Abhijit Baruah and Santanu Dutta: Project Summary and Overview
Diunggah oleh
Santanu DuttaJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Abhijit Baruah and Santanu Dutta: Project Summary and Overview
Diunggah oleh
Santanu DuttaHak Cipta:
Format Tersedia
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Project Summary and Overview
Vis Breaking Propylene Polymers: A Brief Overview and Critical Survey
Abhijit Baruah and Santanu Dutta
MIRAC (Machino Innovative Research and Application Center)
Machino Polymers Limited Plot No 2, Sector 33 37th KM Milestone, NH-8 Gurgaon-122001, HR, India
Authors Declaration
This is the basic introductory part of technical project taken up and under process at Machino Innovative Research and Application Center. The project report is yet to be done. This is the basic introduction and literature review part. The first part of literature review is taken up in the article that covers till the year 2001-2002. The more recon once with the updated work reports at MIRAC will be coming up in parts 2 and 3. The picture collected mostly from open documents in internet and from www.freepatentsonline.com
Introduction
Polypropylene has long been a material for study with great interest by the effect of peroxide. This is mainly because of the unique behavior of polypropylene on free radical reaction. This can be attributed to the molecular structure of Polypropylene and the monomer propylene.
-1Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Fig1. Schematic diagram of structural formulae: (a). Propylene monomer, (b) Propylene polymer. If we start the basic chemistry of propylene monomer we see that the same on free radical reaction produces on allylic free radical which is stabilized by resonance effect to a high degree and further chain reaction forming a polymer is energetically unfavorable. A Basic reaction scheme is shown in Fig 2. In fact propylene monomer can successfully be polymerized to form a usable high polymer only by coordination polymerization such as Zeigler-Natta system or metallocene catalyst system. The schematic shown in Fig 2 gives a brief glance what happen when propylene monomer is reacted by free radical reaction. The free radical can be generated by thermal degradation of organic peroxide
Fig 2: Reaction Scheme for Peroxide Initiation on Propylene Monomer Producing Stable Allylic Free Radical. Thus the resonance stabilized allylic radical has a very high stability and consequently very less reactivity. On the other hand decomposition of peroxide continuously leads to the formation of free radicals until and unless all the radical generators are consumed. This effectively results in the medium rich in radicals which are in direct contradiction to polymerization to an effective degree. In that case the most probable out-come is radical recombination or chain transfer resulting in the end of the life of the radical and consequently a break in the free radical chain reaction. But in spite of all these reactions the initial radicals can react with the monomer as shown in Fig 3.
-2Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Fig 3: Probable pathway for free radical reaction on Propylene Monomer. Each Step is energetically unfavorable. This way the reactions may precede but that is again energetically unfavorable because this requires reaction of an allylic radical to form secondary radicals which are far less stables and also owing to relative high concentration of radicals. Apart from the energetic constraint in terms of high energy radical formation, there is another possibility that the high energy radical actually can also lead to a -scission or a hydrogen radical obstruction from the -position, thereby forming another unsaturated species. But in no case a suitable high polymer of propylene monomer results. This way a number of complex possibilities are present. A very good and brief overview of free radical reaction on propylene monomer is available elsewhere [1, 2] That propylene monomer is not polymerized in Polypropylene by free radical means, and also most of the polyolefin are cross linkable by free radical reactions ,lead to a great deal of studies onto free radical reaction of PP. The most important aspect of free radical reaction on polyolefin emerged after tremendous success of cross linked polyethylene. Cross linking of polyolefins are proven to enhance: 1. Chemical resistance. 2. Solvent resistance 3. ESCR 4. High temperature compression set resistance 5. Improved tension set and creep recovery. 6. Improvement in stiffness, elongation and impact strength balance. 7. Control on processibility and processibility window Free radical cross linking of polyolefin is comparatively an easier task to accomplish as well as the control is not so difficult, as is most often encountered in the process of Elastomers.
-3Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Keeping in mind all these observations, polypropylene has long been tried for cross linking with very limited success. In fact free radical reaction on PP is seen to performed dates back. Soon after XLPE got a tremendous success by late 1980s, efforts followed for the same in PP. The reason behind lies in much superior property profile of Polypropylene as compared to Polyethylene that includes: 1. Lower specific gravity, which accounts for approximately 7.5% weight savings. 2. Much higher physical-mechanical properties. In fact PP can compete very closely with medium profile engineering thermoplastics. 3. Much better processibility with a broader processing window. This is because of smoother and more Newtonian flow behavior of PP as compared to HDPE with same molecular weight distribution. However all the initial efforts on free radical initiated cross-linking nearly resulted in some material with uncontrolled flow and very high degree of brittleness. Investigation on to the happening of the same revealed that by free radical reaction, PP has actually undergone uncontrolled scission reaction. Whereby the high molecular weight, strong relatively tough polymer has converted to a material with very low molecular weight and which is very brittle. Sequential and systematic research efforts onto such degradation soon followed and that resulted in a new class of PP materials, so called vis-broken or controlled rheology PP. The basic reaction mechanism that is established and widely accepted is that, first, the generated free radical in the reaction system abstracts a -hydrogen from the linear propylene polymer chain. The free radical can be generated by thermal energy or by thermal oxidation, where oxygen is the attacking species, or by hemolytic cleavage of a radical generator, such as peroxide. Thus:
Fig 4. Established Mechanism of Free Radical Reaction on PP and followed by beta-scission. Since the mechanism of Polypropylene vis-breaking got established on a firm basis, the same has long been applied for manifold utilities. The late 1990s or early 2000s has also seen a growth in the technology of PP cross-linking. A large number of works has been done as can be seen from the number of publications and still is going on the count. However it is very unfortunate not to have a detail account of the works done and how they are related and dependent on each other. A reader shall come across a large
-4Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
number of review articles that deals with various aspects of polypropylene, but in this particular field such reviews are very scarce. In this work the authors aim is to make and present a critical review on the field of science and thus to bridge in a major gap.
