Some Friedel-Crafts Reactions of Y-Butyrolactone
Diunggah oleh
dntwntJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Some Friedel-Crafts Reactions of Y-Butyrolactone
Diunggah oleh
dntwntHak Cipta:
Format Tersedia
Aust. J. Chem.
, 1978, 31, 341-6
Some Friedel-Crafts Reactions of y-Butyrolactone
Cheryl A. Kerr and Ian D. Rae
Department of Chemistry, Monash University. Clayton, Vic. 3168.
Abstract Benzene reacts with y-butyrolactone and aluminium chloride to give tetralone, but no detectable amount of 3-methylindan-1-one. p-Dichlorobenzene, p-difluorobenzene and m-dichlorobenzene give exclusively the indanones in good yield. Chlorobenzene gives a mixture of tetralones and indanones in ratio 78 : 22 and the major product of each type arises from alkylation ortho to chlorine. y-Butyrolactone is isomerized to crotonic acid by treatment with excess aluminium chloride at 150" and the indanones probably arise by this route.
Introduction The preparation of tetralone (3,4-dihydronaphthalen-l(2H)-one)from benzene and y-butyrolactone in the presence of aluminium chloride (Scheme 1) was reported1 by Truce and Olsen in 1952. We have explored the scope of this annellation reaction because the tetralones which might be prepared would be useful precursors for a range of substituted naphthalenes. Despite the publicity given to this high-yielding reaction,' it does not seem to have found very much application nor to have attracted further investigation.
Results
The reaction of p-dichlorobenzene with y-butyrolactone and aluminium chloride gave the indanone (1) in 84% yield, and the l H n.m.r. spectrum of the crude product showed that no tetralone had been produced. Similarly the difluoroindanone (2) was produced in high yield from p-difluorobenzene, and no tetralone was formed. Indanone (2) had previously been prepared from p-difluorobenzene and crotonic acid.3 At this stage, we repeated the preparation of tetralone from benzene as
Olsen, C. E., and Truce, W. E., J. Am. Chem. Soc., 1952,74, 4721. Olsen, C. E., and Bader, A. R., Org. Synth., 1963, Coll. Vol. IV, 898. Burgess, D. A,, Rae, I. D., Smith, L. K., and Snell, J. D., Aust. J. Chem., 1976, 29, 1435.
C. A. Kerr and I. D. Rae
described by Truce and O l ~ e n , ' ,and examined the l H n.m.r. spectrum of the crude ~ product for evidence (methyl doublet) that some indanone might have formed. None was detected and we place the lower limit of detection at about 2%. With chlorobenzene, a mixture of cyclic ketones was obtained and the 'H n.m.r. spectrum indicated that it contained 78 % tetralones and 22 % indanones. Separation by gas chromatography gave five fractions in the ratio 17 : 5 : 49 : 14 : 15 (order of elution) which were identified as follows. The first was 4-chloro-3-methylindan-I-one (3) which was identified by melting p ~ i n tand analysis of the 270-MHz 'H n.m.r. ~,~ spectrum. Fraction two was an approximately 1 : 1 mixture of 5- and 6-chloro3-methylindan-1-one (compounds (4) and (5) respectively). The resonances of the aromatic hydrogens were mostly separated in the 270-MHz spectrum and analysed nicely for the two 1,2,4-trisubstitution patterns. Fractions three, four and five were identified as tetralones ((6)-(8) respectively) by means of melting points and 90-MHz n.m.r. spectra. In the tetralones, the H 8 signal appeared at much lower field than the others and the analyses of the spectra were quite straightforward. The 6-chloro-3-methylindan-1-one (5) has been reported6 as a liquid but the comparison sample prepared in this work was a low-melting solid. The n.m.r, spectrum of our authentic sample matched the peaks which had been assigned to the 6-chloro compound in the mixture. 3-Methylindan-1-one was nitrated and the 9 : 1 mixture of 6- and Cnitroindanones was separated by fractional crystallization. Reduction to the amine followed by diazotization and reaction with cuprous chloride gave the 6-chloro-3-methylindan-1-one (5). The 5-chloroindanone (4) appears to be new, but proper characterization was not possible because of the small amount and the difficulty of separation or separate synthesis.
m-Dichlorobenzene gave a single product, the indanone (9) which is a known c ~ m p o u n d p-Xylene reacted with y-butyrolactone at 80' or 110' to give a mixture .~ of ketones for which the gas chromatogram showed at least seven peaks. The 'H n.m.r. spectrum showed no sign of the methyl doublet which would characterize an indanone, and the carbonyl absorption in the infrared was at 1675 cm-I with no sign of a
'Friedlanders Fortschritte der Teerfarbenfabrikation' Vol. 16, p. 688; Ger. Pat. 485,309. Mayer, F., Phillips, H., Ruppert, F. W., and Schmidt, A. T., Bey. Dtsch. Chem. Ges., 1928,61, 1966. Braun, J. V., and Heider, K., Ber. Dtsch. Chem. Ges., 1916, 49, 1268.
