Case Study Tomodirect Breast
Diunggah oleh
api-264047496Deskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Case Study Tomodirect Breast
Diunggah oleh
api-264047496Hak Cipta:
Format Tersedia
1
Stephanie Olson
DOS 771 Case Study
April 15, 2015
Treatment for left breast cancer using TomoDirect
History of Present Illness: BS is a 63 year-old female diagnosed with Stage IIA predominant
invasive lobular carcinoma with focal ductal carcinoma of the left breast. She first presented on
screening mammogram in May 2014 which showed possible architectural distortion in the left
lateral aspect of the left breast. At that time, it was recommended that additional mammography
views be taken. At the end of June 2014, BS underwent a diagnostic mammogram that revealed
a five mm mass at the one oclock position within the left breast. There was also a questionable
mass 15 mm medial to this mass which was not visualized on ultrasound. BS underwent a
biopsy of the identified mass which showed evidence of invasive carcinoma with mixed ductal
and lobular features. In late June 2014, BS underwent a left breast lumpectomy and sentinel
node biopsy. Pathologic analysis of the surgical specimen demonstrated the findings
summarized above.
Past Medical History: BS has past medical history of depression, sleep apnea, migraine,
degenerative disc disease, osteopenia, mitral valve prolapse, impaired glucose tolerance, bowel
obstruction, seasonal affective disorder, allergic rhinitis, hyperlipidemia, obesity, osteoarthritis,
GERD, and CAD. In addition, past surgical history includes cholecystectomy, carpal tunnel
release and arthroscopy of the right knee. BS has no known allergies.
Social History: BS is married and lives with her partner of 28 years. She is a registered nurse
manager by training but was not working at the time of consultation. She denies tobacco use or
alcohol consumption. BS reported that her mother had a history of cervical cancer with onset at
the 60 years of age and her father had a history of coronary heart disease and hypertension. The
patient also reported two cousins with a history of breast cancer but the age of diagnosis was
unknown.
Medications: BS uses the following medications: Acetaminophen, Alprazolam, Aspirin,
Calcium, Cetirizine, Estazolam, Lamotrigine, Magnesium, Melatonin, Multivitamin, Omega 3
fatty acid, Omeprazole, Oxycodone, Sumatriptan, Wellbutrin.
Diagnostic Imaging: In June 2014, BS underwent a diagnostic mammogram after an initial
screening mammogram from May 2014 showed possible architectural distortion in the lateral
aspect of the left breast. After completion of her diagnostic mammogram, BS underwent an
image guided biopsy which showed invasive carcinoma with mixed ductal and lobular features.
At the end of June 2014, BS underwent a left lumpectomy and sentinel node biopsy.
Radiation Oncologist Recommendations: After reviewing pathologic, radiologic and clinical
data with BS, who was by herself at consultation, the radiation oncologist discussed the
utilization of radiation therapy to treat her infiltrating lobular carcinoma of the left breast. The
main focus of the discussion was local treatment options for breast cancer including mastectomy
versus lumpectomy in conjunction with radiation therapy. Also discussed was the role of
radiation therapy in the breast conservation setting, which reduces the risk for in-breast tumor
recurrence by approximately two-thirds, typically from 30% to 6-10%.1
The Plan (prescription): The radiation oncologists treatment recommendation to BS was a
hypofractionated whole breast irradiation approach followed by a boost to the lumpectomy
cavity. The prescription recommended for this plan was a dose of 40.05 Gy at 2.67 Gy per
fraction for 15 fractions to the whole breast with an additional boost to the lumpectomy cavity of
10 Gy at 2 Gy per fraction for 5 fractions. For the purpose of this case study, only the initial
whole breast plan will be discussed.
Patient Setup/Immobilization: In July 2014, BS underwent a CT simulation scan for radiation
therapy treatment planning. The patient was placed in the supine position using a Civco wing
board with a standard clear head sponge. The patient had both arms up holding on to handles on
the wing board. Her head was turned to her right and away from the affected side of treatment
(Figures 1). A sponge was placed under her knees for support. Planning CT images were
acquired using 2.5 mm slices. The scanning parameters included from the level of the chin and
extended to include the entire thoracic cavity. The patient was marked using one set of 3-point
markings on her chest. Image defining wires were placed on the patients scars at both the
lumpectomy and nodal biopsy sites. After completion of the CT scan, permanent tattoos were
placed on the patients skin in the chest region at the points of fiducial placement.
