate 2Dokumen6 halamanPH CH 126.1 Fischer Esterification of Methyl Benzoate 2Tammy CacnioBelum ada peringkat
- Experiment 4: The Aldol Condensation Reaction: Preparation of Benzalacetophenones (Chalcones)Dokumen3 halamanExperiment 4: The Aldol Condensation Reaction: Preparation of Benzalacetophenones (Chalcones)Dang Humairah100% (1)
- NaBH4 Reduction of CyclohaxanoneDokumen5 halamanNaBH4 Reduction of Cyclohaxanonenurul1110Belum ada peringkat
- Experiment 32Dokumen14 halamanExperiment 32Morgan Elizabeth Lepley100% (6)
- Report 4 GCDokumen26 halamanReport 4 GCNurhafizah Abd JabarBelum ada peringkat
- Atomic Adsorption SpectrosDokumen14 halamanAtomic Adsorption SpectrosRzi Danil Ishutin0% (1)
- Chm557 Exp2Dokumen4 halamanChm557 Exp2Rap DutaBelum ada peringkat
- CHM557 Exp 3Dokumen22 halamanCHM557 Exp 3syafBelum ada peringkat
- CHM510 - SpeDokumen7 halamanCHM510 - SpeafifiBelum ada peringkat
- Experiment 7 Analysis of Chlorpyrifos in Water by Solid-Phase Extraction (SPE) and Gas Chromatography - Electron Captured Detector (ECD)Dokumen6 halamanExperiment 7 Analysis of Chlorpyrifos in Water by Solid-Phase Extraction (SPE) and Gas Chromatography - Electron Captured Detector (ECD)Maxvicklye RaynerBelum ada peringkat
- Experiment 7Dokumen8 halamanExperiment 7Shinichi KudoBelum ada peringkat
- Experiment 2 Sodium Borohydride Reduction of CyclohexanoneDokumen6 halamanExperiment 2 Sodium Borohydride Reduction of CyclohexanoneSarah HannisBelum ada peringkat
- Determination of Lead in Anchovies by Cold Vapour Generation Atomic Absorption SpectrometryDokumen30 halamanDetermination of Lead in Anchovies by Cold Vapour Generation Atomic Absorption SpectrometryIbrahim Muhamad100% (2)
- Lab Report PDFDokumen21 halamanLab Report PDFLutfi Azmi0% (2)
- Chemistry of Carbonyl CompoundsDokumen28 halamanChemistry of Carbonyl CompoundsRhondene WintBelum ada peringkat
- The Friedel-Crafts Reaction PDFDokumen7 halamanThe Friedel-Crafts Reaction PDFIsaac Nicholas NotorioBelum ada peringkat
- UiTM Organic Chemistry Aldol Condensation ExperimentDokumen7 halamanUiTM Organic Chemistry Aldol Condensation ExperimentNurul izzatiBelum ada peringkat
- CHM 510 Exp 1 GCDokumen8 halamanCHM 510 Exp 1 GCNurul HaziqahBelum ada peringkat
- Sodium Borohydride Reduction of CyclohexanoneDokumen6 halamanSodium Borohydride Reduction of CyclohexanoneIqmal HakimiBelum ada peringkat
- Robinson Annulation Reaction of 3-Nitrochalcone With Ethyl AcetoacetateDokumen9 halamanRobinson Annulation Reaction of 3-Nitrochalcone With Ethyl AcetoacetateAmirul Azhar100% (5)
- Experiment 3Dokumen8 halamanExperiment 3ohhiBelum ada peringkat
- Experiment 3 Esterification Reactions of Vanillin: The Use of NMR To Determine A StructureDokumen1 halamanExperiment 3 Esterification Reactions of Vanillin: The Use of NMR To Determine A StructureAyish MataBelum ada peringkat
- Determination of Water Hardness using Atomic Absorption Spectroscopy (AASDokumen7 halamanDetermination of Water Hardness using Atomic Absorption Spectroscopy (AASAfiqah SamanBelum ada peringkat