Some Friedel-Crafts Reactions of 7-Butyrolactone
peak in the indanone position near 1730 cm-l. Butyrolactone itself did not react with aluminium chloride at temperatures below 10O0, but when excess aluminium chloride was used with nitrobenzene as solvent at 150" the lactone was converted into crotonic acid.
Discussion It is plain that the original Truce and Olsen reaction is of rather limited scope. When deactivating substituents, such as halogens, are present on the aromatic ring, there is extensive rearrangement of the C 4 fragment so that 3-methylindan-1-ones are produced. The rearrangement of the alkylating moiety during a Friedel-Crafts reaction is known to occur to a greater extent when the aromatic ring is less reactive,' but the conversion of y-butyrolactone into crotonic acid by aluminium chloride at 1.50" suggests that prior rearrangement of the alkylating agent may also be important in this case. Such rearrangement can explain the formation of 4,7-dihydroxy-3methylindan-1-one from hydroquinone when it was heated with butyrolactone and a mixture of sodium chloride and aluminium ~ h l o r i d e . ~ The temperature of the melt, 180-20O0, is well above that required for the y-butyrolactone-crotonic acid rearrangement. At more modest temperatures, anisole reacts to give y-(4-hydroxypheny1)- or y-(4-methoxypheny1)-butyric acidg which can be cyclized to the tetralone.'' 2-Methoxy-4-methylphenol reacts with y-valerolactone and boron trifluoride etherate to give two tetralones in 23 % overall yield.'' Thus it is only reactive aromatic rings which may be annellated to tetralones, but in many cases redistribution of the ring methyls and dealkylation of methyl ethers are brought about by aluminium chloride.12 Our experience with p-xylene suggests that these reactions will not be preparatively useful when methyl substituents alone are involved. The expected product of this latter reaction, 5,8-dimethyltetralone, has been shown to rearrange to the 5,7-isomer in the presence of A1G1,/HC113 but our mixtures were even more complex. Dealkylation of methyl ethers has been reported in the cases of anisoleg and the methoxy phenol above.ll Truce and Olsenl felt that tetralone formation proceeded by alkylation to give y-phenylbutyric acid as an intermediate. The conversion of m-chlorobenzene into dichloroindanone (9), rather than the alternative 5,7-dichloroindanone, supports this alkylation proposal for the indanone formation also. m-Dichlorobenzene would almost certainly sustain electrophilic attack at the 4-position and hence we identify the initial step as alkylation rather than acylation. This proposal also helps to account for the isomer distribution which we observed for the products, both tetralones and indanones, of the reaction of chlorobenzene with y-butyrolactone. In each case, about 70% of alkylation occurs ortho to the chlorine, i.e, almost indiscriminate ortho-para attack. The same mixture of indanones was obtained when we repeated the reaction of chlorobenzene with crotonic acid and aluminium chloride.14 The
Norman, R. 0. C . , 'Principles of Organic Synthesis' p. 366 (Methuen: London 1968). Bruce, D. B., Sorrie, A. J. S., and Thomson, R. H., J. Chem. Soc., 1953, 2403. Kadyrov, C . S., and Barashkin, V. A,, Zh. Ovg. Khim., 1968, 4, 1302. Birch, A. J., Jaeger, R., and Robinson, R., J. Chem. Soc., 1945, 582. Yuste, F., and Walls, F., Aust. J. Chem., 1976, 29, 2333. 1z Ramadas, S. R., and Sukarman, K. B., Indian J. Chem., 1970, 8,470. l 3 Baddeley, G., J. Chem. Soc., 1944, 232. l4 Ashraf, M. C., Jackson, W. R., and Rash, D., Aust. J. Chem., 1975,28, 197,
C. A. Kerr and I. D. Rae
p values for alkylation of the aromatic ring are known to be small,15e.g. for acetylation, - 9 1; ethylation, - 2.4. A small p value minimizes the substituent contribution
(expressed in its o value) to the reaction rate, and thus high proportions of ortho attack are to be expected in alkylation reactions. Nonetheless, 70 % seems unusually high when compared to the products of ethylation of chlorobenzene in the presence of gallium bromide:I5 ortho, 42 %; meta, 16 %; para, 42 %. By contrast, acetylation of chlorobenzene takes place 99 % in the para position.''
