1 s2.0 S0016236110000670 Main
Diunggah oleh
saadiisDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
1 s2.0 S0016236110000670 Main
Diunggah oleh
saadiisHak Cipta:
Format Tersedia
Fuel 89 (2010) 20882095
Contents lists available at ScienceDirect
Fuel
journal homepage: www.elsevier.com/locate/fuel
Production of hydrocarbons in FischerTropsch synthesis with Fe-based catalyst: Investigations of primary kerosene yield and carbon mass balance
Kazuhiro Kumabe a, Takuya Sato b, Kozo Matsumoto c, Yasuyuki Ishida d, Tatsuya Hasegawa b,c,*
a
Division of Environmental and Renewable Energy Systems (ERES), Graduate School of Engineering, Gifu University, 1-1 Yanagido, Gifu, Gifu 501-1193, Japan Department of Aerospace Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8603, Japan c EcoTopia Science Institute (ESI), Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8603, Japan d Department of Biological Chemistry, College of Bioscience and Biotechnology, Chubu University, Matsumoto-cho, Kasugai 487-8501, Japan
b
a r t i c l e
i n f o
a b s t r a c t
The FischerTropsch synthesis (FTS) of syngas was carried out using Fe-based catalysts in order to produce hydrocarbons (HCs) equivalent to kerosene, which is used as an alternative aviation fuel. The FTS was conducted in a downdraft continuous-ow-type xed-bed reactor under a temperature of 533 573 K and a pressure of 3.0 MPa. The effects of reduction gases and time of the Fe-based catalyst, reaction temperature and the chemical species included in the Fe-based catalyst on the FTS were studied by focusing on primary kerosene yield and the carbon mass balance. The carbon mass balances in the study were almost 100%. In C6 + HCs, the selectivity of CO to the C11C14 HCs equivalent to kerosene was found to be the second highest, the highest being its selectivity to C20 + HCs equivalent to wax. The amount of primary kerosene produced was maximum under the following conditions: the prepared Fe catalyst did not contain other chemical species, the feed ratio of the reduction gases H2:CO:N2 was 2:1:3, the catalyst reduction time was 8 h, and the FTS reaction temperature was 553 K. 2010 Elsevier Ltd. All rights reserved.
Article history: Received 17 March 2009 Received in revised form 15 February 2010 Accepted 15 February 2010 Available online 23 February 2010 Keywords: FischerTropsch synthesis Fe-based catalyst Kerosene yield
1. Introduction The development of aircrafts has dramatically increased the movement of people and goods. According to a market research report released by the Boeing Company in 2007 [1], the number of air passengers is expected to increase by 4.5% every year and the air cargo market is likely to show an average growth rate of 6.1% per year over the next 20 years. However, the depletion of fossil-fuel resources has increased the necessity for reducing oil consumption [2]. In addition, CO2 emissions from the aviation sector have to be reduced since the report from the Intergovernmental Panel on Climate Change (IPCC) [3] estimates that the ratio of CO2 emissions from aircrafts to the total CO2 emission will increase from 2.3% in 2000 to 3.723% in 2050 depending on the circumstances. In 2006, the European Commission adopted a proposal for legislation to include aviation in the European Union Emission Trading Scheme (EU-ETS). From 2011, emissions from all domestic and international ights between EU airports will be covered, and from 2012, the scope will be extended to cover all international ights from or to anywhere in the world that arrive at or depart from an EU airport. The civil aviation industry is under an increasing pres-
* Corresponding author. Address: EcoTopia Science Institute (ESI), Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8603, Japan. Tel./fax: +81 52 789 4506. E-mail address: t-hasegawa@esi.nagoya-u.ac.jp (T. Hasegawa). 0016-2361/$ - see front matter 2010 Elsevier Ltd. All rights reserved. doi:10.1016/j.fuel.2010.02.018
