Important Instructions For The School Principal: (Not To Be Printed With The Question Paper)
Diunggah oleh
sharvan_creativeDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Important Instructions For The School Principal: (Not To Be Printed With The Question Paper)
Diunggah oleh
sharvan_creativeHak Cipta:
Format Tersedia
Important Instructions for the School Principal
(Not to be printed with the question paper)
1) This question paper is strictly meant for the use in School Based Summative AssessmentII, March-2012 only. This question paper is not to be used for any other purpose except mentioned above under any circumstances. 2) The intellectual material contained in the question paper is the exclusive property of Central Board of Secondary Education and no one including the user school is allowed to publish, print or convey (by any means) to any person not authorised by the Board in this regard. 3) The School Principal is responsible for the safe custody of the question paper or any other material sent by the Central Board of Secondary Education in connection with School based SA-II, March-2012, in any form including the print-outs, compact-disc or any other electronic form. 4) Any violation of the terms and conditions mentioned above may result in the action criminal or civil under the applicable laws/byelaws against the offenders/defaulters.
Note:
Please ensure that these instructions are not printed with the question paper being administered to the examinees.
Page 1 of 13
SUMMATIVE ASSESSMENT II, 2012 II, 2012
SCIENCE /
SC 2020
Class X /
Time allowed : 3 hours 3 General Instructions :
(i) (ii) (iii) (iv) (v) (vi) (vii) (viii) (ix)
X
Maximum Marks : 80 80
The question paper comprises of two Sections, A and B. You are to attempt both the sections. All questions are compulsory. There is no overall choice. However, internal choice has been provided in all the five questions of five marks category. Only one option in such questions is to be attempted. All questions of Section-A and all questions of Section-B are to be attempted separately. Question numbers 1 to 4 in Section-A are one mark questions. These are to be answered in one word or in one sentence. Question numbers 5 to 13 in Section-A are two marks questions. These are to be answered in about 30 words each. Question numbers 14 to 22 in Section-A are three marks questions. These are to be answered in about 50 words each. Question numbers 23 to 25 in Section-A are five marks questions. These are to be answered in about 70 words each. Question numbers 26 to 41 in Section-B are multiple choice questions based on practical skills. Each question is a one mark question. You are to select one most appropriate response out of the four provided to you.
(i) (ii) (iii) (iv) (v) (vi) (vii) (viii) (ix) 1 5 14 23 26 4 13 22 25 41 30 50 70
Page 2 of 13
SECTION-A / 1. Name the functional group present in CH3COCH3 and state the name of this compound. CH3COCH3 Name the part responsible for the power of accommodation of the human eye.
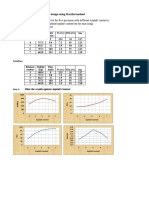
2.
3.
Choose one consumer each that belongs to the second and third trophic levels from the organisms given below. Eagle, frog, tiger , rabbit, fox
4.
Pesticides added to a field is seen in increased amounts in the crop and in the birds that feed on them. What is this phenomenon called ?
5.
State the modern periodic law. How many groups and periods are there in the modern periodic table ?
6.
Out of the two elements X and Y which has bigger atomic radius ? Give reason to justify your answer. (i) X has atomic number 18 and atomic mass 40 (ii) Y has atomic number 20 and atomic mass 40 X Y (i) (ii) X Y 18 20 40 40
7.
Mention the functions of (a) placenta (b) fallopian tube in the human female reproductive system. (a) (b) List any two contraceptive methods practiced only by women. Mention how these methods work.
8.
9.
What is meant by radius of curvature of a spherical mirror? How is it related to the focal length of the mirror ?
10. What is meant by least distance of distinct vision ? How does this vary between the very young and old people ?
11. Explain why the colour of the clear sky is blue. Page 3 of 13
12. List two disadvantages of building large dams over rivers.
13. Name two fossil fuels. List one product each formed when these are burnt in (a)sufficient oxygen and (b)insufficient oxygen. (a) (b) 14. An organic compound A of molecular formula C2H4 on reduction gives another compound B of molecular formula C2H6. B on reaction with Chlorine in the presence of sunlight gives C of molecular formula C2H5Cl. (a) Name the compounds A, B and C (b) Write chemical equation for the conversion of A to B and name the type of reaction. A C2H4 B C2H6 C2H5Cl (a) (b) A, B A B C B C
15. Given below is a part of the periodic table. Li Be B C N Na Mg AI Si P As we move horizontally from left to right :(a) what happens to the atomic size? Justify your answer. (b) what happens to the metallic character of the elements ? Li Na (a) (b) 16. List three advantages of growing plants by vegetative propagation. Be Mg B AI C Si N P
O S
F Cl
O S
F Cl
17. Trace the F1 generation formed by crossing two plants with separate traits for shape and seed colour- round green (RRyy) and wrinkled yellow(rrYY). Mention the characteristic exhibited by it . (RRyy) (rrYY) F1
18. How is the sex of a new born determined genetically in humans? 19. (a) Name two factors that could lead to the rise of new species. Page 4 of 13
(b) (a) (b)
Why are the wings of birds and bats considered analogous organs?