Conclusion
The reaction of y-butyrolactone with aromatic compounds in the presence of aluminium chloride seems likely to give tetralones when the aromatic ring is activated towards electrophilic substitution, albeit with production of mixtures when methyl and methoxy groups are present. From dihalobenzenes, the 3-methylindan-1-ones are produced in good yield. Chlorobenzene is an intermediate case where both tetralones and indanones are produced, and the major products arise from initial alkylation ortho to chlorine. Experimental
Infrared spectra were recorded for paraffin mulls or liquid films on a Perkin-Elmer 257 grating infrared spectrometer. 'H n.m.r. spectra were recorded for dilute solutions in CDC13 on Perkin-Elmer R32 (90 MHz) and Bruker HX270 (270 MHz) spectrometers, the latter at the National N.M.R. Centre, Canberra.
4,7-Dichlovo-3-methylindan-I-one) (I
p-Dichlorobenzene (8 .O g, 0.05 mol), y-butyrolactone (1 .O g, 0.01 mol) and anhydrous aluminium chloride (6.0 g, 0.05 mol) were heated together and worked up as described below for the difluoro (1.8 compound to give 4,7-dichloro-3-methylindan-1-one g, 84% yield) as pale brown crystals, m.p. 95-97" (lit.16 100-101"). N.m.r. spectrum: 1.43, 3H, d, J 7.0; 2.38, IH, dd, J 19.0 and 1.5; 3.06, lH, dd, J 19.0 and 7.5; 3.58, IH, m; 7.27, lH, d, J 8.5; 7.50, lH, d, J 8.5.
Anhydrous aluminium chloride (6.0 g, 0 05 mol) was added, over 5 min, to a solution of y-butyrolactone (1 .O g, 0.01 mol) inp-difluorobenzene (6.2 g, 0.05 mol) and the mixture was heated under reflux for 16 h. The mixture was cooled and mixed with ice and hydrochloric acid, and the product was extracted into methylene chloride (3 x 20 ml). The methylene chloride solution was washed successively with 5% sodium carbonate solution and with water, and dried over MgS04. The solvent was removed under reduced pressure to leave a dark brown solid which was recrystallized (2) from hexane to yield 4,7-difluoro-3-methylindan-1-one (1.3 g, 59% yield) as pale brown crystals, m.p. 41-43" (]its3 46-48"). The infrared and IH n.m.r. spectra were identical with those of an authentic sample.
8
This compound was prepared by the method described above in 61 % yield from y-butyrolactone (1.0 g, 0.01 mol). The m.p. 75.5-76" agreed well with that reported4 (77-80"). N.m.r. spectrum: 1.46, 3H, d, J 7 . 0 ; 2.38, lH, dd, J 1 9 . 0 and 2.0; 3.04, lH, dd, J 19.0 and 7.5; 3.63, lH, m; 7.63,2H, S.
l5 l6
Stock, L. M., and Brown, H. C., Ada Phys. Ovg.Chem., 1963, 1, 35. Braun, W., and Ruske, M., Fr. Pat. 1,377,962, Nov. 6, 1964 (Chern. Abstr., 1965, 62, 7709h)
Some Friedel-Crafts Reactions of y-Butyrolactone
Chlovotetvalones and Zndanones
Chlorobenzene, treated as described for the compounds above, gave a mixture of chloro ketones which was subjected to gas chromatography at 280" on a 20 ft by 318 in. column packed with 20% SE 30 on a support.
This compound was isolated from fraction one as colourless crystals, m.p. 47-47.5" Oit.' 55" and liL4 47'). N.m.r. spectrum: 1.46, 3H, d, J 7 . 0 ; 2.35, lH, dd, J 19.0 and 3.0; 2.95, l H , dd, J 19.0 and 8.0; 3.47, lH, m; 7.35, lH, t, J 7 . 5 ; 7.53, lH, dd, J 7 . 5 and 1.0; 7.65, lH, dd, J 7.5 and 1.0.
