Flow Table
Diunggah oleh
คุณชายธวัชชัย เจริญสุขHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Flow Table
Diunggah oleh
คุณชายธวัชชัย เจริญสุขHak Cipta:
Format Tersedia
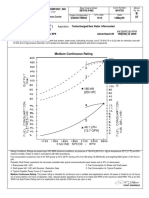
FL-1 00 SERIES, FL-1 10, FL-1 20
Now Available
COMPUTERIZED
FLOW TABLES
FOR OMEGA@ ROTAMETERS
A new R L D project at Omega Instruments has
produced a new computerized procedure for
generating complete Row tables for any suitable
fluid of known viscosity and density.
Tables of flow are available for calibrated and
correlated flowmeters at every scale division from 1 to 100.
Similar tables are available for compact
flowmeters at every direct flow reading of
scale.
condiVolume flow rates are given at the
tions of flow and corrected to volumes
measured at standard conditions.
Each chart for a correlated and calibrated
flowmeter is specific to the actual serialized
meter and float material.
The flow rate can be given in any units
desired including mass flow.
New correlation method uses complex analytical equations programmed to achieve
maximum accuracy.
Resultant accuracy is at least twice as good
as the best previous correlation developed,
namely:
Average error = -t 2% or f 0.5 S.D. whichever is the greater (compared to -+ 4% for
previous best method)
Maximum Error = -t 6% or 2 1.5 SD. whichever is the greater (compared to -+ 12%
for previous best method)
NEW CORRELATION *
he new computerized method is based on
he use of the flow equation given in terms
If q as a function of CR and K, A new more
!xtensive correction is used for
CR and
cf as follows:
1. From the latest available flow data and
flowmeter characteristics ~(10 + log C,) IS
plotted as a function of ~(10 + log K,) with
Parameter Ft. where,
KR IS independent of R and a function of the
physlcal properties of the fluid and the flowmeter only. It is proportional to the square root
of the Archimedes number (a dimensionless
quantity used in fluid dynamics analysis).
division for each particular flowmeter.
2.Two new functions related toy and u are defined as,
a. W = log w. where w = log z log z0
and log z = - log (y, y)
y,=yasu a=
z,=zatSt=5
b.V=logv,wherev=u-u,
and u,=uatSt=5
5. The relationship between C, and St IS simplified in the Stokes region as before to give:
3. The analytical relatlonship between W and
V is given by,
W = n, + n,V + n,V* i n,V3
where St<5
6. The correction factor, K, is defined by:
This polynomial expression is evaluated using the
best values of the four coefficients, n,. n,. n2 and
n3 determined by a graphical least squares analysis of cross plots and derivattve plots These
coefficients In turn are determined by a similar
analysis to yield polynominals In R as follows:
n,=a,+q,R+q2R2
n, =a,,+a,,R+a,,RZ
n2 = aZO + a,,R + a&+
n3 = a, + a,,R + aZ2RZ
Relerence: Gitmont 8 Wechster.
Meas. 8 Control V21, No. 6, P.148 (Dec. 1987).
GB-104350
ingi;98D,
where, R, is a simple function of v; namely,
The coefficients of the linear function above are
again evaluated by a graphical least squares
analysis of cross plots of data to yield:
m,=m,+m,,RJ
and m, = m,, + mrp R2
C,
Substitut-
4-R[A + 21
K ,
yields the volumetnc rate at condltlons of
flow, whfch in turn is converted to the standard rate
by the program. If one attempted these computations with a scientific calculator, it would require
an inordinate amount of time to obtain the complete table produced by the computer program as
illustrated above.
IiOW TO,ORDER
When ordenng your specific computerized table
please supply the following information:
a. At operating conditions of specified
pressure and temperature
b. At standard conditions of 1 atm. and 70F
3. Float material
4. Flowmeter size and serial number where
5. Units of flow desired
Ml 072/0390
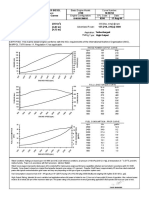
DIRECTIONS
OMEGA@ ROTARilETERS
The procedure for calculating the flow of any fluid of known density and
viscosity are based on the new standard correlation curve supplied
with the new serialized flowmeter. NOTE: Owners of old serial num
bers may write for a new chart at no extra charge, by supplying us with
the size and serial number of the flowmeter.
iIGH-ACCURACY, UNSHIELDED
Computer tables are now included at no extra charge
- four tables in ail, air and water at standard conditions using glass and stainless steel floats. For other
fluids and any conditions of flow.