20. An image 2/3rd the size the object is formed by a convex lens at a distance of 12cm from it. Find the focal length of the lens. 12cm 2/3
21. Draw ray diagram and describe the nature of the image formed by a concave mirror when the object kept__________ (a) between pole and focus of the mirror (b) Between infinity and centre of curvature of the mirror
(a) (b) 22. State the cause of dispersion, when white light enters a glass prism. Explain with a diagram.
23. (a)
(b) (c) (a)
Draw the structures for the following compounds. (i) 2- Bromopentane (ii) 2 methylpropane (iii) Butanal (iv) 1 Hexyne Draw the electron dot structure for ethanoic acid. Draw and explain the structure of the micelle. Which end of the micelle dissolves in dirt ? (i) (iii) 2(ii) (iv) 2 1
(b) (c) OR/ Write the name for the following compounds: (i) CH3CH CH2CH3 (ii) CH3CH=CHCH2CH3 CH3 (iii) CH3CCH2CH3 (iv) CH3CH2CHCH3 Cl (b) (i) Take about 3mL of Ethanol in a test tube and warm it gently in a water bath. (ii) Add a 5% solution of alkaline potassium permanganate drop by drop to the solution. (iii) What happens to the colour of KMnO4 added initially and then in excess? Give reason. Name the product of this reaction. Page 5 of 13
(c) (i)
What is scum ? (ii) (iv) CH3CH=CHCH2CH3 CH3CH2CHCH3 Cl
CH3CH CH2CH3 CH3 (iii) CH3CCH2CH3 (b) (i) (ii) (iii) (c) 24. (a) (i) (b) (c) (a) (i) (b) (c) (a) (b) (c) (a) (b) (c) 25. (a) (b) (ii)
3mL 5% KMnO4
Draw the longitudinal section of a flower and label the following parts. stigma (ii) style (iii) anther (iv) ovary Why are papaya flowers called unisexual ? After fertilization in a flower, mention the structures that develop into the embryo and seed. (iii) (iv)
OR/ In the male reproductive system, where is the organ that produces male germ cells situated ? Why? Mention the role of prostate gland and seminal vesicles in the human male reproductive system. How are the male and female germ cells produced in the human body different from each other ?
(c) (a) (b)
Define power of a lens. An object is kept at a distance of 18cm, 20cm, 22cm, and 30cm, from a lens of power +5D. (i) In which case or cases would you get a magnified image? (ii) Which of the magnified image can be got on a screen? List two widely used applications of a convex lens. +5D (i) (ii) / / 18cm 20cm, 22cm, 30cm
(c) Page 6 of 13
OR/ (a) A very thin narrow beam of white light is made incident on three glass objects shown below. Comment on the nature of behaviour of the emergent beam in all the 3 cases.
(b) (c)
There is a similarity between two of the emergent beams. Identify the two. When light enters from air to glass the angles of incidence and refraction in air and glass are 45and 30 respectively. Find the refractive index of glass. (Given that sin 45 =
1 ; sin 30=1/2) 2
(a)
(b) (c) sin 45 = 45 30
1 ; sin 30=) 2
SECTION - B / 26. The freshly prepared aqueous solution of ferrous sulphate appear (a) dark green (b) pale green (c) light blue (d) dark blue (a) (c) (b) (d)
27. A student dropped Zinc granules in a test tube containing FeSO4 solution. The observations made by him after some time would be (i) No change has taken place (iii) The solution has turned turbid (a) i and ii (b) i and iii (ii) (iv) (c) Zn metal turns black The solution has turned colourless i and iv (d) ii and iv
(i) (iii)
(ii) (iv) Page 7 of 13
(a)
ii
(b)
iii
(c)
iv
(d)
ii
iv
28. A student kept a bottle of acetic acid along with bottle containing Al2(SO4)3 solution. Which of the following substances can he use to identify the bottle containing acetic acid ? (a) NaOH (b) Na2CO3 (c) H2O (d) HCl Al2(SO4)3 (a) NaOH (b) Na2CO3 (c) H2O (d) HCl