Vis-breaking basics:
In basics terms vis-breaking refers to a process of breaking down the large molecular weight. The first instance this operation may be attributed to centuries back. This was the process of mastication of natural rubber of intensive shearing action on a two roll mechanical mixing mill. Breaking the polymer chain to smaller molecular weights can be achieved by several ways such as1. Mechanical high shear mixing. 2. Thermal degradation. 3. Degradation by introducing pro-degradant, such as transition metal complexes. 4. Degradation may be initiated by a chemical species that generates radicals and attack the polymer chain. Degradation induced by electromagnetic waves such as X-rays, -rays or -particles. In this review, we shall restrict the coverage on degradation done by free radical generators and electromagnetic radiations. A brief coverage on degradation by pro-degradant, such as transition metal complexes, will also be included. Since the early days of introduction that PP is degraded with Peroxide forming a material with higher melt flow, this process was termed as cracking. This term was quite well applied since, before knowing the very minute details of the process involved and the chemistry of the reaction, control on the same was very poor. In most cases, uncontrolled reaction leads to materials with unpredictable and erratic behaviors. However since late 1980s gradual change in the entire scenario took place. That resulted in good control on breakdown of the polymer chains with controlled and predictive behaviors on the resulted material. This controlled process has been termed as vis-breaking. However, a clear cut demarcation between these two terms are still absent. The term cracking is still very often employed to mean for the process of vis-breaking. In theory vis-breaking requires generation of an active site in the material itself. For Polypropylene, abstraction of the tertiary hydrogen leads to the formation of a macro radical of polypropylene, wherein the tertiary carbon atom is devoid of one electron and is the active site. This is shown in reaction scheme as shown in Fig 4. The radical once formed has a high degree of reactivity in the ways as presented. PP macro radicals can combine with any radical present in the matrix resulting in the death of the macro radical active species. This is shown in the reaction scheme as in Fig 5. Two Polypropylene macro radicals can combine with each other, thereby causing the loss of two reactive polymer chains at a time. This also leads to the formation of a crosslink in two chains. This may be termed as the precursor of cross linked PP.
-5Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Fig 5: Schematic mechanism of Cross Linked PP formation by Peroxide Induced Free Radical reaction. The most common way of vis-breaking of Polypropylene employed in post polymerization is extrusion at the manufacturers end. The end users such as a compounding unit or a raffia fabricator occasionally employ this technique in house often to achieve the desired level of rheological properties. The most common material employed for vis-breaking is organic peroxide. While the raw material manufacturers, generally have an applied on-line rheometer coupled with the peroxide dosage port to check the on line flow behaviors and consequent control on the introduction rate of the organic peroxide to control the overall process. This type of sensitive and precision devices is being lacked by the converter of the resin such as a compounder or raffia manufactures. Thus for the sake of the converter a clear cut understanding is required for the selection of the peroxide as per the material and the process they have adopted. In table 1 we are providing a list of commonly available organic peroxides, together with the structure of the same. Table 1: Commonly used Organic Peroxides and their chemical structures
-6Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
A relative plot of half life time vs. temperature is presented in Fig.6. This clearly indicates all the peroxide varies by the onset temperature for degradation as well as the thermal activation energies. In general the thermal degradation of peroxides forming free radicals follows a first order kinetics. Increase in the reaction temperature by every 10C causes a 2-3 fold increase in the rate of decomposition. Thus table-2 and fig-2 clearly indicates if one uses a high profile highly active peroxide such as hydroperoxides, peroxy esters or ketones peroxides or acylperoxides with PP, at the processing temperature which is at least 190C-210C.
Fig 6: Half Life Times of different organic Peroxides as a function of temperature (Adopted from Arkema Technical Data Set). For the same, this peroxide does have an extremely short half life. In fact, Fig 6 suggests the half life time is 0.1 sec. only even at temperatures below 150C. This clearly leads to the choice of peroxides that are relatively high temperature resistant and have a slow rate of decomposition. Here the most common choice is Dicumyl Peroxide (DCP). In fact DCP covers around 30% of the total peroxide consumed by the polymer industry. This material is largely and very closely followed by 2, 5-dimethyl, 2, 5-di (tertiary butyl Peroxy hexane (DHBP). Peroxide ditertiary butyl peroxide also coming into the same category, but this suffers from faster reaction rates at high temperatures and a lower flesh point. Fig.6 and table 1 clearly suggests t1/2 is in the order of approximately 10 sec. at 220C temperature. So this is strongly recommended to go for visbreaking at 210C or more temperature. This is to ensure that the total amount of the peroxide being consumed before the material is leaving the reactor. In this case the extruder is being referred. It is strongly advised to set the process temperature and parameter set such that efficient usage and consumption is being made before the material is passing through the vent and vacuum, this leaving a material necessarily free or at a very low level of reaction products, that may be a source of potential problem in final application. For example if the material is applied for injection molding the entrapped reaction products such as volatiles or unreacted radicals may pose of problems of: 1. Unforeseen and unpredictable melt flow. 2. Secondary degradation leading to inferior physical and mechanical properties. 3. Volatile ejection leading to aesthetic defect. 4. Undue stress concentration near the gates or critical design parts leading to potential failure chances.
-7Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
The authors in a sequence of investigation [ Ref ] have seen that the residence time in a twin screw extruder varies with the feeding rate exponentially as can be shown in Fig. 7
Residence Time
Output
Fig 7: Plot of Residence Time against output in a twin screw extruder Fig 7 clearly indicates residence time in twin screw extruder clearly follows a power law equation with the feeding rate or output as sown by equation 1, T= A.F-b . (1)
In equation 1 the constants A and b are dependent on the extruder profile that includes the L/D ratio, the inner and outer diameter of the screw, material density and screw RPM. T is the residence time and F is the feeding rate. Fig 7 and equation 1 clearly Indicates for such vis-breaking operation a very low feed rate is recommended for best results since this will have the h ighest possible residence time in the extruder. However too low a feed rate results in lower productivity and starved feeding of the extruder. Starved feeding on the other hand presents practical problems of extrudate handling once the visbreaking material leaves the extruder die with relatively low melt strength. Thus this is advisable to go for on as high a temperature as possible, keeping in mind the onset of secondary decomposition induced by thermal energies, with a balanced feed rate, so that residual peroxide traces are not there and all the reaction products are either consumed or released before the material leaves through the extruder die. In the same set of experiments the authors have seen that melt flow of a 5 MFI PP resin with 0.1% peroxide varies with temperature as shown in Fig 8. A plot of MFI vs. residence time at three different temperatures is shown in Fig 9. A repeat MFI or reprocess of the clearly indicates that a lower temperature and higher outputs associated to lower residence times results in increase in MFI for further cycles at a higher rate. On the other hand a process temperature of 220-2300c with a residence time of 12-15 seconds gives a fairly constant level of MFI.
-8Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
31 MFI Change ( gm/10 mins) 30 29 28 27 26 25 50 40 30 20 Re sidence Time ( se c) 10 0
Zone f or Ef f ective Residence time
Fig 8 Change in MFI on visbreaking: Effect of Extruder Residence Time. Material used 3.0 MI PPHP, Peroxide 0.1% of Luperox 101-P45, Temperature 230 deg. C Extruder Berstorff ZE25 twin screw system (Data adopted from in house experimental results) Thus we can conclude on the basis of present day observation on vis-breaking chemistry and the technology developed as under--Select peroxide with relatively high temperature stability and controlled degradation rate. The stability and degradation should also be not too slow, in that all the peroxides and the generated reaction products are not consumed before material leave the vent port and vacuum and passing through the die.
