REAKTOR MASS BALANCE
Diunggah oleh
makaqlali0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
184 tayangan2 halamantentang neraca massa pada reaktor
Judul Asli
Neraca Massa Reaktor
Hak Cipta
© © All Rights Reserved
Format Tersedia
DOCX, PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen Initentang neraca massa pada reaktor
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai DOCX, PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
184 tayangan2 halamanREAKTOR MASS BALANCE
Diunggah oleh
makaqlalitentang neraca massa pada reaktor
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai DOCX, PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 2
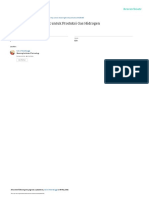
M6
Reaktor
M3
M5
M4
Keterangan :
M3 = massa larutan NaOH dari tangki pelarut
M4 = massa gas Cl2
M5 = massa liquid keluar reaktor
M6 = massa gas Cl2 sisa
Karena perbandingan feed masuk adalah 2 : 1 maka dapat dihitung :
NaOH
500
12,5 kgmol/jam
40
Cl2
1
12,5 6,25 kgmol/jam
2
Konversi reaksi = 94% terhadap NaOH
2 NaOH
Cl2
NaOCl + NaCl + H2O
Mula-mula :
12,5
6,25
Bereaksi
11,75
5,875
5,875
5,875
5,875
Sisa
0,75
0,375
5,875
5,875
5,875
NaOH masuk
= 500 kg/jam
NaOH bereaksi
= 11,75 x 40 = 470 kg/jam
NaOH sisa
= 0,75 x 40 = 30 kg/jam
Cl2 masuk
= 6,25 x 71 = 443,75 kg/jam
Cl2 bereaksi
= 5,875 x 71 = 417,125 kg/jam
Cl2 sisa
= 0,375 x 71 = 26,625 kg/jam
NaOCl produk
= 5,875 x 74,5 = 437,6875 kg/jam
NaCl prod. samping = 5,875 x 58,5 = 343,6875 kg/jam
H2O berlebih
= 5,875 x 18 = 105,75 kg/jam
Neraca Massa pada Reaktor
Bahan Masuk (kg/jam)
Bahan Keluar (kg/jam)
Dari Tangki Pelarut NaOH (M4)
Ke storage produk (M5)
NaOH
: 500
NaOH
H2O
: 2.000
NaOCl
437,6875
NaCl
343,6875
H2O
: 2.105,75
2.500
Dari storage Cl2 (M3)
Cl2
: 443,75
443,75
30
2.917,125
Ke Absorber (M6)
Cl2
26,625
26,625
Total
: 2.943,75
Total
: 2.943,75
Anda mungkin juga menyukai
- Tuhan Inilah Proposal HidupkuDokumen8 halamanTuhan Inilah Proposal HidupkuAbiyyu AhmadBelum ada peringkat
- Lampiran A Neraca Massa (6-12-2020)Dokumen33 halamanLampiran A Neraca Massa (6-12-2020)Zaki Themonk31100% (1)
- Data Brine PDFDokumen10 halamanData Brine PDFNovia PurwitasariBelum ada peringkat
- Contoh Soal Kristalisasi Senja Dewi KinantiDokumen2 halamanContoh Soal Kristalisasi Senja Dewi KinantiArdynaApriSapoetriBelum ada peringkat
- EvaporasiDokumen26 halamanEvaporasiSepptiiBelum ada peringkat
- T2 NewDokumen11 halamanT2 NewUntung SetiawanBelum ada peringkat
- AppendixDokumen314 halamanAppendixRega LinzaBelum ada peringkat
- Prarancangan Pabrik Aseton Dari Isopropil Alkohol Dengan KapasitasDokumen14 halamanPrarancangan Pabrik Aseton Dari Isopropil Alkohol Dengan KapasitasakbarynpBelum ada peringkat
- Kimia KhairatDokumen4 halamanKimia KhairatDion Siagian100% (1)
- Appendix PDFDokumen195 halamanAppendix PDFbirg MF MelBelum ada peringkat
- LAMPIRAN II Rapi Neraca Panas AjiDokumen35 halamanLAMPIRAN II Rapi Neraca Panas AjiAlan BayuBelum ada peringkat
- OPTIMASI UTILITAS PABRIK GARAMDokumen43 halamanOPTIMASI UTILITAS PABRIK GARAMandre p.lBelum ada peringkat
- Prarancangan Pabrik Anilin Kapasitas 10.000 TonDokumen182 halamanPrarancangan Pabrik Anilin Kapasitas 10.000 TonIrvan Eko SaputraBelum ada peringkat