- LONG CAFFEINE UVLong Caffeine UvDokumen8 halamanLONG CAFFEINE UVLong Caffeine UvzaBelum ada peringkat
- NMR & IR spectroscopy tutorialDokumen2 halamanNMR & IR spectroscopy tutorialUmmu UmairahBelum ada peringkat
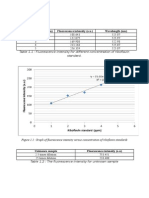
- Determination of Riboflavin in Soft Drinks by Fluorescence SpectrophotometryDokumen9 halamanDetermination of Riboflavin in Soft Drinks by Fluorescence SpectrophotometryNur IzzatieBelum ada peringkat
- NaBH4 Reduction of Cyclohexanone to Cyclohexanol (87Dokumen8 halamanNaBH4 Reduction of Cyclohexanone to Cyclohexanol (87hahadindongBelum ada peringkat
- Covid-19 Laboratory Report Exp 5Dokumen7 halamanCovid-19 Laboratory Report Exp 5Nasuha AriffinBelum ada peringkat
- Laboratory Report: Chm457 - October 2020 - Odl Lab AssessmentDokumen5 halamanLaboratory Report: Chm457 - October 2020 - Odl Lab AssessmentHakim SunaeBelum ada peringkat
- Preparation of Acetaline Notes PDFDokumen6 halamanPreparation of Acetaline Notes PDFAnonymous Wwxatt3oIK100% (1)
- Spectrophotometric Analysis of Transition Metal CationsDokumen5 halamanSpectrophotometric Analysis of Transition Metal CationsFAtma HAnysBelum ada peringkat
- ISOLATION OF CAFFEINE FROM A TEA BAGDokumen5 halamanISOLATION OF CAFFEINE FROM A TEA BAGsarra nazamBelum ada peringkat
- Riboflavin Concentration Fluorescence SpectroscopyDokumen5 halamanRiboflavin Concentration Fluorescence SpectroscopySyahriezan HaminBelum ada peringkat
- Preparation of Colloidal Dispersion ExperimentsDokumen8 halamanPreparation of Colloidal Dispersion ExperimentsFairuz Naim Z100% (1)
- Radical PolymerizationDokumen9 halamanRadical PolymerizationAtie Iekah100% (1)
- ALDOL Synth - DibenzalacetoneDokumen7 halamanALDOL Synth - DibenzalacetoneJames CookeBelum ada peringkat
- Preparation of 1 - ButanolDokumen6 halamanPreparation of 1 - ButanolEdon EduinBelum ada peringkat
- Lab Report 1Dokumen10 halamanLab Report 1sheril nur hazianiBelum ada peringkat
- Robinson Annulation Reaction of NItrochalconeDokumen10 halamanRobinson Annulation Reaction of NItrochalconeMohd Nakirudin Muhamad NorBelum ada peringkat
- Ignition of The PrecipitateDokumen12 halamanIgnition of The Precipitateasep wandi nugraha100% (3)
- Preparation of 4-Methylcyclohexene from AlcoholDokumen10 halamanPreparation of 4-Methylcyclohexene from AlcoholJohanBelum ada peringkat
- NITRATION OF METHYL BENZOATE (ELECTROPHILIC AROMATIC SUBSTITUITION - Idayu Razali - Academia - Edu PDFDokumen7 halamanNITRATION OF METHYL BENZOATE (ELECTROPHILIC AROMATIC SUBSTITUITION - Idayu Razali - Academia - Edu PDFyawsBelum ada peringkat
- Lab Oc Experiment 3Dokumen10 halamanLab Oc Experiment 3Amar SafwanBelum ada peringkat
- COD Test Determines Organic PollutantsDokumen4 halamanCOD Test Determines Organic Pollutantskh!mBelum ada peringkat
- Formal LabDokumen4 halamanFormal Labljeanja2100% (1)
