Alcohol
Diunggah oleh
Atul VermaDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Alcohol
Diunggah oleh
Atul VermaHak Cipta:
Format Tersedia
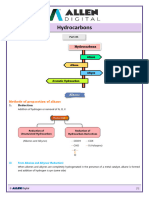
ALCOHOL
Preparation
(1) Hydrolysis of alkyl halides This reaction is useful only with reactants that do not undergo E2 elimination readily. It is really used for the synthesis of alcohols where alkyl halides are primary in nature.
(2) Reaction of Grignard reagents with aldehydes and ketones A method that allows for alcohol preparation with formation of new carbon bonds. Primary, secondary, and tertiary alcohols can all be prepared and stepping up to two carbon atom also can takes place by the help of cyclic epoxides.
(3) Reaction of organolithium reagents with aldehydes and ketones Organolithium reagents react with aldehydes and ketones in a manner similar to that of Grignard reagents to form alcohols. Other reagents which are less reactive than alkyl lithium is dialkyl cadmium, R2Cd where active species is carbanian, and forms similar kind of product.
(4) Reaction of Grignard reagents with esters Produces tertiary alcohols in which two of the substituents on the hydroxyl- bearing carbon are derived from the Grignard reagent. If we want to stop this reaction at carbonyl compound we must use less reactive reagent like dialkyl cadmium R2Cd. Remember the alkyl group in ester goes out finally as alcohol.
Chemical properties of alcohol (i) Acidic nature of alcohol Even alcohol are neutral to litmus and do not reacts with alkali like NaOH but contain active hydrogen atom so reacts with Na or K metal.
(ii)
Reaction
with
Thionyl
chloride
and
phosphorus
Halides
Dehydration of alcohols Alcohols when are reacts with any dehydrating reagent to form alkenes with removal of water. Intermediate carbocation formation takes place where rearrangement occurs by alkyl shift, hydride shift and ring expansion.
In smaller ring always ring expansion takes place due to molecular strain and they
tends
to
convert
to
high
stability
with
large
ring.
In Dehydration of alcohols
(vi) Alcoholation of alkyne When alcohols reacts with alkyne in the presence of BF3 and HgO, according to markonikoffs rule they forms ethers.
d) Catalytic dehydrogenation: It involves the passing of vapours of alcohol over reduced copper at 300C and the product thus formed is identified. (i) Primary alcohols give aldehydes (dehydrogenation). (ii) Secondary alcohols give ketones (dehydrogenation). (iii) Tertiary alcohols form olefins (alkenes). This is dehydration
Anda mungkin juga menyukai
- Advances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookDari EverandAdvances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookArmando J. L. PombeiroPenilaian: 5 dari 5 bintang5/5 (1)
- Ligand Platforms in Homogenous Catalytic Reactions with Metals: Practice and Applications for Green Organic TransformationsDari EverandLigand Platforms in Homogenous Catalytic Reactions with Metals: Practice and Applications for Green Organic TransformationsBelum ada peringkat