MFI Change ( gm/ 10 mins)
40 30 20 10 0 170
190
210
230
250
Run Temperature ( Deg. C)
Fig 9 Change of MFI as a function of run temperature. Material used 3.0 MI PPHP, Peroxide 0.1% of Luperox 101-P45, Temperature 230 deg. C Extruder Berstorff ZE25 twin screw system [Data adopted from in house experimental results ref]. Feeding rate in the extruder to be controlled so that material has sufficient residence time in the extruder and yet no starved feeding happens. Balance in output and screw RPM are the two primary guiding factors for the same.The temperature profile is to be selected so that half life time is short enough so that all the radicals are consumed and yet it is not too fast so as to lead to uncontrolled reaction. The same principle
-9Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
applies to the choice of the free radicals generator, which in most of the cases of choice is organic peroxide. The most widely used materials are DCP, DHBP and DTBP. However other techniques of visbreaking is also available in literature such as radical generation by the use of redox reaction through transition metal complexes or by the use of electromagnetic reactions .When a transition metal complexes at a high valence state is employed which is relatively unstable, the same can abstract an electron and get reduced to a lower valence state and can initiate degradation asPoly---H + Mn+ (L-A)x -------------Poly. + H+ + M (n-1) +(L-A)x
The polymer macro-radical can then undergo degradation as normal way. Reaction by electromagnetic radiation employs then the theory of weak bond breakage by a radiation of sufficient energy. The radiation most often employed is rays or -particles. Gamma-rays have got sufficient electromagnetic energy so as to break the tertiary C-H bond to form polymeric free radicals. The tertiary polymeric radical continues the reaction. The continuing free radical degradation is attributed to -scission of the macroradical resulting in reduction in molecular weight. The reaction is thus controlled by the rate of radical generation. In case of peroxide induced decomposition, thus this is the step that leads to breakdown of the peroxides, as R-O-O-R --------------R-O. + .O-R
The rate of reaction can be expressed asR=Kp [R-O-O-R] So the reaction rate is directly proportional to the peroxide content, and the rate of decomposition of peroxide. Thus any factor that controls the rate of this step has an overall control on the entire reaction. On the other hand in case of reaction that involves metal complexes or ionizing radiation, the polymer is directly involved in the main rate determining step. Therefore here only the control on amount of polymer inserted in the reaction has the major influence. Since the amount and intensity of radiation can be controlled by the radiation generating devices. This is one major advantage of radiation induced degradation of polypropylene over peroxide initiated reactions. Another major importance is that the former can be done after fabrication of the component by passing through the reaction chamber, whereas degradation with peroxides requires high temperature and must be made in the melt mixing or peroxide stage. However peroxide induced degradation technically and by equipment is easy to accomplish since this needs only a melt mixing devise such as an extruder or melt mixer. Radiation induced reaction on the other hand requires special radiation generating devise and auxiliary equipments, which are much more expensive and mostly not been affordable by a compounder or raffia manufacturer. Resin producers also mostly employ the former techniques. Effect of Compounding Ingredients: In the next section we shall put a focus onto the usage of compounding ingredients and its effect on free radical degradation of Polypropylene. Polypropylene like any thermoplastics material is compounded to meet the end use requirements. In fact, polypropylenes that are applied to automotive segments are almost always an outcome of a compounding unit. Also the same is applied to the appliances segment, but to a lesser extent. As compounding in mostly required for meeting the end use performance criteria, the most
- 10 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
common things are fillers or reinforcing agents and elastomers or impact modifiers. Generals compounds always contain other additives such as categorized under: 1. Antioxidants or process stabilizers These materials are hindered phenols, phosphates or hindered hydroxylamine or a combination. These materials are always added as a stabilizer against natural air aging or thermo mechanical degradation as is likely to encounter in processing operation. 2. UV Stabilizers: These are complex hindered amines or hydroxylamines of high molecular weight or a combination. 3. Dispensing Agents or Flow Modifiers. These are medium to high molecular weight stearamides or olefin waxes. 4. Colorants and Nucleating agents/Clarifiers. All these materials presented above are mostly organic compounds and are susceptible to free radical reactions in one way or others stabilizers, such as antioxidants and UV-stabilizers directly take part in free radical induced degradation and start a cyclic reaction, where the active species consumes the degradable polymer radical if formed and consequently retard or quench such degradation. A schematic of such reaction is shown in Fig 10. The cyclic reaction in Fig 10, clearly indicates that the stabilizers take part in the reaction and arrest the degradation, which is in direct contradiction to the usage of peroxides or any degradant employed for breaking the high molecular weight polymer chain to shorter length and thus generate a higher flow controlled rheology material. The above constraint is of high degree of importance, since by the same neither one can get full effect of the degradation, nor the additives can function at their peak level, especially in after process service life. To overcome this constraint, technological innovations have led to the adoption of specialized control rheology process. In this polymer is first mixed with the peroxide dispersed in a suitable carrier, such as talc or calcite, and the same is melt mixed in a mixer such as an extruder. Care is taken for applying full residence time. The extrudate in the downstream can be compounded with other compounding ingredients as used. But the same requires a double process or two extruders connected in series operated in synchronization mode. In this way one can control the vis-breaking reaction at the maximum level as well as the additives are employed with their full effectiveness.
- 11 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Fig 10 Cycle showing oxidative degradation and quenching of the same by stabilizers. Adopted from Technical Bulletin, CIBA Specialty Chemicals However the main disadvantage associated to the same are capital cost incurred in machinery installation as well as the cost associated to double step production and very low output. This practical constraint, has led most compounders or raffia manufactures to use a single step process as applied in normal compounding process. Care should be taken to use peroxides at some higher dosage and to employ highest possible temperature with lowest possible feed rate and low RPM of the screw. However in spite of these the peak result is very difficult to achieve. In a series of experiments the authors have seen that melt flow of the extrudate changes with peroxide content, when in presence of additives and in their absence, as shown in Fig 11.
70 60 MFI gm/ 10 mins 50 40 30 20 10 0 0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 Peroxide ( %) Luperox 101-P45
Poly. (Without Antioxidant) Poly. (With Antioxidant Irg B225 0.2%)
Fig 11 Visbreaking of Polypropylene by Peroxide: Effect of Antioxidant. Material used 3.0 MI PPHP, Peroxide 0.1% of Luperox 101-P45, Temperature 230 deg. C Extruder Berstorff ZE25 twin screw system Antioxidant, 0.2% Irganox B225 from CIBA. MFI measured at 220 deg. C.
- 12 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Fig 11 clearly indicates that the effect of peroxide on base material is markedly higher when no antioxidant or UV stabilizers are present. In fact a careful examination of the system 2, showed the occurrence of many disturbing species with lower molecular weights, branches and some product capable of starting a second degradation. The same observation has been reported by Urs Stadlar [Ref]. On examination of a set of antioxidants and UV stabilizers it has been reported that general purpose phenollic antioxidants or amine based stabilizer packages show strong degree of interfering reaction in terms of peroxide catalyzed vis breaking reaction of PP. In Fig 13 is shown the changes in MFI with peroxide content under different stabilizer packages. .