- Nur Sarah Hannis - Exp3Dokumen1 halamanNur Sarah Hannis - Exp3Sarah HannisBelum ada peringkat
- Temperature Programmed Desorption With ReactionDokumen42 halamanTemperature Programmed Desorption With ReactionaquacobiaBelum ada peringkat
- The Biochemical Roles of Transition Metals: Chm579: Advanced Inorganic ChemistryDokumen15 halamanThe Biochemical Roles of Transition Metals: Chm579: Advanced Inorganic ChemistryNurul HusnaBelum ada peringkat
- Experiment 20: Sodium Borohydride Reduction of A KetoneDokumen12 halamanExperiment 20: Sodium Borohydride Reduction of A KetonenurhazwaniBelum ada peringkat
- Lab Report 3.0Dokumen7 halamanLab Report 3.0Husna Insyirah Bt SamadBelum ada peringkat
- Chm580 Experiment 3Dokumen9 halamanChm580 Experiment 3ohhiBelum ada peringkat
- Experiment Baking SsodaDokumen7 halamanExperiment Baking Ssodaatynzaty0% (1)
- Phase Transfer 0Dokumen3 halamanPhase Transfer 0Jeannine CoxBelum ada peringkat
- Introductory Titrimetric and Gravimetric Analysis: The Commonwealth and International Library: Chemistry DivisionDari EverandIntroductory Titrimetric and Gravimetric Analysis: The Commonwealth and International Library: Chemistry DivisionBelum ada peringkat
- Retained SampleDokumen1 halamanRetained Samplenaim rashidBelum ada peringkat
- Effect of Annealing and Time of Crystallization On Structural and Optical Properties of PVDF Thin Film Using Acetone As SolventDokumen6 halamanEffect of Annealing and Time of Crystallization On Structural and Optical Properties of PVDF Thin Film Using Acetone As Solventnaim rashidBelum ada peringkat
- Reactions With BenzeneDokumen25 halamanReactions With BenzeneVicky75% (4)
- ConclusionDokumen1 halamanConclusionnaim rashidBelum ada peringkat
- Carbon Compounds ModuleDokumen16 halamanCarbon Compounds ModuleAaron Asne100% (1)
- Hydroxy and Carbonyl CompoundsDokumen11 halamanHydroxy and Carbonyl CompoundsFatima SiddiquiBelum ada peringkat
- Ald&Ketone IIDokumen51 halamanAld&Ketone IIheraldas2421Belum ada peringkat
- Frequency Range (CM) Absorption (CM) Appearance Group Compound Class CommentsDokumen3 halamanFrequency Range (CM) Absorption (CM) Appearance Group Compound Class CommentsNazratul NajwaBelum ada peringkat
- Basic Concepts in Organic Chemistry: Jeevanantham P I MSC Chemistry, SRMV College of Arts and Science, CoimbatoreDokumen47 halamanBasic Concepts in Organic Chemistry: Jeevanantham P I MSC Chemistry, SRMV College of Arts and Science, CoimbatoreRama GaurBelum ada peringkat
- TECHNOLOGICAL INSTITUTE OF THE PHILIPPINES FINAL EXAMINATIONDokumen3 halamanTECHNOLOGICAL INSTITUTE OF THE PHILIPPINES FINAL EXAMINATIONWinsletJoyDauagBelum ada peringkat
- 13AMIDE PPT CcoDokumen35 halaman13AMIDE PPT CcoAngelo AstudilloBelum ada peringkat
- SCH 206-Carboxylic Acids PDFDokumen48 halamanSCH 206-Carboxylic Acids PDFShivani DamorBelum ada peringkat
- Aldehyde and Ketones McMurryDokumen43 halamanAldehyde and Ketones McMurryShamira100% (1)