?? The standard calibration chart for air and water using
the glass float now contains the values of R for use
with the new generalized correlation.
?? Calibration curves for any fluid and float can be
obtained at the operating conditions of flow using
the new generalized correlation. Only the density and
viscosity of the fluid need be known.
?? Each serialized meter is statistically calibrated with
air at three points (an improvement over the previous
two point calibration) to assure an accuracy of
f 2%
or f 1 Scale division (whichever is the greater).
. Readings are reproducible to f 1% or * 0.5 scale
divisions (whichever is the greater).
?? Other features have been maintained as follows:
1. Specially designed Teflon stops accept cones.
ponding tapered joints and O-Rings to make a
vacuum tight seal.
2. Permanent black ceramic scale and white
background for easy reading.
3. Corrosion resistant-fluid comes in contact with
glass and tefion only (when using glass float and
plain end meter).
4. Glass and stainless steel floats are supplied with
each meter. A conversion chart indicates pressure
drops for each float and converts flow rates with the
glass float to those with stainless steel.
These flowmeters are manufactured from tapered
(-t .OOOlprecision bore tubing to ultimate tolerances
.0002) which give maximum precision attainable
for spherical float rotameters.
The tolerances on
the floats are of comparable magnitude.
The latest generalized correlation is based on a new
dimensionless quantityKi(related to the Archimedes
number). Prediction of fluid flows with an accuracy at
least twice as good as any previous method is now
possible (see page 1). See DIRECTIONS of sample
calculations using graphical and analytical methods.
* U.S. Patent No. 3,183,713
** Reference: Gilmont 8 Maurer. Instr. & Control Sys. V.34.
p.2070 (1941).
??
Reference:
p.148 (Dec.
Gllmont
1987).
8 Wechsler.
Meas.
&Control
V.21, N
GRAPHICAL SOLUTION
Select the desired value of Rat the corresponding value of
scale division from the calibration curve supplied.
2. Calculate the value of KR:
1.
K,= ,.;, [
SIZE NO
Cat. No.
F-2004
F-2005
F-2031
F-1133
F-1134
1 Ring 101 JOl",
S-1105
S~l105-E
I-Ring EPR
-LOAT
Pf FC Fw
;1ass
253
F-2032
;?I
8 02 18 i 1 F-2032 S
rantttt
166 26 32
149 24
3OF-2032.TC
lCttt
pf = density,
ttt suppled
Cat. No.
F-1104
F-1105
F-1131
F-1133
F-1134
S-1105
S-ll05~E
F~ll32
F-1132-S
F-1132-T
Cat. No.
F~l204
C, = .0852K,R5
4.
Determine the value of the correction factor. K,. For gases ant
values of R < 10, the value of K, may be taken as unity. If not
letermine K, as follows:
a. Calculate the value of v:
b. From the Correction Factor Chart on the page following thr
Correlation Chart, obtain the value of
100/k?, correspondin<
to the values of R and Y.
K,:
c. Calculate the value of
PI
R,
Calculate the volumetric rate of flow,
q:
q= CRK4R f. + 2
[
Kq = 59
K,
1PPt
6 D, !%- )
s
[
snd D, = Diam. float in inches from data.
3. The above.value of q is at the conditions of flow which may be
.educed to a volume measured at standard conditions, q :q
= qpfp
Nhere. p0 = density at standard conditions
SAMPLE CALCULATIONS
FL-105
Shown with
resDective ioint
6,
Cat. No.
Flowmeter, Size
#l. FllOO, Glass Float,
W, = .00530 g, p, =
2.53 glee. 0, = .0625 in. Water at 40
C. p = ,653 cp., p = .99i
g/cc. p. = .99d g/cc. Values of R at 5, 10 & 25
F-7032
F-7032-E
F-1232
F-1232~s
F-1232-T
F~l332
2119-S
1332-T
F~l432
F 1432-S
F-1532
F-1532-S
F-2504
F-1505
F-1531
F-1533
F-1534
F 1432.TC
F-1532.TC
F-1x35~
F-1535-E
R
5
10
25
I!