- Alcohols, Diols, TriolsDokumen32 halamanAlcohols, Diols, TriolsShivam GuptaBelum ada peringkat
- Alcohols: Methods of PreparationDokumen15 halamanAlcohols: Methods of PreparationKarthik SharmaBelum ada peringkat
- Alcohols: Methods of PreparationDokumen15 halamanAlcohols: Methods of PreparationKarthik SharmaBelum ada peringkat
- Name Reactions 1Dokumen81 halamanName Reactions 1Anjish PatelBelum ada peringkat
- Alcohols From Carbonyl Compounds: Oxidation-Reduction and Organometallic CompoundsDokumen31 halamanAlcohols From Carbonyl Compounds: Oxidation-Reduction and Organometallic CompoundslongchinBelum ada peringkat
- Formulae For: AL Dehydes, Ketones & CarboxylicDokumen16 halamanFormulae For: AL Dehydes, Ketones & CarboxylicSâmïr Kumar MundariBelum ada peringkat
- Class XII: Chemistry Chapter 12: Aldehydes, Ketones and Carboxylic Acids Top ConceptsDokumen15 halamanClass XII: Chemistry Chapter 12: Aldehydes, Ketones and Carboxylic Acids Top ConceptsAshaBelum ada peringkat
- Alkohol Dari Senyawa KarbonilDokumen31 halamanAlkohol Dari Senyawa KarbonilAshfie MarwaBelum ada peringkat
- Revision Notes For Class 12 CBSE Chemistry, Aldehydes, Ketones and Carboxylic Acids - TopperlearningDokumen15 halamanRevision Notes For Class 12 CBSE Chemistry, Aldehydes, Ketones and Carboxylic Acids - TopperlearningRishabh Bhandari100% (1)
- Aldehydes, Ketones&CarboxylicacidDokumen13 halamanAldehydes, Ketones&CarboxylicacidDUHA GORASHIBelum ada peringkat
- Alcohol, Ester, Carboxylic Acid PDFDokumen17 halamanAlcohol, Ester, Carboxylic Acid PDFJustin LukmanBelum ada peringkat
- UNIT - 11. Alcohols Phenols and Ethers - NotesDokumen17 halamanUNIT - 11. Alcohols Phenols and Ethers - NotesAngelina DaisyBelum ada peringkat
- Chemistry of The Alcohols Alcohols: Monohydric C H OHDokumen23 halamanChemistry of The Alcohols Alcohols: Monohydric C H OHAyodele AdeyonuBelum ada peringkat
- Alcohol and Phenol 2Dokumen18 halamanAlcohol and Phenol 2Nayem Uddin ToukirBelum ada peringkat
- 07 Ch04.1-2 Alcohols and Ethers 32Dokumen32 halaman07 Ch04.1-2 Alcohols and Ethers 32NH Khánh NhiiBelum ada peringkat
- Aldehydes Ketones Carboxylic AcidsDokumen119 halamanAldehydes Ketones Carboxylic AcidsKashvi KhandelwalBelum ada peringkat
- Cetonas y Aldehidos - 19Dokumen36 halamanCetonas y Aldehidos - 19Moisés ChucBelum ada peringkat
- CHM 102 - Alcohols-2Dokumen7 halamanCHM 102 - Alcohols-2Philip OkunoyeBelum ada peringkat
- The Alchemy of Alcohols:: A Beginner's Guide in Organic ChemistryDokumen17 halamanThe Alchemy of Alcohols:: A Beginner's Guide in Organic ChemistrypentojochaunceyBelum ada peringkat
- Presentation AlcoholDokumen11 halamanPresentation AlcoholMust AkimBelum ada peringkat
- Alkanols and Their ReactionsDokumen11 halamanAlkanols and Their ReactionsEsther OgelekaBelum ada peringkat
- 18273-JEE Main Hydrocarbons Revision Notes - Free PDF DownloadDokumen20 halaman18273-JEE Main Hydrocarbons Revision Notes - Free PDF Downloadpsmicky09Belum ada peringkat
- Chapter 18 - Carbonyl CompoundsDokumen9 halamanChapter 18 - Carbonyl CompoundsNabindra RuwaliBelum ada peringkat