29. Which of the following test tube represents the complete reaction of Na2CO3 with Acetic acid ?
(a)
(b)
II Na2CO3
(c)
III
(d)
IV
(a)
(b)
II
(c)
III
(d)
IV
30. The correct experimental set up for the reaction of Na2CO3 with CH3COOH is
Na2CO3
CH3COOH
Page 8 of 13
31. A student is to find the focal length of (i) a concave mirror (ii) convex lens by focussing the image of a distant object on a screen. He will observe that the screen is on the same side as that of the object in. (a) both cases (b) case (i) but not in case (ii) (c) case (ii) but not in case (i) (d) neither case (ii) nor in case (i) (i) (a) (b) (c) (d) (i) (ii) (ii) (ii) (i) (i) (ii)
32. In an experiment to determine the focal length of a convex lens a student obtained a sharp inverted image of a distant tree on the screen behind the lens. He then removed the screen and looked through the lens in the direction of the tree. He will see. (a) A blurred image on the wall (b) An erect image of the tree on the lens. (c) No image as there is no screen (d) An inverted image of the tree at the focus of the lens.
(a) (b) (c) (d) 33. Four students set up an apparatus consisting of a screen, concave mirror mounted on a stand and meter scale to carry out measurements of focal length of a concave mirror as shown in figures. A,B, C, and D given below. Best result will be obtained by student.
Page 9 of 13
(a)
(b)
B A, B, C D
(c)
(d)
(a)
(b)
(c)
(d)
34. Four students traced the path of a ray of light passing through a glass slab. The correct trace is that of student.
(a)
(b)
II
(c)
III
(d)
IV
Page 10 of 13
(a)
(b)
II
(c)
III
(d)
IV
35. On the basis of experiments performed by students with rectangular glass slabs the correct interpretation about the incident ray, refracted ray and the emergent ray would be(a) i >e (b) e<r (c) emergent ray is parallel to the refracted ray (d) incident ray and emergent ray are parallel to each other
(a) (b) (c) (d)
i >e e<r
36. While observing a student will find that the shape of amoeba is (a) round (b) oval (c) irregular (a) (b) (c)
(d) (d)
rod like
37. In which of the following is binary fission observed ?
(a)
(b)
(c)
(d)
(a)
(b)
(c)
(d)
D Page 11 of 13
38. The following are the sketches made by some students.
The sketch not illustrative of budding in yeast is : (a) A (b) B (c) C (d) D
(a)
(b)
(c)
(d)
39. In budding (a) cell divides transversely (b) cell divides longitudinally (c) nucleus divides followed by the development of protuberance (d) a small protuberance develops followed by nuclear division. (a) (b) (c) (d) 40. Extra water on the surface of soaked raisins is wiped off gently before final weighing using. (a) filter paper (b) dry cotton wool (c) dry cotton cloth (d) hot air blower (a) (b) (c) (d)
41. While determining the percentage of water absorbed by raisins a student recorded the following observations. (a) Mass of water taken in the beaker = 50g (b) Mass of dry raisins = 6.0g (c) Mass of wet raisins 7.5g (d) Mass of water left in the beaker 42g On the basis of above observations the percentage of water absorbed by raisins is 7.5g - 6.0g 7.5g - 6.0g (a) (b) 100 100 6.0g 7.5g
Page 12 of 13
(c)
50g - 42g 100 42g
(d)
50g - 42g 100 50g
= 50g = 6.0g 7.5g 42g
(a) (b) (c) (d) (a) (c)
7.5g - 6.0g 100 6.0g 50g - 42g 100 42g
(b) (d)
7.5g - 6.0g 100 7.5g 50g - 42g 100 50g
Page 13 of 13
Anda mungkin juga menyukai
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- IGCSE Chemistry Section 5 Lesson 3Dokumen43 halamanIGCSE Chemistry Section 5 Lesson 3Bhawana SinghBelum ada peringkat
- Marshal HMA Mixture Design ExampleDokumen2 halamanMarshal HMA Mixture Design ExampleTewodros TadesseBelum ada peringkat
- NLL - Elementary - Coursebook 2019 PDFDokumen24 halamanNLL - Elementary - Coursebook 2019 PDFgilmolto100% (1)