Anatomical Contouring: After completion of the CT simulation scan, the CT data set was
transferred into the Philips Pinnacle3 treatment planning system (TPS) for contouring. The
radiation oncologist contoured the lumpectomy gross tumor volume (GTV) which was expanded
by 1.5 cm in all dimensions to create a lumpectomy planning tumor volume (PTV). The location
and size of the involved disease was verified from the mammography report performed in June
2014 and by the location of the clips placed during the lumpectomy surgery. In addition to the
GTV and PTV, the left breast tissue was contoured by the radiation oncologist. The medical
dosimetrist contoured organs at risk (OR) that included the spinal cord, heart, right and left lung,
humerus, and esophagus. Planning at risk volumes (PRV) created included the spinal cord and
esophagus.
Beam Isocenter/Arrangement: Once all contours were finalized, the radiation oncologist
completed a treatment planning order which identified the prescription, dose constraints and
other pertinent treatment planning information. These instructions were given to the medical
dosimetrist to begin treatment planning. Due to the patients anatomy, the physician requested a
TomoDirect treatment plan to help decrease the dose to the heart, lungs and soft tissue region
adjacent to the breast. For the intensity modulated radiotherapy (IMRT) plan using TomoDirect,
the patients data set and contoured structures were transferred from the Pinnacle3 treatment
planning system to the TomoTherapy Hi-Art version 5.0 planning system. TomoTherapy uses a
dual laser coordinate system. The machine has a fixed isocenter which defines the true isocenter
of the machine. There is also a virtual isocenter which corresponds to the isocenter for which the
patient is aligned to each day. The placement of the virtual isocenter is defined as the location of
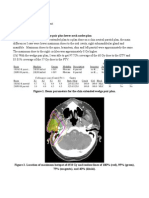
the 3-point isocenter placed at the treatment planning CT (Figures 2-4). Four tangential beams
plus one anterior-posterior (AP) beam with jaw field widths of 5 cm were used. The pitch value
was set to the default of one-tenth that of the field width or 0.500 cm per projection. Medial
tangential beam angles were 313 and 320 and lateral tangential beam angles were 126 and
133 while the AP beam angle was 0 (Figures 5-9). Beam angles were selected to minimize
dose to OR and avoid dose to the contralateral breast. To help ensure proper target coverage
from intra-fractional motion, three leaves of the multi-leaf collimator (MLC) were opened on the
anterior aspects of the tangential beams. By opening the leaves anteriorly, the risk of missing the
target due to breathing motion is reduced and is a common standard of practice when using
TomoDirect for breast cancer treatment planning. TomoTherapy allows a maximum expansion
of five leaves, each having a width of 0.625 cm, which gives the possibility of adding up to an
additional 3.125 cm total.2 TomoTherapy is a mono-energetic treatment modality; therefore the
plan was computed using 6 megavoltage (MV) treatment beams. TomoDirect uses static gantry
angles, similar to those of conventional 3-dimensional (3D) conformal radiation therapy, along
with couch translations and MLC modulation to deliver lower doses to the OR.
Treatment Planning: The radiation oncologist instructed dose prescription and objectives,
which were to cover 95% of the breast PTV with 95% of the dose and a maximum breast PTV
dose of less than 110% was desired. Heart dose constraints included a mean dose of less than 4
Gy, the volume receiving greater than 20 Gy to be kept less than 5% and the volume receiving
greater than 10 Gy to be kept less than 30%. The ipsilateral lung dose constraints included the
volumes receiving greater than 20 Gy, 10 Gy and 5 Gy to be kept less than 15%, 35% and 50%
respectively. Contralateral lung volume receiving 5 Gy was desired to be less than 10%. Before
optimization of the plan occurred, target and OR constraints were defined according to their
importance. Maximum dose and dose volume histogram (DVH) doses and volumes were
specified and maximum dose and DVH penalties were assigned (Figure 10). Greater dose
penalties were assigned to OR which were more critical and for which the optimizer needed to
work harder to meet the given constraint. Once all the constraint objectives were in place,
iterations were run to optimize the plan. As the plan optimized, target and OR constraints were
adjusted as needed until an optimal plan was computed. Once adequate coverage was
established, the medical dosimetrist reviewed the DVH (Figure 11), isodose lines (Figure 12) and
OR with the radiation oncologist. The DVH reflects that 95% of the breast PTV was covered by
100% of the prescription dose. The heart received a mean dose of 1.31 Gy, the volume receiving
20 Gy was 1% and the volume receiving 10 Gy was 3%. The ipsilateral lung volume receiving
20 Gy, 10 Gy and 5 Gy were 6%, 9% and 16% respectively. The contralateral lung received a
maximum dose of 0.61 Gy. After reviewing the plan, the radiation oncologist gave approval for
treatment.