CR
.0842
,233
,526
100/R,
1
.097
,693
.0469
.0625
.125
,250
,375
,500
.P64
,962
5.48
-OFI ANALYTICAL SOLUTION see page following the
r:ON CHART.
.%33
,956
5.45
CORRELA.
exer.
sised not to lose the ball, especially for the smaller sizes It is
POSSi.
aru
318 to replace the ball with negligible error because the diameter
density of the ball are held to very close tolerances. Normal method:
3f cleaning are recommended using mild detergents and drying witt
acetone.
JOINT SETS: It is recommended that short lengths of flexible
tubin<
[such as tygon or teflon) be used on each end to hold the joints se
curely to the flowmeter especially with the smaller sizes
(#O and #1)
To protect the tiny teflon stops of these smaller sizes a suitable nee
dle may be inserted in the orifice upon assembly and disassembly
SET OF
FLOAT
DIAM.
4.44
2.30
K,
1
.923
,870
.06836. Kq = .2130
CLEANING: When cleaning the meter, great care should be
FA and Fw are magmfylng
factors far
ar & wakr
wlfh conversion char, for flow and pressure drop
FLOWUETERS, PLAIN ENDS
RANGES MLlMlN tt
AIR
CAT. NO.
WATER
0.2-100
,002-l .l
FL-100
l-280
.Ol-4.0
FL-101
10-1900
0.2-36
FL-102
200s14,000
3-300
FL-103
l OOO-36,000
1 O-850
FL-104
3000-77,000
30-l 900
FL-105
1 =
y = 1.021 .00530(2.53 - .992).992 O5
R
,653 L
2.53
5
Cat. No.
Cat. No.
at low flows, may cause erratic readings due
to electrostatic charge build-up.
[I
K , = ,-- !T2
P
whers,
NOTE: Extremely dry gases,
SIZE
IOQR
F-2404
F-1405
F 1431
F-1433
F 1434
F 1435-v
F-1435E
F-1231
F-l 233
F-i 234
v = IogK, - ,350 t
F-1304
F-1305
F-1331
F-1333
F-1334
S-1205
S 1205-E
F-1205
where, p = viscoscity of fluid in cp
W, = weight of float in g/cc
p, = density of float in g/cc
p = density of fluid in glee
note, W, and P, are given in the calibration curve data.
3. From the correlation chart on the next page obtain the value o
28 corresponding to R and Kn. When C, < .19. use the equation
SPARE IJAR1 rs LIST
Description
.etlan Stop, Top
retIon Stop. Bottom
:lowmeter Tube
klmt, inner
loIni. Outer
W ,(p:j-pipl
the
TUBE
O.D. n
5116
5116
5116
7116
11116
15/16
TUBE
LENGTH w
7 l/2
7 /2
7 /2
7%
JOlNTSt
CAT. NO.
IO%0
10130
12130
14135
19138
24140
FL-J1
FL-J1
FL-J2
FL-J3
FL-J4
FL-J5
Each meter is supplied with complete directions and correlation charts for calculating the calibration curve for any fluid whose density and viscosity are
known. Owners of serialized flowmeter made prior to the new computerized correlation, may order any one of the four computer tables,
specify desired tables.
Just supply us with flowmeter size and serial number and
tconsists of one inner and outer joint plus two O-rings.
ttFlow ranges for glass float stated above may be extended by factors of approx 2 to 3 by using heavier floats. See Spare Parts List.