sure to impose strict regulations on the emission of greenhouse gases (GHGs) [4]. The vision of the International Air Transport Association (IATA) for 2008 is to achieve carbonneutral growth in the medium term and to develop zero-carbon emission technology within the next 50 years [5]. Therefore, there is a need for the development of alternative aviation fuels. Several studies have been carried out on alternative aviation fuels such as hydrogen [68], coal, oil shale and tar sands [9] in the past, especially in the 1970s when there was an oil crisis. However, the social and technological situations prevalent today are totally different from what they used to be. In addition, it is difcult to use gaseous fuels, solid fuels or electric motors for aircrafts without making any modications to the current aircraft structure because of their fuel capacity, engine weight, etc. Thus, liquid fuels such as dimethyl ether (DME), biodiesel, bioethanol and synthetic hydrocarbon (HC) have been selected as possible alternatives [10]. Synthetic HC fuel, which is called as coal-to-liquid (CTL), biomassto-liquid (BTL) or gas-to-liquid (GTL), is produced by the Fischer Tropsch synthesis (FTS) of syngas via the gasication of coal or biomass or the steam reforming of natural gas. The American Society for Testing and Materials (ASTM) species that the most common fuel for civil aviation is JET A-1, which is described in ASTM D1655 [11]. The major component of JET A-1 is kerosene, which is also used as lamp oil. The difference between JET A-1 and lamp oil is that the former contains several additives such as a deicing agent and corrosion inhibitor and is devoid of water and impure
K. Kumabe et al. / Fuel 89 (2010) 20882095
2089
substances. We have reported that kerosene in HC fuel produced by FTS is suitable as aviation fuel because its characteristics are similar to those of JET A-1 [10]. Therefore, the production process of alternative aviation fuel by CTL, BTL or GTL has to be developed. Kerosene for aviation fuel mainly consists of parafn (about 85%) [12]. In FTS, olen is relatively much produced with an Febased catalyst, whereas parafn is relatively much produced with a Co-based catalyst [13]. Thus, a Co-based catalyst has an advantage for the production of aviation fuel by FTS from a viewpoint of desired products. However, the cost of an Fe-based catalyst is generally lower than that of a Co-based catalyst [14]. This allows an Fe-based catalyst to produce parafn by the hydrogenerated reaction in another reactor after olen production by FTS. Therefore, in the present study, we focus on the FTS of syngas using an Fe-based catalyst. Recently, many investigators have studied the effectiveness of Fe-based catalysts in the FTS. For example, Yoneyama et al. have developed new hybrid catalysts Fe FTS catalysts mixed with H-ZSM-5 zeolite for use in the FTS in order to selectively produce isoparafns in one step [15]. Hayakawa et al. have investigated the activation behavior of precipitated Fe-based catalysts, especially the effects of the addition of sodium silicate and copper oxide on the reduction behavior and catalytic performance such as activity, selectivity and productivity [16]. Farias et al. have studied the dependencies of FTS liquid hydrocarbon product distribution on the operating pressure and temperature by using three K-promoted Fe catalysts with different K molar contents [17]. Zhang et al. prepared a bimodal Fe-based catalyst by a new one-step impregnation method and used the active components as brick to directly build the small pores inside the large pores of support; this catalyst was quite different from previous bimodal catalysts that were prepared using the concept of bimodal support [18]. Thus, many studies on the FTS using Fe-based catalysts have mainly focused on the preparation and characterization of the catalysts and the kinetics of the FTS reaction [1945]. So far, however, there have been no detailed reports both on the yield of alternative aviation fuel equivalent to kerosene and the carbon mass balance in the FTS involving Fe-based catalysts. In order to develop the highly-efcient, i.e. lower in cost, process of alternative aviation fuel production by CTL, BTL or GTL, the primary kerosene yield and carbon mass balance in the FTS have to be investigated to obtain the data for the process simulation performed in future. In the present study, the FTS of syngas using an Fe-based catalyst is experimentally examined in terms of the primary kerosene yield and carbon mass balance in order to produce HCs such as alternative aviation fuel. In other words, the present study is a fundamental one in order to guarantee the quantitative characteristic of FTS. The effects of the reduction gases, time of Fe-based catalyst, reaction temperature and the chemical species included in the Febased catalyst on the FTS are investigated.
