Evaporação Por Transferência de Massa
Diunggah oleh
Rui Costa0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
13 tayangan16 halamanLei de Fick
Judul Asli
Evaporação por Transferência de Massa
Hak Cipta
© © All Rights Reserved
Format Tersedia
PDF atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniLei de Fick
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
13 tayangan16 halamanEvaporação Por Transferência de Massa
Diunggah oleh
Rui CostaLei de Fick
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF atau baca online dari Scribd
Anda di halaman 1dari 16
APPENDIX C- DROPLET EVAPORATION
Alll the physical properties are calculated at 15° Cand 1.5 bar.
The mass transfer rate is calculated through:
dm
SP = K, Ay (Yas Yao) «ay
where K, is the mass transfer coefficient per unit of area: Y4s and ¥4q are the mass
concentrations at saturation and at the operating conditions (respectively). and Ay
is the mass transfer area
For a sphere of mass m,, the mass ’flowing’ into the surrounding fluid, equals
the mass leaving the sphere, dm,/dt:
(C.2)
which, for a sphere of mass 4/3p,7r3, becomes:
7 dnp, C3)
a ATS a (c3)
Combining Equation C.3 with Equation C.1, one obtains:
dr, Yas — Yao
ark AS (C4)
It remains to calculate the mass transfer coefficient, K,.
‘The general equation for droplet evaporation, due to Froessling, reads:
Sh = 2+ 0.6 Re Sc¥ (C.5)
being:
Sh Sherwood number
Re Reynolds number
Se~Schmidt number
Equation C.5 becomes:
140
2+o5 (2 fetter) (4) C6)
where © 4, is the diffusion coefficient.
kis related to K, through:
Kec
C7.
O-¥a)in en
where (1-Y,)jq is the drift factor:
(C8)
=Ck (C9)
with:
Po Yas¥ao) In (
c (C10)
a « a (C10)
and k given by Equation C.6.
‘The motion of the sphere is considered through the simple relationship:
Pa \ l4o-H,1 (
ee em
The differential Equations C.9 and C.11 are solved together in order to calculate
the evaporation time (time necessary for the sphere to reduce its radius from ryy to
0.
With the initial conditions u,=0 and r,=r,q for :=0, the solution was obtained
for water droplets in air with various degrees of saturation. These were assumed to
be constant throughout the calculation, because air is not recycled in the rig.
Variables of interest are listed below:
141
PG = 1.8 ke/sm?
P, = 998.4 ke/sm?
P, = 0.01704 bar
IM, = 18.02 kelkeye
Mg = 28.96 kelkonore
Dgy = 1599x1075 m/s
‘®qp is calculated in Appendix F and P, is the saturation pressure of water vapour.
The mole fraction of water vapour 4s, calculated through the Rzoult’s law, is
related to the mass fraction of water vapour through:
5 Ms
Yas = Sas qo (C.12)
Calculations were carried out for various droplet sizes (up to 10 um) considering
cither the full solution (including droplet acceleration) or the solution obtained for
a stagnant medium (ug=u,). Results are shown in Figure C.1. The following
conclusions can be drawn from this:
1. There is very little difference between both solutions, because droplets,
(very small) accelerate very quickly to the gas velocity, approaching the
stagnant medium solution.
2. The life time of droplets increases with the relative humidity.
3. Droplet initial velocity is not an important parameter, due to
justifications given under item (1) above.
4, Small water droplets produced by a nebulizer (up to 10 yt) will not reach
the measuring section. For a gas velocity of 44 m/s the flight time of a
droplet inside the rig is around 0.09 s which is very close to its life time.
142
ool
oun uontiodvaa 1adoud “1-9 “Dkr
{wily sajawDig yadoug
SZ os Gz 0
10
700
700
‘900
(S) @wiy uoijdsodpaz
(374 oe=")) 1 aqd0NG GaLvaaTaIIV — — 10
WNId3N LNVNOWES —
APPENDIX F- INFLUENCE OF HUMIDITY ON THE
PHYSICAL PROPERTIES OF AIR
For reference all the physical properties are calculated at 15°C and 1.5 bar.