rt
C ORRELA T/ON CHART
10
Density a Viscosity of
Gases at 70F a 1 atm
p in g/Li
GAS
KR
c1 in cp
1.087
1.200
.7155
1.655
2.510
2.481
1.035
1.160
2.989
1.259
1.170
.1657
.0834
1.522
1.429
3.382
.6653
2.139
.035a
1.244
1.161
1.836
1.326
1.075
2.717
5.307
cetylene
.ir
.mmonia
.rgon
utane (n)
utane (iso)
:arbon dioxide
:arbon monoxide
:hlorine
thane
thylene
leiium
lydrogen
lydrogen chloride
lydrogen sulfide
.rypton
lethane
lethyl chloride
leon
litric oxide
litrogen
litrous oxide
)xygen
ropane
iulfur dioxide
:enon
.OlOl
.0181
.00986
.0221
.0076
.0076
.0148
.0174
.0133
.00913
.OlOl
.0194
.0087
.0144
.0126
.02 514
.0109
.0107
.0312
.0188
.0175
.0144
.0203
.00803
.0125
.0226
TABLE II
Density a Viscosity of
Liquids at 70F 8 1 atm
LIOUID
p In g/ml
Acetic Acid
Acetone
Anilme
Benzene
Butyl Acetate
n-butanol
CCI 4
Chlorobenzene
Chloroform
Diethyl Ether
Ethyl Acetate
Ethanol
Ethylene Br
Ethylene Cl
Ethylene Glycol
Fluorobenzene
Heptane
Methyl Acetate
Methanol
Nitrobenzene
n-octane
Propanol
Toluene
o-xylene
m-xylene
p-xylene
Aqueous Solutions
10% HCI
30% HCI
10% HNO,
40% HNO3
10% HzSO~
60% HsSOa
0.2
0.3
0.4
0.5
0.7
1.049
,790
1.022
,879
,083
,610
1.594
1.106
1.483
,714
,900
,709
1.460
898
1.109
1.023
,684
,933
791
1.204
,703
,804
867
,880
.864
861
p in cp
1.221
.32
4.40
,652
,732
2 946
,969
,799
.58
,233
,455
1 20
1.72
.79
19.90
.598
409
381
,597
2 03
,542
2 256
,590
,810
,620
,648
(% by weight)
1.048
1 149
1.054
1.247
1.066
1.499
1.15<
1.70;
1.04;
1 55E
1 22E
5 90:
1.0 1.
CR-
FOR CR < 0.19, CR = .0852 KRR.~
CORRECT/ON FACTOR CHART
SAMPLE CALCULATIONS FORANALyrCAL SOLUTION
ANALYTICAL SOLUTION OF FLUID FLOW
1. Same as step 1 of GRAPHICAL SOLUTION on previous page
2. Same as step 2 of GRAPHICAL SOLUTION
3. Calculate the following:
a) St = KR2R3
b) St,, = 5 + .777R
4. Determine the region of flow as follows:
a) If St < 5 region is Stokes, skip to step 9
b) If .Sb 5 St F 5 region is transitional, skip to step 6
c) If St > St0 region is turbulent, proceed to step 5
5. For the turbulent region, calculate the following:
a) V = log[log Kn - ,350 + 1.5 IogR] (KRfrom step 2)
b) W ,=no+n,V+n2V2+~V3
where, no = -.197 t .00418R- .000155R2
n, = 1.065
- .0189R + 001375R*
n2 = ,929 - .1510R+ .004025R2
n3 = ,542 - .06185R. skip to step 7
6. For the transitional region calculate the following:
a) V, = log[O.5 log S10 - .350] (St,, from step
3b)
b) W, = n,, + n,V, + n2V02 + n3V03 (coeff. the same as in 5b)
c) W, = W0 - V, + V (Vas calculated in Step
5a)
7. Calculate the followino:
a) WC = log-
W,
b) zc = log- (w,+ 0.1193)
C) yc = 10.040 -(l/z,)
d) CR= log-
(y,- 10)
Determine the value of K,as follows: (K, = 1 for gases)
a) v = log- V ( V from step 5a)
8.
1.
R:
af R
7.61
12.77
transitional
5. a) v:
b) W,:
0
:
n, :
2 :
3 :
6. a) V, :
b)
;
c) w,:
7.
a) w,:
b) zc:
c) Yc:
d) C,:
8. a)
Y:
b) R,:
100
c)
where, m, = 4.81 - .138
25
Kn = .08836 for all values
St = .00781 R3
,975
a) sr:
b) St,:
6.805
4.
Region:
Stokes
2.
3.
1
--.1707
1.0315
-.1785
- 0765
-.1592
-.4249
-.1894
1.4519
-.3304
-1.004 3
-.6923
-.9325
-1.061 3
-1.256 5
.0554
1.495
9.371
,235
1
.3760
3.128
9.72
,525
.09632
22.54
IQ:
m,
K,:
::
122.0
24.4
turbulent
23
931
E4
138
71
R3
1000
m,= -2.62+
,369
[I
R2
100
c) K, = 1 - R * Skip to step
R,
PI
9. For the Stokes region calculate:
a) c, = .0852(St)05
10
(m, from step
10.
q = C,K,R
R1
9. a) c,:
b) ~; i
.0842
4.79
,977
8b)
flow at the conditions of flow:
10.