- 2-Port Antenna Frequency Range Dual Polarization HPBW Adjust. Electr. DTDokumen5 halaman2-Port Antenna Frequency Range Dual Polarization HPBW Adjust. Electr. DTIbrahim JaberBelum ada peringkat
- Lecture02 NoteDokumen23 halamanLecture02 NoteJibril JundiBelum ada peringkat
- Fernandez ArmestoDokumen10 halamanFernandez Armestosrodriguezlorenzo3288Belum ada peringkat
- Simply Put - ENT EAR LECTURE NOTESDokumen48 halamanSimply Put - ENT EAR LECTURE NOTESCedric KyekyeBelum ada peringkat
- Personalised MedicineDokumen25 halamanPersonalised MedicineRevanti MukherjeeBelum ada peringkat
- Hyper-Threading Technology Architecture and Microarchitecture - SummaryDokumen4 halamanHyper-Threading Technology Architecture and Microarchitecture - SummaryMuhammad UsmanBelum ada peringkat
- Thin Film Deposition TechniquesDokumen20 halamanThin Film Deposition TechniquesShayan Ahmad Khattak, BS Physics Student, UoPBelum ada peringkat
- ServiceDokumen47 halamanServiceMarko KoširBelum ada peringkat
- Logic and Set Theory PropositionDokumen3 halamanLogic and Set Theory PropositionVince OjedaBelum ada peringkat
- Evaluating Sources IB Style: Social 20ib Opvl NotesDokumen7 halamanEvaluating Sources IB Style: Social 20ib Opvl NotesRobert ZhangBelum ada peringkat
- Exercises 6 Workshops 9001 - WBP1Dokumen1 halamanExercises 6 Workshops 9001 - WBP1rameshqcBelum ada peringkat
- Quantification of Dell S Competitive AdvantageDokumen3 halamanQuantification of Dell S Competitive AdvantageSandeep Yadav50% (2)
- Do You Agree With Aguinaldo That The Assassination of Antonio Luna Is Beneficial For The Philippines' Struggle For Independence?Dokumen1 halamanDo You Agree With Aguinaldo That The Assassination of Antonio Luna Is Beneficial For The Philippines' Struggle For Independence?Mary Rose BaluranBelum ada peringkat
- Onan Service Manual MDJA MDJB MDJC MDJE MDJF Marine Diesel Genset Engines 974-0750Dokumen92 halamanOnan Service Manual MDJA MDJB MDJC MDJE MDJF Marine Diesel Genset Engines 974-0750GreenMountainGenerators80% (10)
- Clark DietrichDokumen110 halamanClark Dietrichikirby77Belum ada peringkat
- Marine Engineering 1921Dokumen908 halamanMarine Engineering 1921Samuel Sneddon-Nelmes0% (1)
- Forensic Science From The Crime Scene To The Crime Lab 2nd Edition Richard Saferstein Test BankDokumen36 halamanForensic Science From The Crime Scene To The Crime Lab 2nd Edition Richard Saferstein Test Bankhilaryazariaqtoec4100% (25)
- Numerical Methods Chapter 10 SummaryDokumen8 halamanNumerical Methods Chapter 10 SummarynedumpillilBelum ada peringkat
- Trading As A BusinessDokumen169 halamanTrading As A Businesspetefader100% (1)
- Data Sheet: Experiment 5: Factors Affecting Reaction RateDokumen4 halamanData Sheet: Experiment 5: Factors Affecting Reaction Ratesmuyet lêBelum ada peringkat
- Unit 1 TQM NotesDokumen26 halamanUnit 1 TQM NotesHarishBelum ada peringkat
- India: Kerala Sustainable Urban Development Project (KSUDP)Dokumen28 halamanIndia: Kerala Sustainable Urban Development Project (KSUDP)ADBGADBelum ada peringkat
- ITU SURVEY ON RADIO SPECTRUM MANAGEMENT 17 01 07 Final PDFDokumen280 halamanITU SURVEY ON RADIO SPECTRUM MANAGEMENT 17 01 07 Final PDFMohamed AliBelum ada peringkat
- Music 7: Music of Lowlands of LuzonDokumen14 halamanMusic 7: Music of Lowlands of LuzonGhia Cressida HernandezBelum ada peringkat
- ASMOPS 2016 - International Invitation PHILIPPINEDokumen4 halamanASMOPS 2016 - International Invitation PHILIPPINEMl Phil0% (3)
- Polyol polyether+NCO Isupur PDFDokumen27 halamanPolyol polyether+NCO Isupur PDFswapon kumar shillBelum ada peringkat
- Ancient Greek Divination by Birthmarks and MolesDokumen8 halamanAncient Greek Divination by Birthmarks and MolessheaniBelum ada peringkat