ions. The catalyst was dried overnight in an oven at 363 K and calcined in an electrical mufe furnace at 573 K for 4 h. The catalyst was ground to a particle size of less than 0.56 mm. The catalysts prepared in this study are as follows: reduced Fe (commercial reagent), Fe 100% (only Fe(NO3)39H2O as the metal nitrate), and Fe:Cu = 10:1, Fe:Mg = 10:1, Fe:Ca = 10:1, and Fe:K = 10:1 (molar ratio); henceforth, these are referred to as reduced Fe, Fe 100%, Fe/Cu, Fe/Mg, Fe/Ca and Fe/K, respectively. The molar ratio of metal promoters to Fe was determined by Ref. [17]. In the study, structural promoters such as H-ZSM-5 zeolite was not use because we would like to investigate the basic characteristics of the FTS using Febased catalysts without structural promoters [15]. 2.2. Characterization of prepared catalyst The distribution of the particle diameters of the prepared catalysts was determined by using a laser-scattering particle size distribution analyzer (Horiba LA-910). The average particle diameters of the catalysts were observed to be 117, 340, 257, 276, 344 and 343 lm for reduced Fe, Fe 100%, Fe/Cu, Fe/Mg, Fe/ Ca and Fe/K, respectively. Except for reduced Fe, the difference between the distribution characteristics of the particle diameters of the prepared catalysts was very small. The BET surface area of the prepared catalysts was measured by using an adsorption method (Quantachrome Autosorb-1) with N2 as the adsorbent. The surface areas were observed to be 0.11, 26.67, 6.76, 10.65, 6.81 and 10.06 m2/g for reduced Fe, Fe 100%, Fe/Cu, Fe/Mg, Fe/Ca and Fe/K, respectively. Among the prepared catalysts, the surface areas of reduced Fe and Fe 100% were the smallest and largest, respectively. 2.3. In situ catalyst reduction and FTS reaction procedure The in situ catalyst reduction and the FTS reaction were carried out in a downdraft continuous-ow-type xed-bed reactor, as shown in Fig. 1. An amount of 2.5 cm3 of the prepared catalyst was mixed with 2.5 cm3 of quartz sand (Nacalai Tesque, Japan) and the mixture was placed in the reactor, which is denoted by 3 in Fig. 1. The catalyst was reduced in situ by the reduction gas H2:CO:N2 with feed ratios of 1:0:1, 2:1:3 or 1:1:2 at 573 K and 0.1 MPa; the space velocity (W/F) was 1320 g h/mol and the reduction durations were 208, 340 or 480 min, respectively. The FTS reaction was carried out using the syngas H2:CO:N2 with a feed ratio of 1:1:2 at 533, 553 or 573 K and 3.0 MPa; the W/F was 13 20 g h/mol and the FTS reaction duration was 300 min. The gas sampled at the gas-sampling port, denoted by GP in Fig. 1, during the FTS reaction was analyzed online by a GCTCD (Shimadzu GC-14B); the length and inner diameter of the column used were 2 m and 3 mm, respectively; the column was packed with 30/60 mesh Molecular Sieve 5A (MS-5A) with He as the carrier gas. The gas collected in the gas bag, denoted by 7 in Fig. 1, after the FTS reaction was analyzed ofine by two GCTCDs (Shimadzu GC-14B and Yanaco G-1880) having an identical column length of 2 m and an identical inner diameter of 3 mm; the two columns were packed with 30/60 mesh MS5A and 80/100 mesh Porapack Q with He and Ar carrier gases, respectively. The HC gas and liquid products collected in the gas bag and liquid recovery unit, denoted by 7 and 4 in Fig. 1, respectively, were analyzed offline by GCFID (Hewlett Packard 5890A-GC) with a capillary column (Frontier Lab. Ultra Alloy-PY1, 30 m 0.25 mm ID) coated by polydimethylsiloxane (0.25 lm lm thickness) with N2 as the carrier gas; and by the GCMS (Shimadzu QP-5050) with a capillary column (Frontier Lab. Ultra Alloy-1 (MS/HT), 30 m 0.25 mm ID) coated by polydimethylsiloxane (0.25 lm lm thickness) with He as the carrier gas.
2. Experimental 2.1. Preparation of Fe-based FTS catalyst The Fe-based FTS catalysts were prepared by the method given in reference [15]. Fe(NO3)39H2O, Cu(NO3)23H2O, Mg(NO3)26H2O, Ca(NO3)24H2O, KNO3, Na2CO3 and reduced Fe were commercially available (Nacalai Tesque, Japan) and used without further purication. In the precipitation reaction, 400 ml of a mixed aqueous solution of metal nitrates and 400 ml of an aqueous solution of Na2CO3 were added dropwise simultaneously to 400 ml of stirred hot water at 333 K and pH 8; the mixture was then stirred for a further duration of 1 h. The resulting gel precipitate was collected by ltration and washed with 300 ml of distilled water to remove Na+
2090
K. Kumabe et al. / Fuel 89 (2010) 20882095
Valve 1 N2 cylinder Valve 2 Reducing gas cylinder Valve 3 Syngas cylinder 1 SV PG TC 2 3 F 4
1. Mass flow controller 2. Ribbon heater 3. Fixed bed 4. Liquid recovery unit 5. Back-pressure regulator 6. Gas meter 7. Bag for gas recovery 6 7
PG: Pressure gauge SV: Safety valve TC: K-type thermocouple GP: Gas-sampling port F: Filter
5 Valve GP
Fig. 1. Schematic diagram of experimental setup for in situ catalyst reduction and FTS reaction.