K, = .2130 for all values of
,180
4:
+2 K,
[ 100
11. The above flow (q) mky be
conditions (q) as follows:
red&d to a volume measured at standard
q = q p where, p. = density at std.
PO
11.
q :
* NOTE:
cond
.179
,964
5.45
For size x0 and S.S. Float multiply by an addatonal facior K,
K,: = [l-(k)]
(1 -9
Where: R,I 700 for gases
R;-3.55-51.5R+2.37R2 forfiquiis
Anda mungkin juga menyukai
- YOKOGAWA - Low Flow Transmitters With Integral Flow Orifice - TI01C20K00-01EDokumen10 halamanYOKOGAWA - Low Flow Transmitters With Integral Flow Orifice - TI01C20K00-01EChoochart ThongnarkBelum ada peringkat
- Vortex Meters For GasDokumen5 halamanVortex Meters For GasRicardo Zárate GodinezBelum ada peringkat
- Flow in Circular Pipes: ObjectiveDokumen35 halamanFlow in Circular Pipes: ObjectivemamunruetBelum ada peringkat
- Balancing & Flow Control Valves PDFDokumen47 halamanBalancing & Flow Control Valves PDFNoushad P Hamsa100% (1)
- Diff PR Flo MeterDokumen19 halamanDiff PR Flo MeterShoeb ShaikhBelum ada peringkat
- Steady-State Analyses of Fluid Flow Characteristics For AFWS in PWR Using Simplified CFD MethodsDokumen4 halamanSteady-State Analyses of Fluid Flow Characteristics For AFWS in PWR Using Simplified CFD MethodsViswanathan DamodaranBelum ada peringkat
- TB8102 Rupture Disc SizingDokumen9 halamanTB8102 Rupture Disc Sizingbabis1980Belum ada peringkat
- Lockhart&Martinelli ImprovedDokumen12 halamanLockhart&Martinelli Improvedrui_filho_12Belum ada peringkat
- HydraulicsDokumen65 halamanHydraulicsMohsin EhsanBelum ada peringkat
- Compressible FlowDokumen35 halamanCompressible FlowAngelica KartikaBelum ada peringkat
- Pipe Systems DesignDokumen31 halamanPipe Systems DesignAvinash VasudeoBelum ada peringkat
- 03 VacSysCalculationsDokumen24 halaman03 VacSysCalculationssajjad_naghdi241Belum ada peringkat
- Fluid Engineering - Flow in PipesDokumen6 halamanFluid Engineering - Flow in PipesSherif Abdel Hamid FakhryBelum ada peringkat
- Models - Cfd.pipe Elbow PDFDokumen22 halamanModels - Cfd.pipe Elbow PDFacharya_s89Belum ada peringkat
- Balancing Flow Control Valves PDFDokumen47 halamanBalancing Flow Control Valves PDFRami ReddyBelum ada peringkat
- Z G+P V V U Q+W Z G+ P V V U: Basic Concepts of Fluid TransportDokumen6 halamanZ G+P V V U Q+W Z G+ P V V U: Basic Concepts of Fluid TransportBernard BaluyotBelum ada peringkat
- Arts Fluid FlowDokumen142 halamanArts Fluid Flowchem.tahirBelum ada peringkat
- Art's Fluid Flow 1Dokumen64 halamanArt's Fluid Flow 1Jagadeesh UnnamBelum ada peringkat
- Head Loss in Pipe - Lab Report MannualDokumen4 halamanHead Loss in Pipe - Lab Report MannualRampukar SahBelum ada peringkat
- VXD Old SMC 0900766b8094a5b0Dokumen32 halamanVXD Old SMC 0900766b8094a5b0FranzKafkaBelum ada peringkat
- Pressure Loss in PipeDokumen8 halamanPressure Loss in PipesaeidianBelum ada peringkat
- Cav03 OS 4 010Dokumen7 halamanCav03 OS 4 010Emad ElsaidBelum ada peringkat
- Pipe Contraction Pressure LossDokumen8 halamanPipe Contraction Pressure LossblumunchieBelum ada peringkat
- Heat ExchangerDokumen27 halamanHeat ExchangerKashish Mehta75% (4)
- Calculation of Flow Rate From Differential Pressure Devices - Orifice PlatesDokumen26 halamanCalculation of Flow Rate From Differential Pressure Devices - Orifice Platesamirreza_eng3411Belum ada peringkat
- Vortex Flowmeter ManualDokumen31 halamanVortex Flowmeter ManualRoLopBelum ada peringkat
- PCV SizingDokumen8 halamanPCV SizingKhanh PhamBelum ada peringkat