- Aldehydes and Ketones FinalDokumen67 halamanAldehydes and Ketones FinalAnil Kumar VermaBelum ada peringkat
- Alcohols 1630993189Dokumen56 halamanAlcohols 1630993189Sahisa MahatBelum ada peringkat
- Lecture Notes Chapter-12-Aldehydes, Ketones & Carboxylic AcidsDokumen26 halamanLecture Notes Chapter-12-Aldehydes, Ketones & Carboxylic AcidsSHUBHAMBelum ada peringkat
- Chapter 21Dokumen52 halamanChapter 21Seung Eun KimBelum ada peringkat
- Carbonyl Compounds Aldehydes and KetonesDokumen62 halamanCarbonyl Compounds Aldehydes and KetonesSubhabrata MabhaiBelum ada peringkat
- Chemistry NotesDokumen19 halamanChemistry NotesSanidhya RaviBelum ada peringkat
- 12 Chemistry Notes ch12 Aldehydes Ketones and Carboxylicacid PDFDokumen19 halaman12 Chemistry Notes ch12 Aldehydes Ketones and Carboxylicacid PDFshiv payasiBelum ada peringkat
- Notes IVDokumen8 halamanNotes IVAmahBelum ada peringkat
- Gist 2 Xii Chemistry 2023Dokumen15 halamanGist 2 Xii Chemistry 2023sroy191006Belum ada peringkat
- Carbonyl Compounds Aldehydes KetonesDokumen58 halamanCarbonyl Compounds Aldehydes KetonesNur Aliyah Abdul RazakBelum ada peringkat
- Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution ReactionsDokumen55 halamanCarboxylic Acid Derivatives: Nucleophilic Acyl Substitution ReactionsMaria AGBelum ada peringkat
- Alcohols 1Dokumen13 halamanAlcohols 1Suresh VedpathakBelum ada peringkat
- 12 Chemistry Notes Ch12 Aldehydes Ketones and CarboxylicacidDokumen11 halaman12 Chemistry Notes Ch12 Aldehydes Ketones and Carboxylicacidankajkumar100% (1)
- Organic Presentation: Maam Sophia AwaisDokumen30 halamanOrganic Presentation: Maam Sophia AwaisAMMAR AHMEDBelum ada peringkat
- Aldehyde KetoneDokumen21 halamanAldehyde KetoneYoBelum ada peringkat
- Organic ReagentsDokumen11 halamanOrganic ReagentsChinmaya Singh100% (1)
- Hydrocarbons SheetDokumen38 halamanHydrocarbons Sheetpathalam subrahmanyamBelum ada peringkat
- ALKANOLSDokumen25 halamanALKANOLSKoki KingBelum ada peringkat
- SM, Ru and PTDokumen8 halamanSM, Ru and PTPG ChemistryBelum ada peringkat
- Namedreactions H: Aloalkanesandhaloarenes 1Dokumen11 halamanNamedreactions H: Aloalkanesandhaloarenes 1Vishant SinghBelum ada peringkat
- Alchohols Phenols and EthersDokumen5 halamanAlchohols Phenols and EthersPritika Yamini SaiBelum ada peringkat
- Alcohols 2Dokumen15 halamanAlcohols 2Junaid KhanBelum ada peringkat
- Carbonyl CompoundsDokumen29 halamanCarbonyl CompoundsKarthik SharmaBelum ada peringkat
- Aldehyde and KetonesDokumen45 halamanAldehyde and KetonesShivam GuptaBelum ada peringkat
- Carbonyl CompoundsDokumen29 halamanCarbonyl CompoundsKarthik SharmaBelum ada peringkat
- Chapter - 13 Hydro CarbonDokumen22 halamanChapter - 13 Hydro CarbonManan TyagiBelum ada peringkat
- Pdf-Haloalkanes and HaloarenesDokumen159 halamanPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- 1.2 Organic Chemistry 1Dokumen7 halaman1.2 Organic Chemistry 1zedrickBelum ada peringkat
- Preparation of Alcohols & Phenols 2Dokumen10 halamanPreparation of Alcohols & Phenols 2Nandlal PrasadBelum ada peringkat