It will be considered that the gas is a mixture of air and water vapour. In
addition, the air is considered to be saturated. This is the most severe situation and
is a reasonable estimation because both fluids flow at similar temperatures for a
considerable distance.
‘The molar fraction of water vapour in the air is calculated by the Raoult's law
P,
P Saver 1)
For the present conditions, fy = 1. and P, = 0.017039 bar. So
Jy = 0.01136
and ¥, = 0.98864
For the present fluids the properties of interest are listed in Table F.1.
TABLE F.1- PHYSICAL PROPERTIES OF DRY AIR AND WATER
VAPOUR
epee eee cee
| PHYSICAL WATER |
PROPERTY AIR | VAPOUR |
1 ee
MOLAR MASS,
(ke/kgmole) 28.96 18.02
CRITICAL
TEMPERATURE (K)] 132.56 647.15
CRITICAL VOLUME|
(on? fkgmole) os | 0.0563
VISCOSITY
| (kg/ms) 178.7x10~7 | 93x1077 |
159
Density The density of both dry and saturated air were calculated using the
methodologies described in the sheets PMI2 and PMI3 of the HTFS Handbook,
respectively.
For dry air, the density is calculated through
MP
ee Ee F.2)
O° aaazT 2)
where 8134.4 [J/kgmole] is the ideal gases constant. This equation gives a value of
1.85 kg/m? for the density
For a gaseous mixture, the density is calculated by:
MP,
°m = SBAZ, Tr Fs)
The subscript ‘m' refers to mixture properties. For the present fluids,
Pp, = 1.80 kg/m. Thus the difference between both cases is less than 3%.
Viscosity The method used to calculate the mixture viscosity was presented by
Buddenberg and Wilke (1949). For a pair of fluids (1 and 2), the following
‘equation was applied:
ty
Hyp + (F.4)
1422
385 Hh
A Bn wy
where %y2 is the diffusion coefficient between air and water vapour. This
parameter is calculated from (Fuller et al,1966):
“7 pins (1, 1)
1.013x10-7 T* (arti)
i
By) PGP aa? 3)
where P, the pressure, is in ‘bar’ and n, and 7, are special diffusion parameters.
For water vapour in air, the values for these two parameters are 12.7 and 20.1,
respectively.
Making the necessary substitutions, the value of 178.3x10~7 kg/sm is obtained
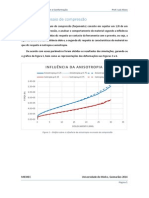
for the mixture viscosity. Figure F.1 shows the effect of the presence of water
vapour in air upon the mixture viscosity. For very small concentrations of water
vapour, its effect is negligible.
160
Hmikg/sm_ x10000)
200
180:
160
140
120
80
60:
00 02 O04 06 08 10
Molar Fraction of Water Vapour
FIG. F.1- Viscosity of a air-water vapour mixture: effect of water vapour
concentration
LIST OF SYMBOLS
4
En)
Particle/droplet radius (m)
Nikuradse sand roughness parameter ©
‘Tube cross sectional area ee (m?)
Light velocity. (m/s)
Friction factor (-)
Droplet concentration in the gas core momen (kgim3)
Constant in the logarithmic law ©
Drag coefficient ©)
Equilibrium droplet concentration in the gas core (kg/m?)
Corrections to the Rosin-Rammler distribution param-
eters a 7 Cc)
Droplev/ particle diameter. 7 (m)
Mass median diameter. (m)
Mean droplet size. sen - (m)
Tube diameter Seer eecreeeCeee (m)
Deposition rate Boe (kg/sm?)
Diffusion coefficient. oe zs! (m/s)
Error associated with the mean diameter. Sree (m)
Light energy ey
Lagrangian energy spectrum function eee (3s?)
190
EB
fi
Syd
fla)
Fr
Entrainment rate : (kelsm?)