Fig 13: Effect of Peroxide content on MFI under different stabilizer packages. That the stabilizers result in inefficient Vis breaking and thus chances of further molecular weight reduction have also been demonstrated. Processing set up also has a high degree of impact on the ultimate result of desired reaction. In Fig 14 and 15 are shown repeat MFI data under the impact of different stabilizer systems and processing conditions.
Fig 14: Effect of Stabilizer systems under Vis Breaking by Peroxide.
- 13 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Fig 15 Impact of Processing Conditions on Peroxide Induced Vis Breaking in presence of phenol based Antioxidant. To overcome this issue peroxide manufacturers have presented a solution in terms of peroxide master batches. Here the peroxide is present at relatively higher concentrations in a PP matrix. In the course of PP melt mixing, the polymeric carrier gets mixed with the main matrix polymer along with peroxides that initiate the degradation. A higher temperature process ensures the full reaction to happen, before the stabilizers are added in the next part. By the same way the effect of other compounding additives can safely be eliminated and those can be used at third fullest possible efficiency. It is observed that flow modifiers or dispersing agents such as stearamides or stearic esters or waxes generally results in a reduction in melt viscosity when employed with peroxides. On the other hand peroxides when added together with nucleating agents or clarifiers the same fail to register the rated increase in HDT, crystallization temperature are well reported elsewhere, but clear cut reasoning is lacking. Apart from the interaction with additives as presented above peroxide also presents a set of side effects on the ground of the secondary reaction products generated. First, the random scission reaction strongly affects long term heat resistance. This happens as a direct consequence of -scission, whereby C=C is generated. Also generation of double bonds may lead to free radical reaction on to the double bond compound, which results in oxidation to carbonyl functions detrimentally affect the organoleptic characterization and potential challenge against voc emission norms. A direct consequence is reduced fogging number. Also the reaction products of peroxides, which in most often is not completely removed are low molecular weight aldehydes and ketones that results in loss of organoleptic properties. However introduction of sterically hindered peroxides that can result in more stable radicals resulted in an improved scenario. For example di-tert-amyl peroxide has been introduced by Arkema. It is claimed that this product offers an excellent solution for low odor, better organoleptic properties and FDA approvals. However this also suffers from less efficiency as compared to DCP and DHCP (special chem. reviews).
- 14 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Effects on Physical Properties:
Effect on physical properties can be directly attributed to the change in molecular structure caused by visbreaking. Vis-breaking results in reduction in molecular weight or weight average molecular weight. The same results in an increase in the number of chain ends or effective number average molecular weight increases. As a direct consequence molecular weight distribution becomes narrow, as shown in Fig.-
Fig: Schematic showing change in molecular weight distribution from broad to narrow after treatment on visbreaking. As a direct consequence of molecular weight reduction and narrowing of molecular weight distribution, the polymer melt gets less entanglement since high end chains are absent or less. Thus a flow pattern becomes more Newtonian and less shear sensitive at higher shear rates, Melt viscosity decreases and melt flow rate increases. This eventually leads to smoother flow through the narrow channel for mould filling. Mouldability increases. For raffia production lowers the viscosity and more Newtonian flow improves drawdown. Other advantages a compounder or the final manufacturers gets are as warpage is reduced. These results from easier channel flow and reduced differential shrinkage between flow and cross flow direction. As molecular weight distribution decreases elongation behavior improves in the controlled region. These factors are all essentially related to the polymer chains being more uniform when the MWD is narrow and as a result react more uniformly to the applied stresses. On the other hand the low shear sensibility may lead to problem in channel flow under high shear rates. Also as a direct consequence of redirection in melt viscosity, melt strength decreases. The same results in a reduction in critical shear limit and melt instability and melt fracture becomes more prominent at high rpm. This upper limit comes down as peroxide content increases. The MFI vs. strand break RPM can be plotted as in Fig.
- 15 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
250 RPM Threshold min-1 200 150 100 50 0 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 Peroxide Content %
Fig Plot for critical rpm required for strand breakage vs peroxide content. Material used 3.0 MI PPHP, Peroxide 0.1% of Luperox 101-P45, Temperature 230 deg. C Extruder Berstorff ZE25 twin screw system Another factor comes from a reduction in molecular weight is that crystallizability decreases. As a direct consequence the skin layer formed immediately after moulding is less oriented and immediate stiffness gained is reduced by approximately 10-15%. These systems need more time for crystallization equilibrium to achieve and rate of stiffness increase is considerably reduced.
Review of Technical Application
This part of this article will be an attempt to throw light on the technological aspects of the science involved in the process of visbreaking. A brief overview and scientific survey of the things that happened will be presented. The technical implication and usage of PP breaking by free radical reaction has been started dates back since PP has got established in the market as a versatile and useful resin. Early studies back in late 1950s through the 60s was performed to obtain a easy to process material out of the reactor made PP resin which was generally of very high molecular weight and was consequently very difficult to process. Patents since 1959 reveal that PP powder was mixed with organic peroxide and oxygen in aqueous slurry and the mixture was heated [JP7221. 1963]. Other proposals indicated heating PP in a solvent with organic peroxide at temperatures ranging 60 deg. C to 250 deg. C in an inert gas which may contain oxygen [JP14490, 1963]. Further proposal came in 1969 in another Japanese patent [JP15186, 1969]. However these all processes suffered from the usage a medium which was either water or a solvent. This created a potential problem of solvent or medium removal at a later stage, high reaction times necessary and inferior and often unpredicted quality of the material. Thus these methods could however provide only an unsatisfactory result in industrial production. Since the advent of vis-breaking it was tried at the academic level as well as the industry level. A large number of patented works is available in the literature. However a limited knowledge about the basic mechanism was one major reason for this to start at a slower pace. In 1975 Baba et al. [USP3887534-1975] revealed a method for producing a modified crystalline propylene polymer. According to this invention, it was shown that PP was mixed with a range of organic peroxides in an extruder at a temperature from 170 to 250 deg. C to attain the desired degree of molecular weight reduction. A set of organic peroxides were compared with varied half lives at 130 deg. C. it was shown that while ultra fast reactants such as t-butyl hydro peroxide, which has a half life of only less than 2 hours was employed the reaction was very fast and resulted in a heterogeneous material with occasional gel
- 16 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
formation. This is attributed to very fast rate of radical formation, resulting in parallel chain radical cross combination along with beta-scission reaction that is normally targeted for. The resulted material was not good for film application, in fact the films made out of the same was seen to contain spots for gel inclusion. The same was consequently not possible for applying in a melt spinning process to form fibers. Similarly it was shown that peroxide with a long half life of more than 10 hours was equally unfavorable in view of incomplete reaction due to relatively short times in the extruder. In fact this way was tried earlier as well. Roberts et. al [USP3143584-1963], Thomson et. al [USP291384-1959], Maragliano et al. [USP30130031961] revealed methods of peroxide breaking of PP resin in extruder in the presence or absence of oxygen. Parallel to the same another set of studies were made by employing oxygen entrapped in the PP resin powder material as it is fed into the extruder. This method was good in control, but was suffering from extreme slow rate. At least two passes was necessary and often three or passes was required in order to attain the desired output of targeted scission [Kawalski, USP3563972-1971] Watson et al. [USP3898209-1975] modified the method well. It was shown that by injecting controlled dosage of atmospheric oxygen in a PP or other alpha olefins such as polybutene-1. Poly-3-methylbutene-1 or copolymers etc., and poly allomers melt, while passing through the extruder it was possible to attain the desired result in a single pass. Using PP resin with a very broad distribution of 5 to 8, it was possible to attain a distribution of as narrow as 2.8 and down to 1.5. Watson et al. in the same has focused a detailed light on the changes in Molecular weight and molecular weight distribution. The same was related to the effects on shear stress and die swell. The same studies presented a viscoelastic grid for polymer processing. The significance of this viscoelastic grid as a starting point for polymer selection for a particular process has also been discussed in detail. Watsons viscoelastic grid is shown in Fig. below. In 1976 Castagna et al. reported more improvement in the technology of controlled degradation of propylene polymers [USP3940379-1976]. The term propylene polymers is used by the inventor to include a range of polymers based on propylene monomer including homo polymer and several classes of copolymers. It was shown that heating propylene polymers with a peroxide and in presence of oxygen or an oxygen containing gas in a sheer mixing zone results in a better control on degradation of the said resin. It was reported that better color and color stability and better odor was achievable by the same. Thereby this invention resulted in a major breakthrough in the field of controlled rheology propylene polymers.