Quality Assurance/Physics Check: The plan was verified using a Delta4 IMRT quality
assurance (QA) phantom. The departmental delivery quality assurance (DQA) method used to
verify TomoTherapy treatment plans was developed based on the Report of the IMRT
Subcommittee of the AAPM Radiation Therapy Committee.3 The approved plan was used to
create a DQA procedure on the TomoTherapy Hi-Art version 5.0 planning system. After the
DQA procedure was created, the DQA RT plan and RT dose were exported to the Delta4
software in preparation for delivery and analysis. The DQA plan was delivered and analyzed to
determine if the DQA passed. A passing TomoTherapy treatment plan must show a consistency
to within 5% of the target dose. The initial whole breast treatment plan was within the 5% dose
difference allowed. The DQA report was generated and imported into the patients electronic
medical record as documentation to this procedure. In addition to the Delta4 IMRT QA, an
initial physics chart check was performed on the treatment plan to verify parameters including
that the correct treatment site was planned for and that the physicians prescription matches the
treatment plan.
Conclusion: Due to the patients anatomy, a TomoDirect treatment plan was created to help
decrease the dose to the heart, lungs and soft tissue region adjacent to the breast. In this case, the
use of TomoDirect was advantageous due to the region of increase skin tissue adjacent to the
breast. To cover the entire breast tissue in the treatment field, a large portion of soft tissue and
ipsilateral lung volume was in the path of the beam. Creating a plan that provided adequate
coverage to the breast PTV was possible through the use of TomoDirect which uses static gantry
angles combined with couch translations and MLC modulation to assist in lowing doses to the
OR. Another advantage is the ability to obtain a daily MVCT scan to ensure proper alignment of
the breast tissue in relation to the lungs and heart. Disadvantages include increased dose to the
patient from daily imaging and increased treatment delivery time due to daily imaging and
overall treatment beam on time. TomoDirect can be a useful tool in delivering radiation
treatments for breast cancer, specifically when patient anatomy presents challenges in
conventional 3D treatment planning.
References
1 NRG Oncology. National Surgical Adjuvant Breast and Bowel Project.
http://www.nsabp.pitt.edu/B-06.asp. Accessed April 8, 2014.
2 TomoTherapy Planning Guide. Madison, WI. 2013.
3 Ezzell G, Galvin J, Low D et al. Guidance document on delivery, treatment planning, and
clinical implementation of IMRT: Report of the IMRT subcommittee of the AAPM radiation
therapy committee. Med Phys. 2003;30(8):2089-115.
http://dx.doi.org.ezproxy.library.wisc.edu/10.1118/1.1591194
Figures
Figure 1. Patient position from CT simulation.
Figure 2. Beams eye view (BEV) of the isocenter from the anterior position using the Philips
Pinnacle3 TPS.
Figure 3. BEV of the isocenter from the left lateral position using the Philips Pinnacle3 TPS.
Figure 4. TomoTherapy fixed isocenter (green) and virtual isocenter (red) using the
TomoTherapy Hi-Art version 5.0 planning system.
10
Figure 5. Medial tangent beam with a gantry angle of 313.
Figure 6. Lateral tangent beam with a gantry angle 320.
11
Figure 7. Medial tangent beam with a gantry angle of 126.
Figure 8. Lateral tangent beam with a gantry angle of 133.
12
Figure 9. AP beam with a gantry angle of 0.
Figure 10. Target and OR constraint objectives used when optimizing the treatment plan in the
TomoTherapy Hi-Art version 5.0 planning system.
13
Figure 11. DVH used to evaluate the initial whole breast treatment plan.
Figure 12. Transverse, coronal and sagittal views of the isodose distribution for the initial whole
breast treatment plan.