- PULP PROSESDokumen6 halamanPULP PROSESRike NoviantiBelum ada peringkat
- Makalah UtilitasDokumen102 halamanMakalah UtilitasMerrison AkhzulBelum ada peringkat
- 04-Neraca Massa Dengan ExcelDokumen9 halaman04-Neraca Massa Dengan ExcelPuji AstutiBelum ada peringkat
- 21 PDFDokumen391 halaman21 PDFAditya YudantokoBelum ada peringkat
- Pembuatan AmoniaDokumen15 halamanPembuatan AmoniaMutia febrianaBelum ada peringkat
- Pra - Rancangan Pabrik Kimia VCMDokumen32 halamanPra - Rancangan Pabrik Kimia VCMindry nikitasaryBelum ada peringkat
- LC 1 EtanolDokumen6 halamanLC 1 EtanolBagja Malik SyakurBelum ada peringkat
- Laporan PKL Fix 4 Maret 2017Dokumen113 halamanLaporan PKL Fix 4 Maret 2017Aidil FadlyBelum ada peringkat
- Proses Industri Kimia Organik (Aminasi-Kelompok 4) - TRKI 2017Dokumen14 halamanProses Industri Kimia Organik (Aminasi-Kelompok 4) - TRKI 2017Azzah LatifahBelum ada peringkat
- 7 Rabu Lapres KristalDokumen41 halaman7 Rabu Lapres KristalAnna Alif Mu'alimahBelum ada peringkat
- Tugas II Neraca EnergiDokumen13 halamanTugas II Neraca EnergiMbembem Tu IchaBelum ada peringkat
- Laporan PK3 - 2Dokumen14 halamanLaporan PK3 - 2mutiana zainBelum ada peringkat
- OPTIMASI PROSES PEMBUATAN METANOLDokumen11 halamanOPTIMASI PROSES PEMBUATAN METANOLoptimusbee4Belum ada peringkat
- FILTRASI OPTIMALDokumen24 halamanFILTRASI OPTIMALJerome HarrisBelum ada peringkat
- ABSORPSI GAS CO2 DAN H2S DENGAN AMINEDokumen3 halamanABSORPSI GAS CO2 DAN H2S DENGAN AMINEandono kusuma jatiBelum ada peringkat
- OPTIMASI TOWERDokumen8 halamanOPTIMASI TOWERolivia christinBelum ada peringkat
- Bab I - Pengendalian ProsesDokumen14 halamanBab I - Pengendalian ProsesPuji RahmawatiBelum ada peringkat
- Tugas Otk Aplikasi Transfer MassaDokumen4 halamanTugas Otk Aplikasi Transfer Massafarhan atqBelum ada peringkat
- Bab II Seleksi Dan Uraian Proses FixDokumen36 halamanBab II Seleksi Dan Uraian Proses FixFitria PutriBelum ada peringkat
- Laporan Evaluasi Kinerja Reaktor Sintesis Urea 1ADokumen109 halamanLaporan Evaluasi Kinerja Reaktor Sintesis Urea 1ATito Fairuz NuriadiBelum ada peringkat
- NERACA MASSADokumen13 halamanNERACA MASSAJoshita KusumadewiBelum ada peringkat
- 3 - Dasar Kristalisasi (Bag II)Dokumen36 halaman3 - Dasar Kristalisasi (Bag II)haniifprasetiawanBelum ada peringkat
- PRARANCANGAN PABRIK ANILINDokumen55 halamanPRARANCANGAN PABRIK ANILINRefi RahmanBelum ada peringkat
- Analisis Kinerja Reaktor Esterifikasi 1 Line A PT Asia Pacific FiberDokumen63 halamanAnalisis Kinerja Reaktor Esterifikasi 1 Line A PT Asia Pacific FiberAnggi Ilfa SBelum ada peringkat
- Soal UAS EKONOMI TEKNIK Kelas 3 - Reg Sem. Genap 2021-2022Dokumen1 halamanSoal UAS EKONOMI TEKNIK Kelas 3 - Reg Sem. Genap 2021-2022NADIA YULIANA ILHAMBelum ada peringkat
- Praktikum Operasi Teknik Kimia SR HDokumen2 halamanPraktikum Operasi Teknik Kimia SR HKartiniBelum ada peringkat
- Pabrik Metil AkrilatDokumen28 halamanPabrik Metil AkrilatAnggitBelum ada peringkat
- Water Gas ShiftDokumen8 halamanWater Gas ShiftRaven_Belum ada peringkat
- BM-ASAMDokumen24 halamanBM-ASAMAhmad HermanBelum ada peringkat
- Reaktor KimiaDokumen23 halamanReaktor Kimiaumar fauzi100% (1)
- Pengantar Industri KimiaDokumen19 halamanPengantar Industri KimiaMuhammad RizkiBelum ada peringkat
