B 0160612
Deskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
B 0160612
Hak Cipta:
Format Tersedia
International Journal of Engineering Inventions ISSN: 2278-7461, www.ijeijournal.
com Volume 1, Issue 6 (October2012) PP: 06-12
Corrosion Behavior of SS316L in Artificial Blood Plasma in Presence of D-Glucose
S. John Mary1, S. Rajendran2
2
Dept. of Chemistry, Loyola College, Chennai, India -600 034 Corrosion Rsearch centre, P.G. & Research Dept. of Chemistry, G.T.N. Arts College, Dindigul, Tamilnadu, India 624005
AbstractThe Corrosion resistance of SS316L in artificial blood plasma in absence and presence of 50ppm and 100ppm of D-Glucose has been evaluated by electro chemical studies such as polarization study AC impedance spectra. Potentiodynamic polarization study and AC impedance spectra have been used to investigate the corrosion behaviour of SS316L. The order of corrosion resistance of SS316L in artificial blood plasma in absence and presence of 50ppm of DGlucose and presence of 100 ppm of D-Glucose is SS316L + plasma 100 ppm D-Glucose + > SS316L + 50 ppm D-Glucose plasma+ > SS316L + plasma
KeywordsCorrosion, artificial plasma, SS316L, D-Glucose
I.
INTRODUCTION
The performance of any material in the human body is controlled by two sets of characteristics: biofunctionality and biocompatibility. There are wide ranges of materials available for implantation. Many metals and alloys have been used in implantation. The corrosion behaviour of Artificial Blood Plasma has been investigated. Influence of PH and corrosion inhibitors D-Glucose in ABP has been investigated. The selection of materials for medical applications is usually based on considerations of biocompatibility. When metals are considered, the susceptibility of the material to corrosion and the effect the corrosion has on the tissue are the central aspects of biocompatibility. The human body is not an environment that one would consider hospitable for an implanted metal alloy. The materials for the devices generate less concern in their biocompatibility with human body than those for implants. The basic knowledge of metal composition, microstructure and processing is necessary to select a metallic material for a specific application. Metallic materials can have serious corrosion problems in aqueous solution, such as in contact with physiological fluids. Corrosion results in releasing toxic metals ions to body and also weakening implants. An electrochemical reaction involves removing electrons from the anodic to the cathode. When the metal is surrounded by an aqueous solution, oxidation may occur at the location on the metal surface. For the past hundred years various metals are used for implantation in the human body, such as aluminum, copper, zinc, iron and carbon steels, silver, nickel, and magnesium. Corrosion resistance of implant materials may involve qualitative measurements or quantitative electrochemical measurements in simulated body fluid. Now a days various alloys and metallic materials are found and are used as implantation material in human body for long period. Corrosion is one of the major issues resulting in the failure of biomedical implant devices. The nature of the passive oxide films formed, and the mechanical properties of the materials form some of the essential criteria for selection of alternative or development of new materials. Commercially available D-Glucose (Indian pharmacopeias grade) was used in this study. 0.05g and 0.10g of D-Glucose was used in artificial plasma (AP). The present work was undertaken to study the corrosion behaviour of SS316L in artificial blood plasma in absence and presence of D-Glucose, by polarization study and AC impedance spectra. Corrosion parameter such as corrosion potential, corrosion current, linear polarization resistance, charge transfer resistance and double layer capacitance have been derived from these studies.
II.