5-Chloro-3-methylindun-I-one (4) and 6-Chloro-3-methylindan-I-one (5)
Fraction two was a brown oil which contained almost equal amounts of these two compounds. N.m.r. spectrum: 1 e46, 3H, d, J 7.0; 2.35, lH, dd, J19.0and 4.0; 2.95, lH, dd, J l 9 . O and 8.0; 3 .48, lH, m. The following signals in the aromatic region were assigned to compound (4) : 7.356, dd, J 8.0 and 1 .5, H 6; 7.495, br, H 4; 7.662, d, J 8.0, H 7. The following signals were assigned to compound (5): 7.448, d, J 8 . 0 , H 4 ; 7.566, dd, J 8 . 2 and 2.0, H 5 ; 7.68, d, J 2 . 5 , H7.
This compound was isolated from fraction three as colourless crystals, m.p. 63-63.5" (lit.I7 65.5-67'). N.m.r. spectrum: 2.22, 2H, m; 2.70, 2H, t, J 6 . 0 ; 3.08, 2H, t, J 6 . 0 ; 7.28, lH, t, J 8 . 0 ; 7.57, lH, d, J 8 . 0 ; 7.99, lH, d, J8.O.
This compound was isolated from fraction four as a colourless semicrystalline solid (lit." m.p. 30-31"). N.m.r. spectrum: 2.24, 2H, m; 2.35, 2H, t, J 6.0; 3.03, 2H, t, J 6.0; 7.29, lH, s; 7.31, lH, d, J 8 . 0 ; 8.00, lH, d, J 8 . 0 .
PChloro-I-tetralone (8) This compound was obtained from fraction five as colourless crystals, m.p. 93-94" (lit." 94"). N.m.r. spectrum: 2.20, 2H, m; 2.70, 2H, t, J 6 . 0 ; 2.96, 2H, t, J 6 . 0 ; 7.20, lH, d, J 8 . 0 ; 7.45, lH, dd, J 8.0 and 3.0; 8.03, lH, d, J 3.0. Isomerization of y-Butyvolactone
Powdered anhydrous aluminium chloride (6.04 g, 0.05 mol) was added to y-butyrolactone (1.06 g, 0.01 mol) in nitrobenzene (15 ml). No crotonic acid could be detected in the solution after it was heated at 100" for 1 h, but conversion was about 25% complete (as judged by lH n.m.r.) after 30 min at 150". Another solution was heated at 150"for 5 h and then worked up to give crotonic acid (0.32 g, 30%) which was identified by comparison of its n.m.r, spectrum with that of an authentic sample.
3-Methylindan-1-onez0 (12.0 g) was nitrated with KN03/H2S04 as described for the parent i n d a n ~ n e . ~The crude product (15.0 g) showed two methyl doublets in the 'H n.m.r, spectrum, ' with area ratio 9 : 1 (1 .45 and 1 .35 ppm respectively). Recrystallization from hexane gave a number
l7 ls
Badder, F. G., El-Assal, L. S., and Doss, N. A,, J. Chem. Soc., 1959, 1027. Schroeter, G., Gluschke, A., Gotzky, S., Huang, J., Irmisch, G., Laves, E., Schrader, O . , and Stier, G., Bev. Dtsch. Chem. Ges., 1930, 63, 1308. l9 Jungmann, A., Justus Liebigs Ann. Chem., 1927, 451,40. 20 Hochmann, H., and Le Claire, C. D., J. Am. Chem. Soc., 1943, 65, 59. 21 Ingold, C. K., and Pigott, H. A,, J. Chem. Soc., 1923, 123, 1469.
C. A. Kerr and I. D. Rae
of early fractions with m.p. 78-79' which were essentially pure 3-methyl-6-nitro-1-indanone. The nitro compound (2.3 g) was hydrogenated in ethanol (50 ml) over 10% Pd/C (0.2 g) to give the amine, m.p. 101-102", in quantitative yield. This was diazotized and treated with cuprous chloridez2 to give a mixture of compounds from which 6-chloro-3-methylindun-I-one, m.p. 30-31, was isolated by gas chromatography (Found: C, 66.1 ; H, 5.0. C,,H,ClO requires C, 66.5; H, 5.0%). The n.m.r. spectrum showed: 1.40, 3H, d, J 7.3; 2.31, lH, dd, J 19.2 and 3.5; 2.97, lH, dd, J 19.2 and 7.5; 3.43, lH, m; 7.452, lH, d, J 8 . 2 ; 7.567, l H , dd, J 8 . 2 and 2.2; 7.680, 1H, d, J 2.2.
Acknowledgments
This work was supported by the Australian Research Grants Committee. We thank Mr David Burgess for helpful discussion and for some assistance with the experimental section, and acknowledge some discussion with Professor Franz Effenberger.
Manuscript received 18 August 1977
22
Vogel, A. I., 'A Textbook of Practical Organic Chemistry' 3rd Edn, p. 600 (Longmans: London 1964).