- Produksi Butanol dari Glukosa melalui Fermentasi ABEDokumen5 halamanProduksi Butanol dari Glukosa melalui Fermentasi ABEFauzi AbdilahBelum ada peringkat
- Lampiran C Spesifikasi Ekstraktor 401Dokumen10 halamanLampiran C Spesifikasi Ekstraktor 401Milian Asha Bio MuradBelum ada peringkat
- BiokerosinDokumen2 halamanBiokerosinrahmiatiBelum ada peringkat
- Laporan SedimentasiDokumen27 halamanLaporan SedimentasiAde AnjaniBelum ada peringkat
- PABRIK METANOLDokumen11 halamanPABRIK METANOLanjarBelum ada peringkat
- Pertemuan Ke 3 - Konversi Dan SelektifitasDokumen23 halamanPertemuan Ke 3 - Konversi Dan SelektifitasDzion PratamaBelum ada peringkat
- OPTIMALKAN MELTERDokumen16 halamanOPTIMALKAN MELTERBrayonoFloBelum ada peringkat
- SPERALATANDokumen27 halamanSPERALATANnaufalBelum ada peringkat
- Makalah Reaktor Industri KimiaDokumen22 halamanMakalah Reaktor Industri KimiaGhina F. SalimBelum ada peringkat
- KINERJA UNIT CLEAR JUICE EVAPORATOR PABRIK GULA BUNGAMAYANGDokumen22 halamanKINERJA UNIT CLEAR JUICE EVAPORATOR PABRIK GULA BUNGAMAYANGMara SutanBelum ada peringkat
- Neraca Masssa Industri Pulp PDFDokumen299 halamanNeraca Masssa Industri Pulp PDFLenywulandari AyundaBelum ada peringkat
- Laporan Pembuatan HCLDokumen13 halamanLaporan Pembuatan HCLRizka Rismayani100% (1)
- REAKSI KIMIADokumen7 halamanREAKSI KIMIAVika MelindaBelum ada peringkat
- Appendik ADokumen14 halamanAppendik AElok RachmawatiBelum ada peringkat
- OPTIMALKAN_KONVERSIDokumen7 halamanOPTIMALKAN_KONVERSImukhlisoharifah0% (1)
- Laporan HCLDokumen8 halamanLaporan HCLRizal AprianBelum ada peringkat
- Ciri Ciri Mahluk HidupDokumen1 halamanCiri Ciri Mahluk HidupmakaqlaliBelum ada peringkat
- PLN Yogyakarta GAT 2016Dokumen139 halamanPLN Yogyakarta GAT 2016Anita Dwi KusumastutiBelum ada peringkat
- Gambar NM 2Dokumen1 halamanGambar NM 2makaqlaliBelum ada peringkat
- Keplek TegakDokumen1 halamanKeplek TegakmakaqlaliBelum ada peringkat
- Fungsi MS ExcelDokumen13 halamanFungsi MS ExcelEddy SasmiBelum ada peringkat
- 4 Absorber Stripper d3Dokumen10 halaman4 Absorber Stripper d3Sandy SetiyoBelum ada peringkat
- Uud 1945 Dan AmandemenDokumen4 halamanUud 1945 Dan Amandemenanon-52624180% (15)
- Uud 1945 Dan AmandemenDokumen4 halamanUud 1945 Dan Amandemenanon-52624180% (15)
- Analisis Lipida SederhanaDokumen13 halamanAnalisis Lipida SederhanasaduddinachmedBelum ada peringkat
- Ref He 2Dokumen8 halamanRef He 2anon_431686175Belum ada peringkat
- Pertamawati Pengaruh SuhuDokumen5 halamanPertamawati Pengaruh SuhumakaqlaliBelum ada peringkat
- Skripsi-Pembuatan Metil Ester (Biodiesel) Dari Minyak Dedak Dan Metanol Dengan Proses Esterifikasi Dan TransesterifikasiDokumen43 halamanSkripsi-Pembuatan Metil Ester (Biodiesel) Dari Minyak Dedak Dan Metanol Dengan Proses Esterifikasi Dan TransesterifikasiHendrik Lin Han XiangBelum ada peringkat
- Laporan EsterifikasiDokumen10 halamanLaporan EsterifikasiMarina Rosa AnggraeniBelum ada peringkat
- Pembuatan BiodieselDokumen6 halamanPembuatan BiodieselrezkinugrohoBelum ada peringkat
- KONDENSASIDokumen25 halamanKONDENSASIsuckrindjinkBelum ada peringkat
- Indonesian FlyerDokumen1 halamanIndonesian FlyerRahmad BernardBelum ada peringkat