MATERIALS AND METHODS
The present work is undertaken. The metal specimens, namely, SS316L has been chosen for the present study. The composition of SS 316L is (wt %) 18 Cr, 12 Ni, 2.5 Mo, < 0.03 C and balance iron. The metal specimen was encapsulated in Teflon. The metal specimen was polished to mirror finish and degreased with trichloroethylene. The metal specimen was immersed in artificial blood plasma. The chemical composition of the artificial plasma according to PN-EN ISO 10993-15 standard (g/l distilled water) was NaCl 6.8, CaCl2 0.200, KCl 0.4, MgSO4 0.1, NaHCO3 2.2, Na2HPO4 0.126, NaH2PO4 0.026. In electrochemical studies the metal specimen was used as working electrode and Artificial blood plasma was used as electrolyte (10 ml). The temperature was maintained at 37 Potentiodynamic Polarisation: Polarization studies were carried out in a CHI Electrochemical workstation with impedance, Model 660A. A three-electrode cell assembly was used. The working electrode was the metal specimen..A saturated calomel electrode (SCE) was the reference electrode and platinum was the counter electrode. From the polarization study, corrosion
Corrosion Behavior of SS316L in Artificial Blood Plasma in Presence of D-Glucose
parameters such as corrosion potential (Ecorr), corrosion current (Icorr) and Tafel slopes (anodic = ba and cathodic = bc) were calculated. AC Impedance Spectra: The instrument used for polarization study was used to record AC impedance spectra also. The cell setup was also the same. The real part (Z) and imaginary part (Z) of the cell impedance were measured in ohms at various frequencies. Values of the charge transfer resistance (Rt) and the double layer capacitance (Cdl) were calculated from Nyquist plots, impedance log (Z/ohm) value was calculated from bode plots.
III.
RESULTS AND DISCUSSIONS
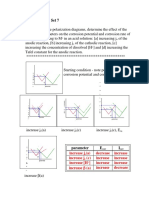
Analysis of potentiodynamic polarization curves The corrosion parameters of SS316L in artificial blood plasma (ABP) are given in Table 1. The potentiodynamic polarization curves are shown in Figs. 1 to 3. To study the corrosion behaviour of SS316L in artificial blood plasma in absence and presence of D-Glucose by polarization study and AC impedance spectra. Corrosion parameters such as corrosion potential, corrosion current, linear polarization resistance, charge transfer resistance and double layer capacitance have been derived from these studies. Glucose C6H12O6 also known as D-Glucose, dextrose,or grapesugar)is simple sugar (monosaccharide) and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate. D-Glucose is one of the main products of photosynthesis and fuels for cellular respiration. DGlucose is the human body's key source of energy, through aerobic respiration, providing approximately 3.75 kilocalories (16 kilojoules) of food energy per gram. It is dissolved in water. The main objective of present study is to investigate how the intake of a D-Glucose solution affects the diabetic people who have undergone implanation. Hence the corrosion behaviour of SS316L in absence and presence of 50 ppm and 100 ppm D-Glucose is studied.
Structure of D-Glucose
Figure1: Polarization curves of SS316L immersed in artificial blood plasma in absence of D-Glucose
Corrosion Behavior of SS316L in Artificial Blood Plasma in Presence of D-Glucose
Figure2: Polarization curves of SS316L immersed in artificial blood plasma in presence of 50 ppm D-Glucose
Figure3: Polarization curves of SS316L immersed in artificial blood plasma in presence of 100 ppm D-Glucose
IV.
ANALYSIS OF POTENTIODYNAMIC POLARISATION CURVES