- Cambridge University Pressorganic Chemistry PDFDokumen47 halamanCambridge University Pressorganic Chemistry PDFluis peixoto100% (1)
- Acrylic Acid Production - CompressDokumen82 halamanAcrylic Acid Production - CompressDavid Berkat H SBelum ada peringkat
- Matriculation Chemistry (Carboxylic Acid)Dokumen68 halamanMatriculation Chemistry (Carboxylic Acid)ridwan50% (2)
- Timetable For Lab Chm412/413 (Mar-July 2020) ORGANIC CHEMISTRY CHM412/CHM413 Venue: Makmal Kimia EDU Exp. Title Date NotesDokumen1 halamanTimetable For Lab Chm412/413 (Mar-July 2020) ORGANIC CHEMISTRY CHM412/CHM413 Venue: Makmal Kimia EDU Exp. Title Date NotesNaurah AisyahBelum ada peringkat
- Pre-Medical: Chemistry Allen: Carbonyl Compounds, Acids and It'S Derivatives Carbonyl CompoundsDokumen18 halamanPre-Medical: Chemistry Allen: Carbonyl Compounds, Acids and It'S Derivatives Carbonyl CompoundsJK JHABelum ada peringkat
- Carbonyl CompoundsDokumen244 halamanCarbonyl CompoundsAbcd EfghBelum ada peringkat
- Kalyani Univ Chemistry - Hons Syllabus 2010Dokumen27 halamanKalyani Univ Chemistry - Hons Syllabus 2010Abbhijit RoyBelum ada peringkat
- CHEM 200 IR SpectrosDokumen35 halamanCHEM 200 IR SpectrosIngrid LacsonBelum ada peringkat
- Overview of The Reactions of Carbonyl Compounds: - Topical Outline of CoverageDokumen54 halamanOverview of The Reactions of Carbonyl Compounds: - Topical Outline of CoverageveronashaqBelum ada peringkat
- Rings, Polymers and Analysis (Unit 4) - OCR Chemistry Notes - Robbie PeckDokumen14 halamanRings, Polymers and Analysis (Unit 4) - OCR Chemistry Notes - Robbie Peckrobbiepeck100% (1)
- Stereo Specificity PDFDokumen15 halamanStereo Specificity PDFRams ChanderBelum ada peringkat
- 12 Chemistry Keypoints Revision Questions Chapter 12Dokumen20 halaman12 Chemistry Keypoints Revision Questions Chapter 12sangam patraBelum ada peringkat
- Synthesis of Benzyl Acetate Through Fischer Esterification ReactionDokumen10 halamanSynthesis of Benzyl Acetate Through Fischer Esterification ReactionAnonymous GO6JVW9Wud100% (4)
- Rules in Naming of Carboxylic Acids and Its DerivativesDokumen12 halamanRules in Naming of Carboxylic Acids and Its DerivativesJr BalagtasBelum ada peringkat
- Kate Coleen D. Galera BS in Chemistry II May 4, 2017 Experiment 11 Acyl Compounds: Soaps and DetergentsDokumen5 halamanKate Coleen D. Galera BS in Chemistry II May 4, 2017 Experiment 11 Acyl Compounds: Soaps and DetergentsKateBelum ada peringkat
- Chem 317 - Lab 7 - Arene II ReportDokumen15 halamanChem 317 - Lab 7 - Arene II Reportapi-251758870100% (4)
- B. Pharmacy I Yr Syllabus 2009-2010Dokumen15 halamanB. Pharmacy I Yr Syllabus 2009-2010harikrishna2009Belum ada peringkat
- 31 IndexDokumen40 halaman31 IndexJohnBelum ada peringkat
- DNSDokumen7 halamanDNSainakmliaBelum ada peringkat
- Organic Reactions and MechanismDokumen51 halamanOrganic Reactions and MechanismAbhay Kumar Nayak75% (8)
- Chemistry 1152 Practice Final ExamDokumen18 halamanChemistry 1152 Practice Final Examdeckbyte865Belum ada peringkat