- Organic Chemistry 3Dokumen2 halamanOrganic Chemistry 3GundanBelum ada peringkat
- Chapter 18 Aldehydes and KetonesDokumen53 halamanChapter 18 Aldehydes and KetonesindraneelBelum ada peringkat
- Organic Chemistry Reaction ListDokumen4 halamanOrganic Chemistry Reaction ListAliSulaimanMohammadBelum ada peringkat
- 1 - Synthesis of Alcohols and PhenolsDokumen2 halaman1 - Synthesis of Alcohols and PhenolsMohamad AzaniBelum ada peringkat
- Aldehydes and KetonesDokumen20 halamanAldehydes and KetonesA BeheraBelum ada peringkat
- Final FinalDokumen36 halamanFinal FinalaprilrosesanchezBelum ada peringkat
- Heat Heat: The SI Unit of Heat Is Joule (J) - However, The Traditional Unit of HeatDokumen8 halamanHeat Heat: The SI Unit of Heat Is Joule (J) - However, The Traditional Unit of HeatAtul VermaBelum ada peringkat
- Practical Practical: Monday TuesdayDokumen15 halamanPractical Practical: Monday TuesdayAtul VermaBelum ada peringkat
- Energy: Work FXDDokumen8 halamanEnergy: Work FXDAtul VermaBelum ada peringkat
- Spherical MirrorDokumen8 halamanSpherical MirrorAtul VermaBelum ada peringkat
- A.I.S.S.C.E.-2019: Chemistry Investigatory Project ONDokumen12 halamanA.I.S.S.C.E.-2019: Chemistry Investigatory Project ONAtul VermaBelum ada peringkat
- GocDokumen108 halamanGocAtul VermaBelum ada peringkat
- Salt Metallurgy (World's Best Cheatsheet) PDFDokumen8 halamanSalt Metallurgy (World's Best Cheatsheet) PDFAtul VermaBelum ada peringkat
- SS 40 Phy 14Dokumen11 halamanSS 40 Phy 14Atul VermaBelum ada peringkat
- Angel Education Society: HALFYEARLY EXAMINATION (2017-20018) Subject: Physics Class - 12 F.M - 70 Time-3 HrsDokumen3 halamanAngel Education Society: HALFYEARLY EXAMINATION (2017-20018) Subject: Physics Class - 12 F.M - 70 Time-3 HrsAtul Verma0% (1)
- 12th WorkshhetsDokumen24 halaman12th WorkshhetsAtul VermaBelum ada peringkat
- S.S. Krotov With Solution 1.1 To 1.61Dokumen12 halamanS.S. Krotov With Solution 1.1 To 1.61Atul VermaBelum ada peringkat
- Chapter 10 - FluidsDokumen18 halamanChapter 10 - FluidsAtul VermaBelum ada peringkat
- General Physics-Bank: Dimension Level - IDokumen4 halamanGeneral Physics-Bank: Dimension Level - IAtul VermaBelum ada peringkat
- 3Dokumen1 halaman3Atul VermaBelum ada peringkat
- A V Classes: Class: Xi Physics Assignment No. Iv Unit: Iv, Chap: Work Energy & Power 1 Mark TypeDokumen1 halamanA V Classes: Class: Xi Physics Assignment No. Iv Unit: Iv, Chap: Work Energy & Power 1 Mark TypeAtul VermaBelum ada peringkat
- A V Classes: Class: Xi Physics Assignment No. Iii Unit: Iii, Chap: Laws of Motion 1 Mark QuestionDokumen1 halamanA V Classes: Class: Xi Physics Assignment No. Iii Unit: Iii, Chap: Laws of Motion 1 Mark QuestionAtul VermaBelum ada peringkat
- Lines and AnglesDokumen29 halamanLines and AnglesAtul Verma100% (2)
- The Fundamental Unit of LifeDokumen19 halamanThe Fundamental Unit of LifethinkiitBelum ada peringkat
- Atul Kumar Verma: Curriculum VitaeDokumen2 halamanAtul Kumar Verma: Curriculum VitaeAtul VermaBelum ada peringkat
- Alternating CurrentDokumen18 halamanAlternating CurrentAtul VermaBelum ada peringkat