SS316L immersed in D-Glucose The Potentiodynamic polarization curves of SS316L immersed in artificial plasma in the absence and presence of 50ppm D-Glucose and 100 ppm D-Glucose are shown in fig 1 to 3. The corrosion parameters namely corrosion potential, corrosion current, (I corr) Tafel slope ba, bc, LPR linear polarization resistance are given in the table (1) Table 1: Corrosion parameters of SS316L immersed in artificial blood plasma in the absence and presence of DGlucose obtained by polarization study Metal System ECorr bc ba LPR ICorr A/cm2 2 mV vs SCE mV/ decade mV/decade ohm cm
SS316L SS316L
SS316L +ABP SS316L +ABP +50ppm DGlucose SS316L +ABP +100ppm DGlucose
-410 -429
154 152
460 523
8.23 x105 9.99 x 105
6.097x 10-8 5.144 x 10-8
SS316L
-412
182
612
1.30 x 106
4.691x 10-8
Corrosion Behavior of SS316L in Artificial Blood Plasma in Presence of D-Glucose
SS316L immersed in artificial plasma in the absence of D-Glucose. When SS316L immersed in artificial plasma, the corrosion potential is -410 mV Vs SCE. The LPR value is 8.23 x 105 ohmcm2 and the corrosion current (I corr) is 6.097 x 10-8 A/cm2. The tafel slope values (bc = 154 mV/decade ba = 460 mV/decade) indicate that the rate of change of corrosion current with potential is much higher during the anodic polarization than during the cathodic polarization. During anodic polarization oxide film is formed on metal surface in presence of SS316L. Corrosion behaviour of SS316L in artificial blood plasma in presence of 50 ppm of D-Glucose. When SS316L immersed in ABP in presence of 50 ppm of D-Glucose the corrosion potential is shifted from -410 to -429 mV Vs SCE. The tafel slopes bc = 152 mV/decade ba = 523 mV/decade further the LPR value increase from 8.23 x 105 ohm cm2 to 9.99 x 105 ohmcm2. The corrosion current (I corr) decreases from 6.097 x 10-8 A/cm2 to 5.144 x 10-8 A/cm2. Thus polarization study confirms the formation of a protective film on the metal surface. The polarization study reveals that the corrosion resistance of SS316L in ABP increases in presence of 50 ppm of D-Glucose. SS316L immersed in artificial blood plasma in the presence of 100 ppm of D-Glucose. When SS316L immersed in artificial blood plasma in presence of 100 ppm of D-Glucose the corrosion potential is shifted from -410 to -412 mV Vs SCE. The tafel slopes bc = 182 mV/decade ba = 612 mV/decade. Further the LPR value is increased from 8.23 x 105 to 1.30 x 106 ohmcm2. The corrosion current (I corr) shifted from 6.097 x 10-8 A/cm2 to 4.691x 108 . Thus the polarisation study confirms the formation of a protective film on the metal surface. The polarization study reveals that the corrosion resistance of SS316 in ABP in presence of 100 ppm increases. SS316 L+ plasma + SS316L + plasma+ SS316 L+ plasma 100 ppm D-Glucose > > 50 ppm D-Glucose AC impedance spectra: The instrument used for polarization study was used to record AC impedance spectra also. The cell setup was also the same. The real part (Z) and imaginary part (Z) of the cell impedance were measured in ohm at various frequencies. Values of the charge transfer resistance (Rt) and the double layer capacitance (Cdl) were calculated. Table 2: Corrosion parameters of SS316L immersed in artificial blood plasma in the absence and presence of DGlucose obtained by AC Impedance Spectra Nyquist plot Bode plot Metal SS316L SS316L System SS316L +ABP SS316L +ABP +50ppm D-Glucose SS316L +ABP +100ppm D-Glucose Rt ohm cm2 129200 177300 Cdl F/cm2 3.94 x10-11 2.87 x 10-11 Impedance log (z/ohm) 5.4 5.5
179600
2.83 x 10-11
5.6
figure 4. AC impedance spectra of SS316L immersed in ABP in absence of D-Glucose
Corrosion Behavior of SS316L in Artificial Blood Plasma in Presence of D-Glucose
Figure 5. AC impedance spectra of SS316L immersed in ABP in presence of 50 ppm D-Glucose