Lens focal length... = eat (m)
Interfacial friction factor... ~ ~ (-)
Probability density function (frequency) nm ©
Probability density function (volume) ()
Froude number (=U, /(dg)°*) oO
Fraction of entrained liquid collected by the plate )
Drag force vector - a ww)
Fraction of entrained liquid driven onto the liquid film )
Cumulative distribution function (frequency) (-)
‘Cumulative distribution function (volume) won ()
Gravitational acceleration. (m/s)
Body forces vector é (mjs?)
‘Mass flux (kg/sm?)
Latent heat of vaporization... Wik)
Light intensity Sere i ©
AC component of Doppler signal light intensity ©)
DC component of Doppler signal light intensity... ©)
Relative turbulence intensity (=u” pug ) zasaaee oO
Mass transfer coefficient asa (m/s)
Flatness £8CtOf eveevnnnnnn Eee a (-)
‘Mass transfer rate per unit area... z (ke/m?s)
mixing length ——- (m)
191
OB
Pu) pv)
unit vectors ... Oo
Light energy distribution ©
Length of the plate (nm)
Turbulence length scale 7 i (m)
Mean film thickness BCP SeeeeeeEeeereeees (m)
Base liquid film thickness s sepnenitheastar())
Loading ratio. )
Mass of sphere z : (kg)
Mass flow rate : (ka(s)
Number of sizing groups of the droplet distribution
function 7 (-)
Molar mass (ke/kgmole)
Width parameter of Rosin-Rammler distribution ©)
Exponent of the power law )
Wave number (= 1/27) &
Number of events... 7 oO
Number of fringes.. ~
Number of lines in the grating saaties —- (-)
Number of fringes crossed by the dropletiparticle
inside the probe volume ei ©
Obscuration nance (“)
Ohnesorge number (=u,/\/op, Dz) vere (=)
Probability distribution functions of velocity com-
POREDS ener i ©
192
Re
Reng
fe
Pressure .... a (Nim?)
Energy dissipation per unit of mass (m?/s)
Saturation pressure of water vapour (W/m?)
Turbulent kinetic energy per unit of mass ... (m/s?)
Volumetric flow rate (m3/s)
Radial distance measured from the centre of the tube (m)
‘Correlation coefficient (Equation 7.22) .. =e ©
Radial distance measured from the centre of the probe
volume 7 (m)
Radius of sphere. a (m)
Dimensionless radius (=r/R) 7 ()
Tube radius (m)
Reynolds number (=D;Up/a) ~)
Particle Reynolds number (=|u,! fd/vy) ©)
Fringe spacing. (m)
Line spacing of the grating a (m)
Skewness factor (-)
Schmidt number (=(W/og@4p)") nove (=)
Sherwood number (=2kr,/24p) Z (-)
Mean droplet size standard deviation ...-.....-—. (m)
Slip ratio (=(ug—Uug)lug ) ~ ns (=)
HME onsen sf (9)
droplet/ particle residence time inside the LDA probe
volume scene oes (s)
droplet/ particle residence time inside the laser beam ()
MALVERN coefficients matrix (Equation 5.1)... (-)
Temperature (kK)
Velocity vector : (m/s)
Fringes vel (m/s)
Instantaneous velocity components in a cartesian
system, eo (m/s)
Fluctuating velocity components (m/s)
Mean values of the velocity components (mys)
RMS values of the velocity fluctuations (m/s)
Reynolds shear stress. asa: (m?/s)
Shear velocity (= V7). o (m/s)
Core dimensionless velocity (=u/u") oO
Central moment k of the velocity component Uce-miorermeenwer (=)
Particle velocity perpendicular to the fringes in the
laser probe volume (ms)
Superficial velocity (=rh/pA 7)... (m/s)
Dimensionless superficial gas-_—_velocity
(UspG'VeD7@, Pe) “ a — ()
Visibility sees woe (=)
Particle/ droplet volume... ot (m3)
Probe volume radius. enrnenne (m)
Weight distribution of droplets....ecco-eeernenne ©
194
x
‘Weber number (=p, U3D;/0) (-)
Weber number defined as (=p, Ug4y/0) (-)
Weber number defined by Equation 7.20 ©
Cartesian coordinates. (m)
Peak parameter of the Rosin-Rammler distribution (m)
Distance between the droplet sample and the
collection lens (Figure 5.5) as (m)
Molar fraction - - ©
Mass fraction ©)
Dimensionless distance from the wall (=yu"/v) (-)
Axial distance - - (mn)
Compressibility ©
Greek symbols
Void fraction ©
Void fraction of droplets in the gas COf€ oe. oO
Plate thickness. a ees (mn)
Interfacial roughness parameter ( =mu" p,/y,).. ©
Empirical coefficients in Equation 2.10 ©
Variation of the liquid film mass flow rate (kg/s)
Dissipation rate of turbulent energy (m?/s3)
Fluid diffusivity... saan x (7/5)
195
6, Particle/droplet diffusivity .