Anda mungkin juga menyukai
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Tomotherapy PresentationDokumen26 halamanTomotherapy Presentationapi-24740282767% (3)
- Typhon Group Easi - Evaluation Survey InstrumentDokumen3 halamanTyphon Group Easi - Evaluation Survey Instrumentapi-264047496Belum ada peringkat
- Service ProjectDokumen3 halamanService Projectapi-264047496Belum ada peringkat
- Typhon Group Ahst SystemDokumen2 halamanTyphon Group Ahst Systemapi-264047496Belum ada peringkat
- Budget ActivityDokumen2 halamanBudget Activityapi-247490129Belum ada peringkat
- Final DraftDokumen30 halamanFinal Draftapi-264047496Belum ada peringkat
- Final 2015 Competency List SummerDokumen1 halamanFinal 2015 Competency List Summerapi-264047496Belum ada peringkat
- Sample BudgetDokumen6 halamanSample Budgetapi-264047496Belum ada peringkat
- Typhon Group Easi - Evaluation Survey InstrumentDokumen3 halamanTyphon Group Easi - Evaluation Survey Instrumentapi-264047496Belum ada peringkat
- Final Qa ChartDokumen10 halamanFinal Qa Chartapi-264047496Belum ada peringkat
- Hippocampal Sparing VMAT Using Pinnacle Treatment Planning SystemDokumen12 halamanHippocampal Sparing VMAT Using Pinnacle Treatment Planning Systemapi-264047496Belum ada peringkat
- Typhon Group Easi - Evaluation Survey InstrumentDokumen3 halamanTyphon Group Easi - Evaluation Survey Instrumentapi-264047496Belum ada peringkat
- Typhon Group Easi - Evaluation Survey InstrumentDokumen3 halamanTyphon Group Easi - Evaluation Survey Instrumentapi-264047496Belum ada peringkat
- Quantec ReviewDokumen64 halamanQuantec Reviewapi-279520503Belum ada peringkat
- SBRT PDFDokumen14 halamanSBRT PDFrubenBelum ada peringkat
- 2nd DraftDokumen28 halaman2nd Draftapi-264047496Belum ada peringkat
- Citation AssignmentDokumen1 halamanCitation Assignmentapi-264047496Belum ada peringkat
- Typhon Group Easi - Evaluation & Survey InstrumentDokumen3 halamanTyphon Group Easi - Evaluation & Survey Instrumentapi-264047496Belum ada peringkat
- Hall 2009article1Dokumen15 halamanHall 2009article1api-264047496Belum ada peringkat
- TallyDokumen2 halamanTallyapi-264047496Belum ada peringkat
- Mentoring ActivityDokumen7 halamanMentoring Activityapi-264047496Belum ada peringkat
- Final 2015 Competency List SummerDokumen1 halamanFinal 2015 Competency List Summerapi-264047496Belum ada peringkat
- Clinical Practicum II Clinical Lab AssignmentDokumen3 halamanClinical Practicum II Clinical Lab Assignmentapi-264047496Belum ada peringkat
- Parotid LabDokumen10 halamanParotid Labapi-264047496Belum ada peringkat
- Typhon Group Easi - Evaluation & Survey InstrumentDokumen3 halamanTyphon Group Easi - Evaluation & Survey Instrumentapi-264047496Belum ada peringkat
- SpringcompsDokumen3 halamanSpringcompsapi-264047496Belum ada peringkat
- Typhon Group Easi - Evaluation & Survey InstrumentDokumen3 halamanTyphon Group Easi - Evaluation & Survey Instrumentapi-264047496Belum ada peringkat
- CaselogsDokumen2 halamanCaselogsapi-264047496Belum ada peringkat
- Csi AssignmentDokumen8 halamanCsi Assignmentapi-264047496Belum ada peringkat
- EvalmayDokumen3 halamanEvalmayapi-264047496Belum ada peringkat
- EvalaprDokumen3 halamanEvalaprapi-264047496Belum ada peringkat
- IMRT by Musaib MushtaqDokumen44 halamanIMRT by Musaib MushtaqDr. Musaib MushtaqBelum ada peringkat
- Jason Laher Medical Dosimetry Resume 11 11 2018Dokumen3 halamanJason Laher Medical Dosimetry Resume 11 11 2018api-375154016Belum ada peringkat
- History of Radiotherapy & Infrastructure in IndiaDokumen106 halamanHistory of Radiotherapy & Infrastructure in IndiaRakesh JadhavBelum ada peringkat