Figure 6. AC impedance spectra of SS316L immersed in ABP in presence of 100 ppm D-Glucose SS316 immersed in ABP in absence of D-Glucose: When SS316L immersed in ABP, the change transfer resistance Rt is 129200 ohmcm2. The double layer capacitance value is 3.94 x 10-11 F/cm2 Fig(4). The impedance value is 5.4 Fig 4, 5, and 6 and the corresponding Bode plot is shown in Fig 7, 8, and 9. SS316 immersed in ABP presence of 50 ppm of D-Glucose: When SS316L immersed in ABP containing 50 ppm D-Glucose the charge transfer resistance Rt increases from 129200 ohm cm2 to 177300 ohm cm2. The Cdl value decreases from 3.94 x 10-11 F/cm2 to 2.87 x 10-11 F/cm2. The impedance value log (2/ohm) increases from 5.4 to 5.5. These results lead to the conclusion that the protective film is formed. SS316 immersed in ABP presence of 100 ppm of D-Glucose: When 100 ppm D-Glucose is added the Rt value increases from 129200 to 177300 ohm cm2 the cdl value decreases from 3.94 x 10-11 to 2.87 x 10-11 F/cm2. The impedance value increases from the 5.5 to 5.6. Thus the AC impedance study leads to the conclusion that the protective film is formed. Thus AC impedance spectra lead to the conclusions that in the absence and presence of D-Glucose, the order of corrosion resistance is SS316L + plasma + 100 ppm D-Glucose > SS316 L+ plasma 50 ppm D-Glucose > SS316L + plasma
10
Corrosion Behavior of SS316L in Artificial Blood Plasma in Presence of D-Glucose
Figure 7. AC impedance spectra of SS316L immersed in ABP in absence of D-Glucose(Bode plot)
Figure 8. AC impedance spectra of SS316L immersed in ABP in presence of 50ppm D-Glucose (Bode plot)
Figure 9. AC impedance spectra of SS316L immersed in ABP in presence of 100ppm D-Glucose (Bode plot)
11
Corrosion Behavior of SS316L in Artificial Blood Plasma in Presence of D-Glucose V. CONCLUSION
The corrosion behaviour of SS316L immersed in ABP in absence and presence of 50 ppm of D-Glucose, 100 ppm of D-Glucose have been studied. Polarization study leads to the following conclusions. SS316L + plasma + SS316L + plasma > SS316L + plasma 100 ppm D-Glucose > 50 ppm D-Glucose AC impedance leads to the following conclusions. SS316L + plasma+ 100 ppm D-Glucose > SS316L + plasma 50 ppm D-Glucose > SS316L + plasma
Acknowledgement:The authors are thankful to their respective management.
REFERENCES
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. American Society for Metals, Metals Handbook, 8Ul edn, Voll, Metals Part, Ohio, (1968). N D. Tomashov and G.P. Chernova, Passivity and Protection of Metals Against Corrosion, Plenum Press, New York (1967). M. Stern, J. Electrochem. Soc, 102 (1955), 663. Eurocor 77, The 6 European Congress on Metallic Corrosion (1977), Society of Chemical Industry, London. Antony, N., Sherine, H.B., Rajendran, S. (2010) Inhibition and Biocide Actions of Sodium Dodecyl Sulfate-Zn2+ System for the Corrosion of Carbon Steel in Chloride Solution.Portugaliae Electrochimica Acta, 28(1): 1-14 Florence, H.R.G., Anthony, N.A., Wilson, S.J., Amalraj, J.A., Rajendran, S. (2005) Indian Journal of Chemical Technology, 12, July, 472-476 Gurappa, I. (2002) Characterization of different materials for corrosion resistance under simulated body fluid conditions. Materials Characterization, 49(1): 73-79 Mareci, D., Nemtoi, G.H., Aelenei, N., Bocanu, C. (2005) The Electrochemical Behaviour of Various Nonprecious Ni and Co based alloys in Artificial Saliva. European Cells and Materials, 10,1 Rajendran, S., Paulraj, J., Rengan, P., Jeyasundari, J., Manivanna, M. (2009) Corrosion behavior of metals in artificial saliva in present of spirulina powder. Journal of Dentistry and oral hygiene, 1, str. 1-8 Rajendran, S., Uma, V., Krishnaveni, A., Jeyasundari, J., Shyamaladevi, B., Manivannan, M. (2009) Corrosion behavior of metals in artificial saliva in presence of D-D-Glucose.Arabian Journal for Science and Engineering, 34(2C) 147-158 Rathish, J.R., Rajendran, S., Christy, J., Devi, S.B., Johnmary, S., Manivannan, M., Rajam, K., Rengan, P. (2010) Corrosion behaviour of metals in Artificial Sweat. Open Corrosion Journal, 3(1): 38-44 Vieira, A., Ribeiro, A., Rocha, L., Celis, J. (2006) Influence of pH and corrosion inhibitors on the tribocorrosion of titanium in artificial saliva. Wear, 261(9): 994-1001
11. 12. 13.