©,
¢, Liquid film roughness ........ (m)
Collection aperture... )
4, Diffraction angle... — - vo (HD
$y “Heat flux Peer ete eee re annonces (Wh?)
n Amplitude ratio (-)
7 Ratio of densities (=p,/p)) ..-. ©)
T Gamma function... ()
T, Tangential flow per unit perimeter .. (kg/sm)
T, Axial flow per unit perimeter (ke/sm)
4. Light wavelength i a (m)
Avaye Disturbance wave wavelength _ (m)
Ap Perturbation wavelength at the end of the plate (m)
Ay Taylor wavelength (=/9;8) (m)
2° Disturbance wave trough length... “ (m)
Viscosity Bee eee (kg/ms)
y Kinematic viscosity... esas (m?/s)
v Frequency .......... Eee vecuscunn (Hz)
¥, Shift frequency... sseee ee (Hz)
TI Offset angle of collection. (-)
8 Diffraction angle ae ©
p Density nee See See (kgim?)
© Surface tension _..... (Nim)
%
Pp
cL
Standard deviation of the Gaussian function =
Shear stress
Interfacial shear stress s
Eddy frequency
Frequency of the most energetic eddies a
Grating rotational speed
Half of the crossing angle of the laser beams
Subscripts
Apparent
Core
At the centre line
Doppler
f Fluid
Gas
Gas core (homogeneous)
Indices in tensor notation
min
Liquid film
Mixture property
Minimum value
197
©
(Nim?)
(Wim?)
(rads)
(rad/s)
(rads)
©)
‘max
pid
yoo B®
Maximum value
Particle/droplet
Radial component
Relative
At saturation
‘Terminal
Wall
Axial component
Under actual conditions
198
Anda mungkin juga menyukai
- Secagem Por Jatos Dyson AirbladeDokumen31 halamanSecagem Por Jatos Dyson AirbladeRui CostaBelum ada peringkat
- Determinação Das Curvas Características de Uma Bomba Centrífuga (Gilkes)Dokumen22 halamanDeterminação Das Curvas Características de Uma Bomba Centrífuga (Gilkes)Rui Costa100% (3)
- Simulação Ensaio de Tração UniaxialDokumen4 halamanSimulação Ensaio de Tração UniaxialRui CostaBelum ada peringkat
- Simulações Dos Ensaios de Compressão e EmbutiduraDokumen12 halamanSimulações Dos Ensaios de Compressão e EmbutiduraRui CostaBelum ada peringkat
- Kart e Lançador de ProjéteisDokumen42 halamanKart e Lançador de ProjéteisRui CostaBelum ada peringkat
- Análise Cinemática Pedaleira TricicloDokumen2 halamanAnálise Cinemática Pedaleira TricicloRui CostaBelum ada peringkat
- Análise Forças TricicloDokumen6 halamanAnálise Forças TricicloRui CostaBelum ada peringkat
- Maquinagem de Uma PeçaDokumen22 halamanMaquinagem de Uma PeçaRui CostaBelum ada peringkat