- 17 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Fig. Viscoelastic grid www.freepatentsonline.com)
as
presented
by
Watson
et
al.
(Taken
after
permission
from
This method also was successful in controlled degradation process at a relatively lower melt temperature and faster rate. A range of peroxides were used, including organic and inorganic. It was reported that peroxides which are high boiling, higher molecular weight and low odor such as t-butyl peroxy isopropyl carbonate gives best balance in the desired set of properties including color. The better success of this method compared to the previous once can be attributed to combined action of peroxide and oxygen in initiating the free radical chain degradation. Lower melt temperatures caused good control over the reaction rate and less undue oxidation, thus taking care of color problem. Also the same invention adopted a primary antioxidant such as that based on Irganox 1010 to care of undue oxidation. This, however as discussed earlier has a negative impact on peroxide action. Still, this compromise seemed to be another key to the success of the said invention. Tobias et al. have shown [USP4051306-1977] a process of controlled environmental degradation of polyolefin materials especially polypropylene. The basic aim of this work was to establish a method for degradable plastic production. The process utilized the use of a degradation promoting polymer or use of a partially degraded organic polymer. Partially degraded polymers are material wastes from the action of light, heat oxidation or products of a separate process. Materials include polyethylene, polypropylene, poly1-butene and so on. It was reported that the rate of environmental degradation will depend on the environmental conditions, such as light, heat, extent of oxygen, pressure etc. it was proposed that the
- 18 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
resulted material had an enhanced degradability. When films were manufactured using a material from this technology the increased degradability of the same was a key for easy removal from a container in the case of recycling. Boyton et al has described another method of vis-breaking a propylene polymer [USP4282076-1981]. Compared to the methods available till then, this method employed the use of pro degradant that is based on a portion of PP resin that is pre activated by ionizing radiation or by oxidation. The essence of this patent is a process for lowering the MW and narrowing down the MWD of crystalline PP material. The result was an environmentally stable thermoplastic composition, wherein large molecular chains are vis broken in a controlled way. Like the method of Tobias et al; this also uses a pro degradant polymer which is activated by high energy ionizing radiation. This activated polymer portion is mixed with a PP material containing standard antioxidant and extruding the mixture thereby ensuring a controlled degradation in the processing stage with the resultant material having higher flow, narrower MWD and is environmentally stable. This invention shows 13 claims and studied the effects of different dosage of ionizing radiation on the preformed pro degradant. A higher dosage of degradant shows greater effect and unlike the other methods available till date this one was capable of producing good and repetitive result with processing set a relatively lower melt temperature, like that shown by Tobias et al. This is because, radiation effected pro degradant is a source of radicals and in melt mixing sufficient radicals are there in the medium to ensure proper extent of reaction. In 1981 Boyton and Eugene [EP19810301809-1981] have reported an improved method of vis breaking PP. It was shown that The molecular weight of crystalline propylene polymer can be lowered, giving an environmentally stable plastic composition having narrow molecular weight distribution by vis breaking a propylene polymer using an activated portion (e.g. 0,5 to 50%) of the propylene polymer as a pro degradant. The pro degradant is formed by activating a portion of the propylene polymer by exposure to ionizing radiation. Vis breaking takes place at 200 to 300 DEG C by shear mixing in an extruder. Marman and Wisneski have shown [USP4451589-1984] a method of improving the processing behavior of polymers and resulting polymer compositions. This was achieved by low dosing of peroxide on PP resin and thermal degradation while extruding the same mixture. This was achieved by using pro degradant high molecular weight PP resin generated by treatment with a peroxide. Peroxides chosen were based on high thermal stability as exemplified by 2,5-dimethyl-2,5-di tertiary butyl peroxy hexane and others. To generate a pro degradant amount of the peroxide varied from 0.01 to 10 percent by weight of the PP resin. This method did not require any post extrusion treatment or a second operation after vis breaking effected by this. The invention also claimed to have reduced the safety hazards present when handling free radical degradant by the polymer processor. It also states avoidance of multiple additions of such materials, while still producing a material that can be processed by the polymer producer or processor. The inventors have compared several peroxides for the same with varying half life periods with the end effect. The same was related to the rate of reaction and the relative rates of decomposition. The variation of melt viscosity as a function of time for different peroxides is shown in Fig below:
- 19 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Fig: Variation of melt viscosity with time of reaction. Adopted from USP4451589, by courtesy, of www.freepatentsonline.com. A plot of relative effects on melt viscosity of different peroxides with the amount is shown in the fig below:
Fig: Plot of inverse of melt viscosity vs percent pro degradant. Adopted from www.freepatentsonline.com Upadhyaya et al. in 1986 [USP4624993-1986] have presented a new method for oxidative degradation of Polypropylene and Poly-a-olefins. This method uses injection of a specific rate of oxygen containing gas bubbles through the polymer melt well above the melting temperature. The inventors claimed that this method is effective, because of no need for a degradation catalyst, comparatively low reaction times, reduced reaction temperature and consequently improved and controlled product ranges. This method was particularly suitable for treatment of amorphous polypropylene, poly-a-olefins and synthetic waxes prepared by the Fischlar_Tropsch process. The end products were also shown to have better odor behavior as undesirable bi products were much reduced. Also a lower reaction temperature results in more control to eliminate or reduce over oxidation to keep good product color. Normally lower reaction temperatures enhance the reaction times markedly. This method taken care of the same issue by selective
- 20 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
injection of oxygen through the polymer melts in selective zones. In essence this method indicates to be of good example of deploying selective chain de-polymerization for generating quality products from a preformed high polymer. Davison et al. [USP4578430-1986] has filed a new method of controlled degradation of C3+ polymers. The patent employs polyolefin ranging from C3 to C7. The method uses controlled injection of peroxide in the polymer melt in an extruder. The rate of injection of peroxide is varied. The rate is varied at a frequency, so that the period associated is longer than the decomposition time of peroxide, but shorter than the residence time of the material in the extruder. This method in essence is successful in getting a good balance in properties vs vis-breaking and control. But, the ultimate beauty of the process lies elsewhere. It is generally established and has been reported by researchers that a controlled degradation of PP is accompanied by reduction in molecular weight. The three molecular weight distributions as Mw, Mn and Mz are designated and related by equations as bellow:
In some cases are inadequate to express a detailed description of the MWD as they are based on averaging process. In such cases a detailed spectral analysis of the MWD is preferred, where separate segments of the MWD are specifically examined. For example any two PP materials with same MFI may have wide differences in the MWD, depending on the manufacturing process as well as the catalyst. On the contrary two PP materials with different melt flow rates may show similar width of MWD curve when made by same technique or process, as can be shown schematically in Fig a and b bellow. Although MFI as stated above may be same of two PP resins the rheological behavior obvious gets different due to the differences in the fractions of molecular weight species present. A material with a narrower distribution generally shows less sheer sensitivity than those of broader distribution. This is attributed to the presence of higher amount of HMW fractions in the later. The difference in the amount of HMW portion can have profound influence on the final properties of the product. This particular method takes care of the same as compared to others available or established earlier. Earlier it was needed to blend two or more resins with different MWD s to meet the end requirement of rheological behavior as well as to performance criteria. This method was successful in getting the independent variability of MWD and desired melt flow. The control on the desired MWD made this method inherently novel. The result is ultimately an controlled vis break PP resin with greater MFI and desired MWD, as was obtainable earlier by blending two or more such species to achieve MFI and MWD. Davison et al. by using pulsing peroxide injection was successful in controlling the degradation reaction to different degrees in different phases of the process. In Fig below is shown the curves of different phases as explained by the inventors
- 21 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Fig [a] indicates average distribution curve with high MWD and Low MED broad distribution. This exemplifies the case with two materials with different melt flow but same MWD. [b] Indicates narrow and broad distribution in same MW range that exemplifies similar MFI with different MWD. . The particular invention had open up its use in the particular application for fiber applications. Both MFI and MWD of PP affect fiber properties. In general strength decreases and draw ratio increases with increase in MFI. The effects of MWD on processibility are profound, but are more difficult to define. Table below shows some general trends of properties as they are related to MFI and MWD.
Fig 5 Alteration of MWD by Cyclic variations of peroxide injection rate. Recommendation: Mixing Time>Cycle Period>Cracking Time. Overall Product MFI to keep constant as indicated by dashed lines. (Adopted with permission from www.freepatentsonline.com).
- 22 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
Invention of Ehrig et al [Ref] states a method preparing controlled rheology polypropylene taking care of the smell issue. This is claimed that small amounts of peroxides which do not decompose to t-butyl alcohol and which have comparable half lives with those of standard peroxides that are widely applied for PP visbreaking, when incorporated can produce controlled rheology polymer with better smell characteristics. This was reported that the peroxides of choice are 2,2-di tertiary amyl peroxy propane and 3,6,6,9,9pentamethyl-3-n-propyl 1,2,4,5-tetraoxy cyclo nonane. Investigations revealed that in these cases the reaction generates a sterically hindered radical eg., t-amyl radical which is stable enough and do not decompose readily to generate aldehydes or ketones with smell issue. Most often the radicals take part in reaction sequence and act as end blocker. This is also shown that the sterically hindered peroxides also have a lower reactivity and hence a lesser efficiency as compared to peroxides like DCP or DHCB. However investigations have reported that this was not of very serious drawback since control on the overall reaction was as good and the products reported to show performance at per. Investigations on the process of reducing the amount of t-butyl alcohol led to the invention of another process by Fodor et al. [Ref USP 5017661]. It was shown that polypropylene resin on treatment with 2, 5dimethyl-2, 5-di tertiary butyl peroxy hexane in presence of string acidic materials results in considerable reduction in the amount of t-butyl alcohol generated. The strong acids reported by Fodor et al include sulfuric acid, nitric acid, or strong organic acids such as trichloro acetic acid trifluoro acetic acid, fluorinated alkyl or aryl sulfonic acid etc. Use of strong acidic materials however causes a practical problem of usage in the equipment due to corrosion effect. The same necessitates changes and modifications in the set up. The problem associated with usage of strong acids effecting corrosion and decay of machines was overcome by applying solid strong acids which can act as a reaction bed. The strong acidic materials were used at a temperature ranging from 170 deg. V to 350 deg. C at a level of 4:1 to 0.25:1 for acid to peroxide.
- 23 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
It is established theoretically that acidic medium strongly interfere with peroxide catalyzed free radical reaction. This was taken care by modification of the process set up. It was reported that treatment with an acidic zeolite based molecular sieve resulted in a product having MFI of 37-39 at the same level of peroxide, whereas the untreated material registered a MFI of 42. The amount of t-butyl alcohol released was reduced to 28 ppm from 66 ppm for the untreated system. However considering overall, the viability of the entire invention remains uncertain considering the requirements of process adjustments as well as process set up modifications required. Also another uncertainty remains in the efficiency of the reaction considering usage of strong acidic material. In another attempt to encounter the limit the amount of the t-butyl alcohol generated under the standard regulation limits Schoenberg [Ref USP5530073-1996] has shown that use of a thioester while melt reaction of PP resin with standard and widely accepted peroxides as shown above, results in CR PP resin with improved melt flow wherein the amount of t-butyl alcohol resulted was considerably les than the regulation limit of 100 ppm. It was shown that the thioester was reacting synergistically with the peroxide employed to produce desired level of controlled rheology material with control on the amount of t-butyl alcohol formed. It was shown that the content of thioester was in the range of 0.01 to 1.0 %. Above the same degradation becomes very much uncontrolled. it was shown that thioesters increase the efficiency and thus result ion higher level of molecular weight reduction at same level of peroxide employed. Fig taken from the data of Schoenberg shows 0.45% DSTDP registers around 80% increase in MFI
80 70 MFI gm/ 10 mins 60 50 40 30 20 10 0 0 0.02 0.04 0.06 Peroxide Loading % 0.08
DSTDP = 0 DSTDP = 0.45%
Fig : Schoenbergs plot showing synergistic effect of thioester in peroxide initiated visbreaking reaction. DSTP content 0.45%
- 24 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
MACHINO POLYMERS LIMITED Plot # 2, Sector 33 37th KM Stone, NH 8 Gurgaon-122001 T : +91-124-4684200 F : +91-124-4684299
40 35 MFI gm/ 10 mins 30 25 20 15 10 5 0 0.001 0.01 DSTP Content % 0.1 1
Fig : Schoenbergs plot showing synergistic effect of thioester in peroxide initiated visbreaking reaction. Peroxide content 0.04%.