- Quick Choice Of: Temperature ControlsDokumen5 halamanQuick Choice Of: Temperature ControlsFlorian DraguBelum ada peringkat
- Pipe Flow Calculations PDFDokumen12 halamanPipe Flow Calculations PDFharrypop418Belum ada peringkat
- Models - Heat.turbulent Heat ExchangerDokumen18 halamanModels - Heat.turbulent Heat ExchangerMaximiliano FaríaBelum ada peringkat
- Ta-Fus1On-C: Combined Control and Balancing Valves With Independent EQM CharacteristicsDokumen16 halamanTa-Fus1On-C: Combined Control and Balancing Valves With Independent EQM CharacteristicsCatalin DinuBelum ada peringkat
- Losses in PipeDokumen9 halamanLosses in PipeNiroexBelum ada peringkat
- OCW Drilling Hydraulics LectureDokumen59 halamanOCW Drilling Hydraulics Lecturefreelancer2earn100% (1)
- Lab 5: Linear Momentum Experiments PurposeDokumen5 halamanLab 5: Linear Momentum Experiments Purposenil_008Belum ada peringkat
- Choose Cast-Iron, Bronze, or Stainless Steel Construction.: Self-Priming PumpsDokumen12 halamanChoose Cast-Iron, Bronze, or Stainless Steel Construction.: Self-Priming PumpscarlofilippinBelum ada peringkat
- Qpedia Apr09 Basic Principles of Wind Tunnel Design9Dokumen3 halamanQpedia Apr09 Basic Principles of Wind Tunnel Design9Ryan FadhliBelum ada peringkat
- Man-Arian Flow Cad SoftwareDokumen27 halamanMan-Arian Flow Cad Softwaresyahmi1337Belum ada peringkat
- 505 - Lec 01 PDFDokumen33 halaman505 - Lec 01 PDFUdara DissanayakeBelum ada peringkat
- Offshore Pipeline Hydraulic and Mechanical AnalysesDokumen25 halamanOffshore Pipeline Hydraulic and Mechanical AnalysesEslam RedaBelum ada peringkat
- Aerothermal Performance Measurements and AnalysisDokumen8 halamanAerothermal Performance Measurements and Analysisraul19rsBelum ada peringkat
- Basic Concepts of Fluid TransportDokumen14 halamanBasic Concepts of Fluid TransportBernard BaluyotBelum ada peringkat
- Laminar & Turbulent Flow in PipesDokumen5 halamanLaminar & Turbulent Flow in PipesDhananjay KadamBelum ada peringkat
- Op Tim IzationDokumen8 halamanOp Tim IzationJulia Turpo SuarezBelum ada peringkat
- Major No. 2 - Design Problems For The Acetone Production FacilityDokumen15 halamanMajor No. 2 - Design Problems For The Acetone Production FacilityJimmy Velarde RodriguezBelum ada peringkat
- Lab 5Dokumen9 halamanLab 5Cem UsmangilBelum ada peringkat
- Lab 4-Friction Losses and Minor LossesDokumen7 halamanLab 4-Friction Losses and Minor LossesJJ Sean CruzBelum ada peringkat
- Flow in Circular Pipes: ObjectiveDokumen25 halamanFlow in Circular Pipes: ObjectivePatrickAndradeBelum ada peringkat
- Vortex FlowmeterDokumen16 halamanVortex FlowmeterBiswajit DebnathBelum ada peringkat
- PNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGDari EverandPNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGBelum ada peringkat
- Applied Process Design for Chemical and Petrochemical Plants: Volume 1Dari EverandApplied Process Design for Chemical and Petrochemical Plants: Volume 1Penilaian: 3.5 dari 5 bintang3.5/5 (3)
- Southern Marine Engineering Desk Reference: Second Edition Volume IDari EverandSouthern Marine Engineering Desk Reference: Second Edition Volume IBelum ada peringkat
- Analysis and Design of Multicell DC/DC Converters Using Vectorized ModelsDari EverandAnalysis and Design of Multicell DC/DC Converters Using Vectorized ModelsBelum ada peringkat
- Cummins Engine Company, Inc M-9720 6BTA5.9-M2Dokumen2 halamanCummins Engine Company, Inc M-9720 6BTA5.9-M2คุณชายธวัชชัย เจริญสุข100% (1)