- Class Solutions As Related To IMRT TreatmentsDokumen4 halamanClass Solutions As Related To IMRT Treatmentsapi-336525339Belum ada peringkat
- Icru Report 83Dokumen103 halamanIcru Report 83Marlon SantosBelum ada peringkat
- Shielding Requirements in Helical Tomotherapy: Home Search Collections Journals About Contact Us My IopscienceDokumen12 halamanShielding Requirements in Helical Tomotherapy: Home Search Collections Journals About Contact Us My IopscienceSayan DasBelum ada peringkat
- 48-50 ICARO Book - of - Synopses PDFDokumen274 halaman48-50 ICARO Book - of - Synopses PDFEgor TitovichBelum ada peringkat
- TG 119 IMRT Commissioning and QA PDFDokumen15 halamanTG 119 IMRT Commissioning and QA PDFmarkBelum ada peringkat
- QT - Importing 3rd Party Treatment Plans Into Eclipse To Add To A RapidPlan DVH Estimation Model - SecuredDokumen4 halamanQT - Importing 3rd Party Treatment Plans Into Eclipse To Add To A RapidPlan DVH Estimation Model - SecuredMIchaelBelum ada peringkat
- Radiotherapy in Cancer Care Facing The Global ChallengeDokumen578 halamanRadiotherapy in Cancer Care Facing The Global Challengeerine5995Belum ada peringkat
- Radiotherapy: Basic Concepts and Recent AdvancesDokumen17 halamanRadiotherapy: Basic Concepts and Recent AdvancesutokaBelum ada peringkat
- Imrt VmatDokumen29 halamanImrt VmatandreaBelum ada peringkat
- Van Asten ResumeDokumen2 halamanVan Asten Resumeapi-499382707Belum ada peringkat
- KV-CBCT MVCTDokumen6 halamanKV-CBCT MVCTapi-280277788Belum ada peringkat
- 1002 04 Mobius3D BrochureDokumen8 halaman1002 04 Mobius3D BrochureObamaBelum ada peringkat
- TG-218 AapmDokumen32 halamanTG-218 AapmGabrielaBelum ada peringkat
- Characteristics and Limitations of A Secondary Dose Check Software For VMAT Plan CalculationDokumen8 halamanCharacteristics and Limitations of A Secondary Dose Check Software For VMAT Plan Calculationkaren CarrilloBelum ada peringkat
- Apollo Proton Cancer Centre (Medical Oncology) For Kenyan PatientsDokumen10 halamanApollo Proton Cancer Centre (Medical Oncology) For Kenyan PatientsApollo Information CentreBelum ada peringkat
- Radiation Characteristics of Helical Tomotherapy: Robert JerajDokumen9 halamanRadiation Characteristics of Helical Tomotherapy: Robert JerajenriquefisicoBelum ada peringkat
- CN Winter 2007Dokumen21 halamanCN Winter 2007kirkwriteBelum ada peringkat
- LINAC HistoryDokumen19 halamanLINAC Historycrusader8880% (1)
- Adaptive RadiotherapyDokumen95 halamanAdaptive RadiotherapyGina RBelum ada peringkat
- Contemporary IMRT PDFDokumen477 halamanContemporary IMRT PDFmabidsaleemBelum ada peringkat
- List of Philhealth Accredited Level 3 Hospital As of October 31, 2019Dokumen35 halamanList of Philhealth Accredited Level 3 Hospital As of October 31, 2019Lex CatBelum ada peringkat
- Intensity Modulated Radiotherapy (Imrt) AND 3 D Conformal Radiotherapy (3D CRT)Dokumen102 halamanIntensity Modulated Radiotherapy (Imrt) AND 3 D Conformal Radiotherapy (3D CRT)kyuleen05Belum ada peringkat
- OSL Source BookDokumen36 halamanOSL Source BookNawel MorjanBelum ada peringkat
- The PACE Trial: Radiotherapy Planning and Delivery Guidelines (Pace-A and Pace-C)Dokumen33 halamanThe PACE Trial: Radiotherapy Planning and Delivery Guidelines (Pace-A and Pace-C)Анастасия АнохинаBelum ada peringkat
- Radiation Oncology Management Decisions 2002 (Perez)Dokumen773 halamanRadiation Oncology Management Decisions 2002 (Perez)abdullmajeed50% (2)
- RadiotherapyDokumen12 halamanRadiotherapyapi-3805764100% (1)