Anda mungkin juga menyukai
- Anomalous Reaction of Epichlorohydrin With Trimethylamine by D. M. BurnessDokumen3 halamanAnomalous Reaction of Epichlorohydrin With Trimethylamine by D. M. Burnessjohn_dominic_4Belum ada peringkat
- Selective N-Dealkylation of Tertiary Amines With Vinyl Chloroform Ate - An Improved Synthesis of Naloxone - Tetrahedron Lett, 1977, No 18, P 1567-1570Dokumen4 halamanSelective N-Dealkylation of Tertiary Amines With Vinyl Chloroform Ate - An Improved Synthesis of Naloxone - Tetrahedron Lett, 1977, No 18, P 1567-1570muopioidreceptor100% (1)
- Lewis Acid Promoted Reactions of N - (Chlorides. Ring-Size Effects in Competitive Intramolecular Acylation of Phenyl and Cyclopropyl SubstituentsDokumen3 halamanLewis Acid Promoted Reactions of N - (Chlorides. Ring-Size Effects in Competitive Intramolecular Acylation of Phenyl and Cyclopropyl SubstituentsthamtusieuquayBelum ada peringkat
- Organic and Biological Chemistry: Reaction of Iron Pentacarbonyl With Gem-DihalidesDokumen4 halamanOrganic and Biological Chemistry: Reaction of Iron Pentacarbonyl With Gem-DihalidesCabBelum ada peringkat
- Annulation of Imidazolines With Bis-Electrophiles: Synthesis of Imidazo (1,2-A) PyridinesDokumen10 halamanAnnulation of Imidazolines With Bis-Electrophiles: Synthesis of Imidazo (1,2-A) PyridinesboksabbBelum ada peringkat
- Some Aspects of The Toluene Pyrolysis'Dokumen4 halamanSome Aspects of The Toluene Pyrolysis'Kuganathan Slowfingers PadmanathanBelum ada peringkat
- Exp't 41: The Reaction of Maleic Anhydride and CycloheptatrieneDokumen5 halamanExp't 41: The Reaction of Maleic Anhydride and CycloheptatrienelovehopeBelum ada peringkat
- Synthesis of 1chloronaphthaleneDokumen2 halamanSynthesis of 1chloronaphthalenechaudhary TahiraliBelum ada peringkat
- Non-Hazardous, Bench Top Experiment An Electron-Deficient CompoundDokumen2 halamanNon-Hazardous, Bench Top Experiment An Electron-Deficient Compoundianchibs96Belum ada peringkat
- Cashew Nut Shell Liquid. The Chromatographic Separation and Structural Investigation of The Olefinic Components of MethylcardanollDokumen6 halamanCashew Nut Shell Liquid. The Chromatographic Separation and Structural Investigation of The Olefinic Components of MethylcardanollXuân BaBelum ada peringkat
- Cathinone SynthDokumen6 halamanCathinone Synthd4rk3Belum ada peringkat
- Haberfield 1969Dokumen3 halamanHaberfield 1969Saurav PaulBelum ada peringkat
- Electrophilic Aromatic SubstitutionDokumen4 halamanElectrophilic Aromatic SubstitutionJoshuaBelum ada peringkat
- Anal. Trans-: Condensations 3-Acetocoumarin With Amides, and With Amides and Ketones1Dokumen5 halamanAnal. Trans-: Condensations 3-Acetocoumarin With Amides, and With Amides and Ketones1ashokBelum ada peringkat
- General Basic Catalysis PDFDokumen7 halamanGeneral Basic Catalysis PDFOliiversito HrnandzBelum ada peringkat
- Couch Man 1964Dokumen9 halamanCouch Man 1964Luana Matos FernandesBelum ada peringkat
- Reactions of The Ethyl Radical v. Addition To The Monomethyl AcrylonitrilesDokumen10 halamanReactions of The Ethyl Radical v. Addition To The Monomethyl Acrylonitrilesfarooq_bagbanBelum ada peringkat
- Synthesis of N-Chloroquinolines and N-Ethynylquinolines (nZ2, 4, 8) : Homo and Heterocoupling Reactions.Dokumen10 halamanSynthesis of N-Chloroquinolines and N-Ethynylquinolines (nZ2, 4, 8) : Homo and Heterocoupling Reactions.kawtherahmedBelum ada peringkat