S. John Mary, S.Rajendran Corrosion Behavior of Ni-Cr In Artificial Blood Plasma In Presence Of Shelcal-Vitamin D Journal of Convergence, ISSN 0972-6489 Vol 12, No.1- 4(2011) pg77-84
12
Anda mungkin juga menyukai
- Design and Implementaion of A Dual Tone Multi-Frequency (DTMF) Based Gsm-Controlled Car SecurityDokumen7 halamanDesign and Implementaion of A Dual Tone Multi-Frequency (DTMF) Based Gsm-Controlled Car SecurityInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- C 0491740Dokumen24 halamanC 0491740International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Data Mining Impact On Business IntelligenceDokumen4 halamanData Mining Impact On Business IntelligenceInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- G 04123844Dokumen7 halamanG 04123844International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- B 0490716Dokumen10 halamanB 0490716International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Relevance of Designing and Developing An Improvised White Board Compass For Teaching Geometrical Construction Concepts in Basic TechnologyDokumen6 halamanRelevance of Designing and Developing An Improvised White Board Compass For Teaching Geometrical Construction Concepts in Basic TechnologyInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- An Imperceptible Blind Image Watermarking Scheme For Image Authentication Using DNA Encoding and Multi-Resolution Wavelet DecompositionDokumen8 halamanAn Imperceptible Blind Image Watermarking Scheme For Image Authentication Using DNA Encoding and Multi-Resolution Wavelet DecompositionInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Saturation Point of Traffic On The Channel Based On The Ship DomainDokumen5 halamanSaturation Point of Traffic On The Channel Based On The Ship DomainInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- C 04111114Dokumen4 halamanC 04111114International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Improvement of Friction Testing Machine and Study On Friction TestDokumen5 halamanImprovement of Friction Testing Machine and Study On Friction TestInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- A 0490106Dokumen6 halamanA 0490106International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Effect of Processing Technique On LDPE Thin Films and SheetsDokumen5 halamanEffect of Processing Technique On LDPE Thin Films and SheetsInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- E 04112135Dokumen15 halamanE 04112135International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- B 04110710Dokumen4 halamanB 04110710International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- A 04110106Dokumen6 halamanA 04110106International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- F 04103138Dokumen8 halamanF 04103138International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Design and Prototype Development of A Cleaning Device For Outdoor AdvertisementDokumen6 halamanDesign and Prototype Development of A Cleaning Device For Outdoor AdvertisementInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Design of A Tele Medical Channel Dedicated To Telemonitoring of Cardiac Insufficiency by Correlative AnalysisDokumen5 halamanDesign of A Tele Medical Channel Dedicated To Telemonitoring of Cardiac Insufficiency by Correlative AnalysisInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- E 04102330Dokumen8 halamanE 04102330International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Correlative Analysis and Determination of The Attenuation Coefficients of The Photoplethysmographic SignalDokumen4 halamanCorrelative Analysis and Determination of The Attenuation Coefficients of The Photoplethysmographic SignalInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- I 04105358Dokumen6 halamanI 04105358International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- G 04103948Dokumen10 halamanG 04103948International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Polygon Oscillating Piston Engine: Kevin Anderson, Steve Cunningham, Martin Stuart, Chris McnamaraDokumen6 halamanPolygon Oscillating Piston Engine: Kevin Anderson, Steve Cunningham, Martin Stuart, Chris McnamaraInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- A 04100105Dokumen5 halamanA 04100105International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- A Solar Powered Smart Helmet With MultifeaturesDokumen6 halamanA Solar Powered Smart Helmet With MultifeaturesInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Design and Prototype Development of A Cleaning Device For Outdoor AdvertisementDokumen6 halamanDesign and Prototype Development of A Cleaning Device For Outdoor AdvertisementInternational Journal of Engineering Inventions (IJEI)Belum ada peringkat