- 6BT (180,2500, 2891, MCD, Aug 04, M-09762)Dokumen2 halaman6BT (180,2500, 2891, MCD, Aug 04, M-09762)คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Cummins Mercruiser Diesel Marine Performance Curves 8206 17-Aug-04 5.9 Liter (359 In) 102 MM (4.02 In) 120 MM (4.72 In) 6 220B M-90760 D402013MX02Dokumen2 halamanCummins Mercruiser Diesel Marine Performance Curves 8206 17-Aug-04 5.9 Liter (359 In) 102 MM (4.02 In) 120 MM (4.72 In) 6 220B M-90760 D402013MX02คุณชายธวัชชัย เจริญสุข100% (1)
- HIno Torque SpecDokumen1 halamanHIno Torque Specคุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Cummins Engine Company, Inc M-90196 4BT3.9-MDokumen2 halamanCummins Engine Company, Inc M-90196 4BT3.9-Mคุณชายธวัชชัย เจริญสุข100% (1)
- 4BTA (250,3000,2197, HO, Aug 04, M-90231)Dokumen2 halaman4BTA (250,3000,2197, HO, Aug 04, M-90231)คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- F002 A10 118 PartDokumen2 halamanF002 A10 118 Partคุณชายธวัชชัย เจริญสุขBelum ada peringkat
- F002 A10 118 Product UseDokumen1 halamanF002 A10 118 Product Useคุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Cummins Engine Company, Inc M-90806 270BDokumen2 halamanCummins Engine Company, Inc M-90806 270Bคุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Test ValueDokumen2 halamanTest Valueคุณชายธวัชชัย เจริญสุข100% (2)
- 105833Dokumen2 halaman105833คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Denso 190000-2341Dokumen2 halamanDenso 190000-2341คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Install Notes 1. Install The Game 2. No Cracks Needed.. 3. Have Fun Playing.Dokumen1 halamanInstall Notes 1. Install The Game 2. No Cracks Needed.. 3. Have Fun Playing.คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- 3360Dokumen2 halaman3360คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Denso 196000-6020Dokumen4 halamanDenso 196000-6020คุณชายธวัชชัย เจริญสุข100% (1)
- 0 400 866 208 TestPlanDokumen2 halaman0 400 866 208 TestPlanคุณชายธวัชชัย เจริญสุข100% (2)
- SV 4.20: TV Issue-17: FP A262A902: FV 1.32: RV 1.10: LV 3.00Dokumen1 halamanSV 4.20: TV Issue-17: FP A262A902: FV 1.32: RV 1.10: LV 3.00คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Denso 090000-7632Dokumen1 halamanDenso 090000-7632คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Denso 190000-8920Dokumen2 halamanDenso 190000-8920คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- f2607-#### f2607 20191203 103218Dokumen2 halamanf2607-#### f2607 20191203 103218คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- f2607-#### f2607 20191203 105836Dokumen2 halamanf2607-#### f2607 20191203 105836คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- f2607-#### f2607 20191203 111644Dokumen2 halamanf2607-#### f2607 20191203 111644คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- f2607-#### f2607 20191203 104707Dokumen2 halamanf2607-#### f2607 20191203 104707คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Delphi28236381 20190611 112907Dokumen2 halamanDelphi28236381 20190611 112907คุณชายธวัชชัย เจริญสุขBelum ada peringkat
- Chemical Process IndustriesDokumen2 halamanChemical Process Industriessonu mahipalBelum ada peringkat
- Guia de Proveedores de PinturasDokumen28 halamanGuia de Proveedores de PinturasEDGrog100% (1)
- (Dr. Munawir) Environmental and Nutrition PathologyDokumen82 halaman(Dr. Munawir) Environmental and Nutrition Pathologyadidarma58Belum ada peringkat
- Drawback of Plastic Industry Cipet Final Report PDFDokumen32 halamanDrawback of Plastic Industry Cipet Final Report PDFSavi sharmaBelum ada peringkat