- Organic Chemistry Lab ReportDokumen12 halamanOrganic Chemistry Lab Reportcyc5326100% (1)
- Experimental: Gas-Phase Synthesis of NitrilesDokumen4 halamanExperimental: Gas-Phase Synthesis of NitrilesAnonymous 3uZzIm43Belum ada peringkat
- Anchimeric AssistanceDokumen7 halamanAnchimeric AssistanceBen Duncan Málaga EspichánBelum ada peringkat
- The Room Temperature Polymerization of Propylene OxideDokumen5 halamanThe Room Temperature Polymerization of Propylene OxidecesarmachucaBelum ada peringkat
- 1,5-Dipolar Cyclizations: 1. LntroducfionDokumen51 halaman1,5-Dipolar Cyclizations: 1. LntroducfionRikta SahaBelum ada peringkat
- Reactions P-Hydroxybenzyl Alcohol Derivatives and Their Methyl Ethers With Molecular Chlorine'Dokumen6 halamanReactions P-Hydroxybenzyl Alcohol Derivatives and Their Methyl Ethers With Molecular Chlorine'Sandipan SahaBelum ada peringkat
- (WWW - Rhodium.ws) : Synthesis of Dimethyltryptamine (DMT)Dokumen2 halaman(WWW - Rhodium.ws) : Synthesis of Dimethyltryptamine (DMT)dextroenantiomerBelum ada peringkat
- C27H4fi03: T., F., E., H., R. S., 17Dokumen17 halamanC27H4fi03: T., F., E., H., R. S., 17Phil DinningBelum ada peringkat
- Organic Aldehyde - Isothiocyanate ChemistryDokumen244 halamanOrganic Aldehyde - Isothiocyanate Chemistrycarlosazucena1100% (2)
- Heterocyclic Chemistry: Dr. Mohamed El-NaggarDokumen124 halamanHeterocyclic Chemistry: Dr. Mohamed El-NaggarDeepshika WahengbamBelum ada peringkat
- Solomon's Chapter 15 SolutionDokumen34 halamanSolomon's Chapter 15 SolutionRobert0% (2)
- Experiment 8 Classification of Organic Halides Formal ReportDokumen5 halamanExperiment 8 Classification of Organic Halides Formal ReportRoxine Florentino56% (9)
- ObjectiveDokumen8 halamanObjectivenaim rashidBelum ada peringkat
- Piperidine Derivatives. XXI. 4-Piperidone, 4-Piperidinol and Certain of Their Derivatives - J Am Chem Soc, 1949, 71 (3), 901-906 - Ja01171a038Dokumen6 halamanPiperidine Derivatives. XXI. 4-Piperidone, 4-Piperidinol and Certain of Their Derivatives - J Am Chem Soc, 1949, 71 (3), 901-906 - Ja01171a038muopioidreceptorBelum ada peringkat
- O Nucleophilic Amino Alcohol Acyl Transfer Catalysts The Effect of Acidity of The Hydroxyl Group On The Activity of The CatalystDokumen4 halamanO Nucleophilic Amino Alcohol Acyl Transfer Catalysts The Effect of Acidity of The Hydroxyl Group On The Activity of The Catalystbruenor304amancaboihBelum ada peringkat
- Preliminary Communications: SP Ophotome Ic Determination of Chromium With Xylenoi Orange and E Yl Ol I&EDokumen3 halamanPreliminary Communications: SP Ophotome Ic Determination of Chromium With Xylenoi Orange and E Yl Ol I&EOdai ShaheenBelum ada peringkat
- R.A. Heacock and M.E. Mahon - The Chemistry of The "Aminochromes" Part II: The Preparation, Paper Chromatography, and Spectroscopic Properties of Pure Adrenolutin The Infrared Spectrum of AdrenochromeDokumen5 halamanR.A. Heacock and M.E. Mahon - The Chemistry of The "Aminochromes" Part II: The Preparation, Paper Chromatography, and Spectroscopic Properties of Pure Adrenolutin The Infrared Spectrum of AdrenochromeGummyColaBelum ada peringkat
- 4aap Method PhenolDokumen5 halaman4aap Method PhenolhitessshhhBelum ada peringkat
- Novel Analgesics and Molecular Rearrangements in The Morphine Thebaine Group IV Acid Catalyzed Rearrangements of Alcohols of The 6 14 Endo EthenotetDokumen11 halamanNovel Analgesics and Molecular Rearrangements in The Morphine Thebaine Group IV Acid Catalyzed Rearrangements of Alcohols of The 6 14 Endo EthenotetSwoll JonesBelum ada peringkat