- B 0490716Dokumen10 halamanB 0490716International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- C 0491740Dokumen24 halamanC 0491740International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- A 0490106Dokumen6 halamanA 0490106International Journal of Engineering Inventions (IJEI)Belum ada peringkat
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- ProValve Knife Gate ValveDokumen12 halamanProValve Knife Gate ValveHarry HonchoBelum ada peringkat
- Stainless Steel TypesDokumen20 halamanStainless Steel Typessushant_jhawerBelum ada peringkat
- OES-INS-P057 Piping Inspection ProcedureDokumen9 halamanOES-INS-P057 Piping Inspection ProcedureMatheus Ribeiro100% (2)
- BS 6349-1-4-2021Dokumen88 halamanBS 6349-1-4-2021MoGH100% (4)
- Grundfosliterature 3153478 PDFDokumen5 halamanGrundfosliterature 3153478 PDFRobin GomezBelum ada peringkat
- Synthesis, Properties and Applications of Inorganic-Organic PolymersDokumen10 halamanSynthesis, Properties and Applications of Inorganic-Organic Polymersbyte20Belum ada peringkat
- Wire Solutions CatalogDokumen8 halamanWire Solutions Catalogpumud15Belum ada peringkat
- Product Datasheet ASSA ABLOY DL6120T enDokumen28 halamanProduct Datasheet ASSA ABLOY DL6120T enAbin RajuBelum ada peringkat
- Klueberoil GEM 1 N 030063 PI GB enDokumen6 halamanKlueberoil GEM 1 N 030063 PI GB ena3.msaputraBelum ada peringkat
- Soluition Charpter 3 - 2 PDFDokumen3 halamanSoluition Charpter 3 - 2 PDFBryan de BarrosBelum ada peringkat
- GE Cosmetic Inspection GuidelinesDokumen31 halamanGE Cosmetic Inspection Guidelines57641Belum ada peringkat
- Analisis TFC FinalDokumen98 halamanAnalisis TFC FinalMartín Dione100% (1)
- LED Spec Sheet ApprovalDokumen20 halamanLED Spec Sheet ApprovalAnonymous G1iPoNOKBelum ada peringkat
- Marine Port Structure GuidanceDokumen12 halamanMarine Port Structure Guidancemulyadi100% (1)
- Comet Gland CatalogueDokumen12 halamanComet Gland CataloguePrabhudatta KarBelum ada peringkat
- The Galvanic Effect of High-Strength Weathering Steel Welded Joints and Its Influence On Corrosion ResistanceDokumen12 halamanThe Galvanic Effect of High-Strength Weathering Steel Welded Joints and Its Influence On Corrosion ResistanceSREEJITH S NAIRBelum ada peringkat
- Erosion-Corrosion of Duplex Stainless Steel Under KuwaitDokumen8 halamanErosion-Corrosion of Duplex Stainless Steel Under Kuwaitorea1Belum ada peringkat
- Boiler Water TreatmentDokumen50 halamanBoiler Water Treatmentak_thimiriBelum ada peringkat
- Refractory Constructions PDFDokumen51 halamanRefractory Constructions PDFHammadBelum ada peringkat
- How Vci WorkDokumen21 halamanHow Vci Work同道文档中心100% (1)
- Types of Corrosion Inhibitors - Cor ProDokumen3 halamanTypes of Corrosion Inhibitors - Cor ProPritha DasBelum ada peringkat
- Surface Treatment Technologies of Aluminum Alloy For AutomobilesDokumen4 halamanSurface Treatment Technologies of Aluminum Alloy For Automobilesharibabu ampoluBelum ada peringkat
- PIP STE05121 (Anchor Bolt Design Guide)Dokumen55 halamanPIP STE05121 (Anchor Bolt Design Guide)bowitchhazelBelum ada peringkat
- Seamanship 3 - Final Module 1)Dokumen5 halamanSeamanship 3 - Final Module 1)Corrine AbucejoBelum ada peringkat
- STP1239 Eb.1415051 1 PDFDokumen283 halamanSTP1239 Eb.1415051 1 PDFpaola100% (1)
- Continental Cylinder Application Guide PDFDokumen6 halamanContinental Cylinder Application Guide PDFJonatan BernalBelum ada peringkat
- Sulfur Species in Oilfield Production - The Good, the Bad and the UglyDokumen20 halamanSulfur Species in Oilfield Production - The Good, the Bad and the UglyaseBelum ada peringkat
- ASTM C1218 Standard Test Method For Water-Soluble Chloride in Mortar and ConcreteDokumen3 halamanASTM C1218 Standard Test Method For Water-Soluble Chloride in Mortar and ConcreteElbert Contreras R80% (5)
- 22-1239 Corrosion Catalog 2022-En LRDokumen12 halaman22-1239 Corrosion Catalog 2022-En LRΓεώργιος ΠαπουτσόγλουBelum ada peringkat
- DAE Civil Engineering Syllabus - NewDokumen225 halamanDAE Civil Engineering Syllabus - Newnargissuhail0% (1)