- 25 Machino Innovative Research and application Center is accredited by Department of Scientific and Industrial Research, Ministry of Science, Govt. of India.
Anda mungkin juga menyukai
- Diffusion in Polymers PDFDokumen322 halamanDiffusion in Polymers PDFJavier FrancesconiBelum ada peringkat
- PWC Annual ReportDokumen46 halamanPWC Annual ReportAigulBelum ada peringkat
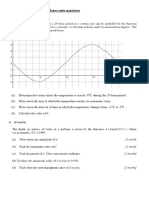
- Sine and Cosine Exam QuestionsDokumen8 halamanSine and Cosine Exam QuestionsGamer Shabs100% (1)
- Additives For PlasticsDari EverandAdditives For PlasticsRaymond SeymourPenilaian: 5 dari 5 bintang5/5 (1)
- IEA The Status of Large Scale Biomass FiringDokumen88 halamanIEA The Status of Large Scale Biomass Firinggakguk100% (1)
- Thermoplastic Characteristics: Figure 1 Molecular Arrangement of Polymer ChainsDokumen49 halamanThermoplastic Characteristics: Figure 1 Molecular Arrangement of Polymer ChainsliyazmaBelum ada peringkat
- The Unseelie Prince Maze of Shadows Book 1 by Kathryn AnnDokumen267 halamanThe Unseelie Prince Maze of Shadows Book 1 by Kathryn Annanissa Hri50% (2)
- Summary Refinery Sulfur Recovery ProjectsDokumen8 halamanSummary Refinery Sulfur Recovery ProjectsAli MBelum ada peringkat
- Modified Polymers, Their Preparation and Properties: Main Lectures Presented at the Fourth Bratislava Conference on Polymers, Bratislava, Czechoslovakia, 1-4 July 1975Dari EverandModified Polymers, Their Preparation and Properties: Main Lectures Presented at the Fourth Bratislava Conference on Polymers, Bratislava, Czechoslovakia, 1-4 July 1975A. RomanovPenilaian: 5 dari 5 bintang5/5 (1)
- Polyurethanes From Renewable ResourcesDokumen46 halamanPolyurethanes From Renewable ResourcesMohammad R ChandanBelum ada peringkat
- PlasticDokumen9 halamanPlasticCarla CampiBelum ada peringkat
- Advanced Ceramics ProcessingDokumen17 halamanAdvanced Ceramics ProcessingLuis Kike RiveraBelum ada peringkat
- Introduction to Fluoropolymers: Materials, Technology and ApplicationsDari EverandIntroduction to Fluoropolymers: Materials, Technology and ApplicationsBelum ada peringkat
- Recent Developments in The Chemistry of Halogen-Free Flame Retardant PolymersDokumen52 halamanRecent Developments in The Chemistry of Halogen-Free Flame Retardant PolymersThinh DangBelum ada peringkat
- Keb Combivis 6 enDokumen232 halamanKeb Combivis 6 enhaithamBelum ada peringkat
- Drying Costs of Woody Biomass in A Semi-Industrial Experimental Rotary Dryer - 2008Dokumen5 halamanDrying Costs of Woody Biomass in A Semi-Industrial Experimental Rotary Dryer - 2008Peter Kimbel100% (1)
- Recent Advance Reactive Extrusion in PolymerDokumen24 halamanRecent Advance Reactive Extrusion in Polymershanshicn8351Belum ada peringkat
- ChristieDokumen130 halamanChristieHuy Nguyen100% (1)
- Bioplastics CompoundingDokumen45 halamanBioplastics Compoundingchristopher_kephart1100% (1)
- Zeus Weathering of PlasticsDokumen7 halamanZeus Weathering of PlasticsDiego Fernado AvendañoBelum ada peringkat
- Theory of Particulate Processes: Analysis and Techniques of Continuous CrystallizationDari EverandTheory of Particulate Processes: Analysis and Techniques of Continuous CrystallizationBelum ada peringkat
- Reactive ExtrusionDokumen23 halamanReactive ExtrusionDIPAK VINAYAK SHIRBHATEBelum ada peringkat
- 2 Plastics IndustryDokumen41 halaman2 Plastics IndustrystephendixBelum ada peringkat
- Characterization of Composite MaterialsDari EverandCharacterization of Composite MaterialsHatsuo IshidaBelum ada peringkat
- Handbook of Plastic Foams: Types, Properties, Manufacture and ApplicationsDari EverandHandbook of Plastic Foams: Types, Properties, Manufacture and ApplicationsPenilaian: 4.5 dari 5 bintang4.5/5 (3)
- Solidification and CrystallizationDari EverandSolidification and CrystallizationDieter M. HerlachBelum ada peringkat
- Development of High-Efficiency Polycondensation Reactor With Structured Flow FieldDokumen2 halamanDevelopment of High-Efficiency Polycondensation Reactor With Structured Flow FieldAnonymous aDBepZBelum ada peringkat
- Scale-Up of Extrusion and SpheronizationDokumen20 halamanScale-Up of Extrusion and SpheronizationkjghlkdfjgBelum ada peringkat
- To Compost or Not To Compost. Carbon and Energy Footprints of Biodegradable Materials Waste Treatment PDFDokumen13 halamanTo Compost or Not To Compost. Carbon and Energy Footprints of Biodegradable Materials Waste Treatment PDFJosmarth Duvan Avila RojasBelum ada peringkat
- Physical Conversion TechnologiesDokumen89 halamanPhysical Conversion TechnologiesIttihad KhanBelum ada peringkat
- Polyvinyl Fluoride: Technology and Applications of PVFDari EverandPolyvinyl Fluoride: Technology and Applications of PVFBelum ada peringkat
- Nomogramas Perry Transp NeumáticoDokumen6 halamanNomogramas Perry Transp NeumáticoIvo AsturiasBelum ada peringkat
- Silane Coupling Agents Used For Natural Fiber - Polimer Composites A ReviewDokumen14 halamanSilane Coupling Agents Used For Natural Fiber - Polimer Composites A ReviewSam GvBelum ada peringkat
- Solid State Shear ExtrusionDokumen2 halamanSolid State Shear ExtrusionPey ManBelum ada peringkat