- Molecules: New 3H-Indole Synthesis by Fischer's Method. Part IDokumen8 halamanMolecules: New 3H-Indole Synthesis by Fischer's Method. Part ILEONETTILENCINABelum ada peringkat
- Op 7001886Dokumen10 halamanOp 7001886TąmąƦą UltƦąsBelum ada peringkat
- E. Dickson Ozokwelu.2014.TOLUENE - Kirk-Othmer Encyclopedia of Chemical Technology.Dokumen24 halamanE. Dickson Ozokwelu.2014.TOLUENE - Kirk-Othmer Encyclopedia of Chemical Technology.Tissa Novida Aulia ZahraBelum ada peringkat
- Heterocyclic Chemistry and Spectroscopy C 22022/CHE 22022: Course Lecturer: Dr. Dinusha UdukalaDokumen26 halamanHeterocyclic Chemistry and Spectroscopy C 22022/CHE 22022: Course Lecturer: Dr. Dinusha UdukalaDidula ThrimannaBelum ada peringkat
- Ref 28 PDFDokumen3 halamanRef 28 PDFbuhalnitaBelum ada peringkat
- Experiment # 8 Six-Step Synthesis Aniline To 1-Bromo-3cholor-5iodobenzeneDokumen10 halamanExperiment # 8 Six-Step Synthesis Aniline To 1-Bromo-3cholor-5iodobenzeneColin CheBelum ada peringkat
- ffr2 63Dokumen6 halamanffr2 63api-253517612Belum ada peringkat
- Com 97 8051Dokumen5 halamanCom 97 8051bruna_0410Belum ada peringkat
- Anie.199510211 Preparation, Structure, and Reactivity of 1,3,4 - Triphenyl-4,5-dihydro-lH-l, 2,4-Triazol-5-Ylidene, A New Stable CarbeneDokumen3 halamanAnie.199510211 Preparation, Structure, and Reactivity of 1,3,4 - Triphenyl-4,5-dihydro-lH-l, 2,4-Triazol-5-Ylidene, A New Stable CarbeneРумен ЛяпчевBelum ada peringkat
- A General Method For C Reductive Alkylation of Indoles: Anu Mahadevan, Howard Sard, Mario Gonzalez and John C. MckewDokumen3 halamanA General Method For C Reductive Alkylation of Indoles: Anu Mahadevan, Howard Sard, Mario Gonzalez and John C. MckewMichell UrzúaBelum ada peringkat
- Exp 7 - Prep of Carbocations by The Friedel-Crafts Rxn-ReportDokumen4 halamanExp 7 - Prep of Carbocations by The Friedel-Crafts Rxn-ReportUyen V. NguyenBelum ada peringkat
- Abdul Alrahman Al-Sumait University: Five Membered Heterocyclic Compound 1 10Dokumen10 halamanAbdul Alrahman Al-Sumait University: Five Membered Heterocyclic Compound 1 10Ali Issa OthmanBelum ada peringkat
- A2 Chemistry Revision NotesDokumen13 halamanA2 Chemistry Revision NotesJobe Bryer50% (4)
- The Thermal Decomposition of AzinesDokumen14 halamanThe Thermal Decomposition of AzinesKamesh ReddiBelum ada peringkat
- Willemsens 69 Investigations PDFDokumen6 halamanWillemsens 69 Investigations PDFSavarinathan Maria RayappanBelum ada peringkat
- Clarkson1930 Primer SPFXDokumen11 halamanClarkson1930 Primer SPFXyulliarperezBelum ada peringkat
- JornalDokumen3 halamanJornalIshu SethiBelum ada peringkat
- Aldhyde Ketone AcidDokumen30 halamanAldhyde Ketone AcidSamratBelum ada peringkat
- Tetrahedron 2010 66 10 01902 - 01910 CiclicoDokumen9 halamanTetrahedron 2010 66 10 01902 - 01910 Ciclicoteodoro11Belum ada peringkat
- Physical Organic Chemistry—Ii: Specially Invited Lectures Presented at the Second IUPAC Conference on Physical Organic Chemistry Held at Noordwijkerhout, Netherlands, 29 April–2 May 1974Dari EverandPhysical Organic Chemistry—Ii: Specially Invited Lectures Presented at the Second IUPAC Conference on Physical Organic Chemistry Held at Noordwijkerhout, Netherlands, 29 April–2 May 1974Th. J. De BoerBelum ada peringkat