- Presentation of Takenate Mar.2017 3Dokumen20 halamanPresentation of Takenate Mar.2017 3huy.dicBelum ada peringkat
- Chapter 16 HWDokumen11 halamanChapter 16 HWsarah_choi_21Belum ada peringkat
- Mass BalanceDokumen6 halamanMass BalanceHaziq AzliBelum ada peringkat
- Effect of Natural Zeolite As Substrate Filler On The Properties of NBREPDM BlendDokumen8 halamanEffect of Natural Zeolite As Substrate Filler On The Properties of NBREPDM BlendhesBelum ada peringkat
- Screening and Isolation of The Soil Bacteria For Ability To Produce AntibioticsDokumen5 halamanScreening and Isolation of The Soil Bacteria For Ability To Produce AntibioticssdBelum ada peringkat
- Zeta-Potential Measurements On Micro Bubbles GeneratedDokumen9 halamanZeta-Potential Measurements On Micro Bubbles Generatedggg123789Belum ada peringkat
- Lipid Digestion and AbsorptionDokumen37 halamanLipid Digestion and AbsorptionIMDCBiochem100% (1)
- Mic180 - Chapter 5 - Carbohydrate - EditedDokumen75 halamanMic180 - Chapter 5 - Carbohydrate - EditedfarBelum ada peringkat
- Additives For Flour Standardisation - Part I: EnzymesDokumen11 halamanAdditives For Flour Standardisation - Part I: EnzymesMilling and Grain magazineBelum ada peringkat
- Non-Chemical Methods For Controlling Diseases in The Home Landscape and GardenDokumen2 halamanNon-Chemical Methods For Controlling Diseases in The Home Landscape and GardenSharad BhutoriaBelum ada peringkat
- Adams RogerDokumen47 halamanAdams Rogerp23cc005Belum ada peringkat
- Critical ConstantsDokumen4 halamanCritical ConstantsDavid0% (1)
- METAL WORKS General CatalogueDokumen695 halamanMETAL WORKS General CatalogueSheila BowenBelum ada peringkat
- Acute Respiratory Distress Syndrome (Ards)Dokumen22 halamanAcute Respiratory Distress Syndrome (Ards)Hani ShafiraBelum ada peringkat
- Alkanes: IB Chemistry Topic 10.2Dokumen20 halamanAlkanes: IB Chemistry Topic 10.2Ravi RanjanBelum ada peringkat
- Optimizing Glycol Dehydration System For Maximum Efficiency A Case Study of A Gas Plant in NigeriaDokumen16 halamanOptimizing Glycol Dehydration System For Maximum Efficiency A Case Study of A Gas Plant in Nigeria1412091090Belum ada peringkat
- MCQ Based Questions Grade 10 - Acids, Bases and SaltsDokumen6 halamanMCQ Based Questions Grade 10 - Acids, Bases and SaltsMahi RajneBelum ada peringkat
- U08 CW03 Acid and Base Properties of Salts Worksheet v2Dokumen2 halamanU08 CW03 Acid and Base Properties of Salts Worksheet v2Muyao ChenBelum ada peringkat
- 7873Dokumen8 halaman7873Faisal AbbasBelum ada peringkat
- PORTFOLIO 6 MEJOS ElaineFaithS. BSMLS1JDokumen4 halamanPORTFOLIO 6 MEJOS ElaineFaithS. BSMLS1JELAINE FAITH MEJOSBelum ada peringkat
- Idoc - Pub - Extraction of Caffeine From Tea Lab ReportDokumen11 halamanIdoc - Pub - Extraction of Caffeine From Tea Lab ReportĐoàn NgọcBelum ada peringkat
- BFR 36-English Version 01-01-12Dokumen16 halamanBFR 36-English Version 01-01-12Rafael García BarasoainBelum ada peringkat
- ASTM Biodiesel Specs - Nov08Dokumen1 halamanASTM Biodiesel Specs - Nov08Alejandra RojasBelum ada peringkat
- Preservation of Fruits by WaxingDokumen10 halamanPreservation of Fruits by WaxingKrishna Kotturi100% (6)
- Report Petrochemical Sec BenchmarkingDokumen174 halamanReport Petrochemical Sec BenchmarkingSachin Chavan100% (2)
- Biochem Exam Practice QuestionsDokumen12 halamanBiochem Exam Practice Questionsihack_101Belum ada peringkat