- Waste Energy Plastic 3Dokumen3 halamanWaste Energy Plastic 3Jorge Alejandro DelaVega Lozano100% (2)
- Soft Computing in the Design and Manufacturing of Composite Materials: Applications to Brake Friction and Thermoset Matrix CompositesDari EverandSoft Computing in the Design and Manufacturing of Composite Materials: Applications to Brake Friction and Thermoset Matrix CompositesBelum ada peringkat
- Epoxy - WikipediaDokumen11 halamanEpoxy - Wikipediaramthecharm_46098467Belum ada peringkat
- Combination of Hydraulic Brake With Power Booster SystemDokumen77 halamanCombination of Hydraulic Brake With Power Booster Systemjyani hitesh100% (1)
- Film Coextrusion Troubleshooting 7832Dokumen29 halamanFilm Coextrusion Troubleshooting 7832OscarLucianoBelum ada peringkat
- Thermal Analysis of PolymersDokumen86 halamanThermal Analysis of PolymersMarister OliveiraBelum ada peringkat
- Naoki Ota 1B 022216Dokumen13 halamanNaoki Ota 1B 022216MagsBelum ada peringkat
- A Model Recycling Process For Low Density PolyethyleneDokumen8 halamanA Model Recycling Process For Low Density Polyethylenekartik521Belum ada peringkat
- The Mechanical Properties of Poly (Ether-Ether-Ketone) (PEEK) With Emphasis On The Large Compressive Strain ResponseDokumen18 halamanThe Mechanical Properties of Poly (Ether-Ether-Ketone) (PEEK) With Emphasis On The Large Compressive Strain ResponseUriel PeñaBelum ada peringkat
- Plastics EngineeringDokumen3 halamanPlastics EngineeringPraveen Pulavarthi100% (1)
- Use of Aerodisp Fumed Silica DispersionsDokumen31 halamanUse of Aerodisp Fumed Silica DispersionswinsonecBelum ada peringkat
- Bioreactor: ECH 3201 Biochemical EngineeringDokumen26 halamanBioreactor: ECH 3201 Biochemical EngineeringWazif ZakwanBelum ada peringkat
- Introduction To Polymer StabilizationDokumen59 halamanIntroduction To Polymer Stabilizationvsi100% (1)
- Biopolymer: IUPAC DefinitionDokumen4 halamanBiopolymer: IUPAC DefinitiondearbhupiBelum ada peringkat
- Properties of Lactic Acid Based Polymers and Their Correlation With CompositionDokumen41 halamanProperties of Lactic Acid Based Polymers and Their Correlation With CompositionAero Fortia Natura100% (2)
- Alice in ChainsDokumen18 halamanAlice in ChainsmexicolaBelum ada peringkat
- Making An Appointment PaperDokumen12 halamanMaking An Appointment PaperNabila PramestiBelum ada peringkat
- Schedule For Semester III, Class of 2021Dokumen7 halamanSchedule For Semester III, Class of 2021Jay PatelBelum ada peringkat
- Anna University CTDokumen3 halamanAnna University CTprayog8Belum ada peringkat
- UNIT- 5 IRSDokumen78 halamanUNIT- 5 IRSganeshjaggineni1927Belum ada peringkat
- LinkedIn Learning - Workplace Learning Report 2021 EN 1Dokumen65 halamanLinkedIn Learning - Workplace Learning Report 2021 EN 1Ronald FriasBelum ada peringkat
- Simple Present 60991Dokumen17 halamanSimple Present 60991Ketua EE 2021 AndrianoBelum ada peringkat
- Rieka Fitri Sutrisno-CGK-SXBHSY-BTH-FLIGHT - ORIGINATINGDokumen2 halamanRieka Fitri Sutrisno-CGK-SXBHSY-BTH-FLIGHT - ORIGINATINGfairuz fanaBelum ada peringkat
- Strategic Marketing FiguresDokumen34 halamanStrategic Marketing FiguresphuongmonBelum ada peringkat
- Documentation Control HandbookDokumen9 halamanDocumentation Control Handbookcrainvictor 45Belum ada peringkat
- PENGARUH CYBERBULLYING BODY SHAMING TERHADAP KEPERCAYAAN DIRIDokumen15 halamanPENGARUH CYBERBULLYING BODY SHAMING TERHADAP KEPERCAYAAN DIRIRizky Hizrah WumuBelum ada peringkat
- Chapter 9 Lease DecisionsDokumen51 halamanChapter 9 Lease Decisionsceoji25% (4)
- BS en 1044-1999 - Brazing Filler MetalsDokumen26 halamanBS en 1044-1999 - Brazing Filler MetalsBorn ToSinBelum ada peringkat
- Chapter 3-The Hospitality & Travel Marketing SystemDokumen14 halamanChapter 3-The Hospitality & Travel Marketing SystemCharis AbadBelum ada peringkat
- Vestax VCI-380 Midi Mapping v3.4Dokumen23 halamanVestax VCI-380 Midi Mapping v3.4Matthieu TabBelum ada peringkat
- A Study On Consumer Buying Behaviour Towards ColgateDokumen15 halamanA Study On Consumer Buying Behaviour Towards Colgatebbhaya427Belum ada peringkat
- E Series CatalystDokumen1 halamanE Series CatalystEmiZBelum ada peringkat
- Organized Educator Seeks New OpportunityDokumen1 halamanOrganized Educator Seeks New OpportunityCaren Pogoy ManiquezBelum ada peringkat
- Pirates and Privateers of the Caribbean: A Guide to the GameDokumen25 halamanPirates and Privateers of the Caribbean: A Guide to the GameLunargypsyBelum ada peringkat
- HandoutDokumen4 halamanHandoutZack CullenBelum ada peringkat
- Raj Priya Civil Court Clerk FinalDokumen1 halamanRaj Priya Civil Court Clerk FinalRaj KamalBelum ada peringkat
- Tips For Effective Presentation Design and DeliveryDokumen2 halamanTips For Effective Presentation Design and DeliveryJames Manrique100% (1)
- Building MassingDokumen6 halamanBuilding MassingJohn AmirBelum ada peringkat
- Colorimetric Determination of ManganeseDokumen16 halamanColorimetric Determination of ManganeseidaayudwitasariBelum ada peringkat
- DesignWS P1 PDFDokumen673 halamanDesignWS P1 PDFcaubehamchoi6328Belum ada peringkat