- Organic Reaction Mechanisms 1982: An annual survey covering the literature dated December 1981 through November 1982Dari EverandOrganic Reaction Mechanisms 1982: An annual survey covering the literature dated December 1981 through November 1982A. C. KnipeBelum ada peringkat
- Sec 4 Chem Prelim Paper 1Dokumen16 halamanSec 4 Chem Prelim Paper 1TeckluckyBelum ada peringkat
- Acid Value 1Dokumen7 halamanAcid Value 1Mujtaba AbbasBelum ada peringkat
- Electrolysis Aqueous SolutionDokumen40 halamanElectrolysis Aqueous SolutionVictor OkosunBelum ada peringkat
- Class 10 Science Chapter NotesDokumen192 halamanClass 10 Science Chapter NotesTarunBelum ada peringkat
- Tiamina, IPDokumen4 halamanTiamina, IPmagicianchemistBelum ada peringkat
- Thermochemistry 2010Dokumen33 halamanThermochemistry 2010Ain FzaBelum ada peringkat
- EnterobacteriaceaeDokumen20 halamanEnterobacteriaceaeNarayani MathivananBelum ada peringkat
- Chemsheets GCSE 1231 AlkanesDokumen2 halamanChemsheets GCSE 1231 AlkanesRobinBelum ada peringkat
- Ammonia, Sulfur, Air N Water - 1Dokumen2 halamanAmmonia, Sulfur, Air N Water - 1bilalBelum ada peringkat
- Unit 7 Review Problem Set 3Dokumen5 halamanUnit 7 Review Problem Set 3api-182809945Belum ada peringkat
- Jee Main 2019 Jan ChemDokumen84 halamanJee Main 2019 Jan ChemBhavesh KriplaniBelum ada peringkat
- Lab Report Experiment 1 Chm624Dokumen11 halamanLab Report Experiment 1 Chm624Hazwan HamimBelum ada peringkat
- 10.0 Carboxylic Acids 2021Dokumen64 halaman10.0 Carboxylic Acids 2021NURUL HIDAYAH SAIFUL ANUARBelum ada peringkat
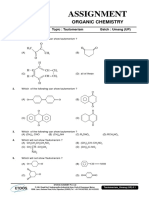
- Assignment: Organic ChemistryDokumen6 halamanAssignment: Organic ChemistryWalid EbaiedBelum ada peringkat
- Staining - Practical and Theoretical (1962) by GurrDokumen654 halamanStaining - Practical and Theoretical (1962) by GurrDan JohnsonBelum ada peringkat
- Trends in Group 2 Elements Alkaline Earth MetalsDokumen52 halamanTrends in Group 2 Elements Alkaline Earth MetalsKemoy FrancisBelum ada peringkat
- Act 6 Benzene PDFDokumen5 halamanAct 6 Benzene PDFDulce Wendolyn BollásBelum ada peringkat
- Synthesis of Acetylsalicylic AcidDokumen7 halamanSynthesis of Acetylsalicylic Acidjeniccax17Belum ada peringkat
- Lecture Notes PDFDokumen60 halamanLecture Notes PDFprakas.rao39695Belum ada peringkat
- Solutions of Soaps in Organic SolventsDokumen4 halamanSolutions of Soaps in Organic SolventsSandry KesumaBelum ada peringkat
- N+factor+Dokumen109 halamanN+factor+Gmail ManBelum ada peringkat
- Baek 1985Dokumen4 halamanBaek 1985FELIPE DANIEL MONTERO BRUNIBelum ada peringkat
- PK Urine Practicum 1 KBK Sem 6 2016 ManualDokumen38 halamanPK Urine Practicum 1 KBK Sem 6 2016 Manualanindita fauziahBelum ada peringkat
- Practice Stoichiometry Test (V1 Jan 2019) FINALDokumen14 halamanPractice Stoichiometry Test (V1 Jan 2019) FINALHo Lam YikBelum ada peringkat
- Chapter 28Dokumen16 halamanChapter 28Muhamad Ivan AbrorBelum ada peringkat
- Lesson Plan in Dec. 05 Carbon Compounds Grade 9Dokumen6 halamanLesson Plan in Dec. 05 Carbon Compounds Grade 9Edessa Masinas100% (1)
- Esters - IntroductionDokumen5 halamanEsters - Introductionnikunj-smcite@rediffmail.comBelum ada peringkat
- HOMEWORK Polymers and SynthesisDokumen21 halamanHOMEWORK Polymers and SynthesisRobert EdwardsBelum ada peringkat
- Eschweiler-Clarke Solventfree PDFDokumen10 halamanEschweiler-Clarke Solventfree PDFRenæ NaeBelum ada peringkat
- SPM Chemistry Form 5Dokumen5 halamanSPM Chemistry Form 5Aileen PoLyBelum ada peringkat