3. Results and discussion 3.1. Effect of reduction gases of Fe-based catalyst on FTS The effect of the reduction gases (H2:CO:N2 with feed ratios of 1:0:1, 2:1:3 and 1:1:2) of the Fe-based catalyst on the FTS was investigated by examining the in situ reduction of the Fe catalyst (Fe 100%) for an FTS time of 480 min and under a reaction temperature of 553 K. The change in CO conversion for each in situ reduction gas with the FTS time is shown in Fig. 2. The CO conversions became almost constant after an FTS time of approximately 1 h. The CO conversions during the FTS after the catalyst reductions with CO remained in more than 90% for 4 h, while the amount of CO converted in the presence of a reduction gas devoid of CO was lower and increased approximately from 15% to 20% during the 4-h constant-state period during the FTS. The overall CO conversions were 26%, 94% and 93% for H2:CO:N2 with feed ratios of 1:0:1, 2:1:3, and 1:1:2, respectively. This suggested that there was a low activity in the Fe-based catalyst when the reduction gas did not contain CO; as a result, the FTS reaction was progressively enhanced. The selectivity of CO to CO2, HCs and other compounds in the FTS for different in situ reduction gases is shown in Table 1. The HCs, which are equal to the sum of parafn and olen, in C1C2, C3C4, C5, C6C10, C11C14, C15C17, C18C19 and C20+ were dened in this paper as fuel gas, liqueed petroleum gas (LPG), solvent, gasoline, kerosene, light oil, heavy oil and wax, respectively,
Table 1 Selectivities of CO to CO2, HCs and other compounds in the FTS for different in situ reduction gases. Total selectivity (%) HCs selectivity (%) Others selectivity (%)
H2:CO:N2 with feed ratio of 1:0:1 20.6 CO2 C1C2 26.6 26.6 C3C4 32.7 32.6 C5 7.9 7.8 C6C10 2.9 1.8 C11C14 3.9 2.2 C15C17 2.4 1.7 C18C19 1.2 0.9 C20+ 4.3 3.5 Total 102.4 77.1
0.0 0.0 0.1 1.0 1.7 0.7 0.3 0.9 4.7
H2:CO:N2 with feed ratio of 2:1:3 36.0 CO2 C1C2 6.7 6.6 C3C4 4.9 4.6 C5 1.7 1.1 C6C10 14.3 8.9 C11C14 11.1 9.1 C15C17 5.7 5.1 C18C19 2.6 2.4 C20+ 10.6 9.9 Total 93.6 47.7
0.0 0.3 0.5 5.5 2.0 0.6 0.2 0.8 10.0
100 90 H2:CO:N2 = 1:0:1 H2:CO:N2 = 1:0:1 H2:CO:N2 = 2:1:3 H2:CO:N2 = 2:1:3 H2:CO:N2 = 1:1:2 H2:CO:N2 = 1:1:2
H2:CO:N2 with feed ratio of 1:1:2 36.4 CO2 C1C2 19.6 19.6 C3C4 14.6 14.4 C5 2.4 2.2 C6C10 7.4 4.3 C11C14 6.6 4.7 C15C17 3.7 3.0 C18C19 2.0 1.6 C20+ 7.3 5.8 Total 100.0 55.7
0.0 0.1 0.2 3.1 1.8 0.7 0.4 1.5 7.9
CO conversion [%] .
80 70 60 50 40 30 20 10 0 0 50 100 150
200
250
300
350
Time [min]
Fig. 2. Change in CO conversion with FTS time for each in situ reduction gas.
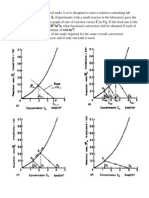
by referring to Refs. [4648]. The carbon mass balances were almost 100%. The others in Table 1 contained alcohol as the major component and ester and aldehyde as the minor components. In C6 + HCs, the selectivity of CO to kerosene at FTS was the second highest, the highest being the selectivity of CO to wax. Among the different feed ratios of the reduction gas, the selectivity to C1C4 was higher for the reduction gases of H2:CO:N2 with feed ratios of 1:0:1 and 1:1:2, while that to C6+ was higher for H2:CO:N2 with a feed ratio of 2:1:3. The errors in the repeatability of the selectivities obtained in the present study are observed to be within approximately 5%. Changes in the selectivity of CO with a carbon number of more than 6 in the FTS for each in situ reduction gas are shown in Fig. 3.
K. Kumabe et al. / Fuel 89 (2010) 20882095
2091
4 3.5 3
H2:CO:N2 = 1:0:1
Others Olefin Paraffin
2.5 2 1.5 1 0.5 0
The chain growth probabilities (a) were 0.89, 0.91 and 0.87 for feed ratios of 1:0:1, 2:1:3, and 1:1:2, respectively. Theoretically, an a value of 0.850.90 is suitable for the production of kerosene and light oil [49]; this is convenient for the present study. However, the a values obtained in the present study were much higher than that in the previous study [18,40]. It is known that the larger pore of a catalyst leads to the greater a value [50]. Thus, this might be caused by that the catalysts prepared in the present study had larger pores, or that it did not really progress to pore blockage with produced C6 + HCs such as wax because the reaction time was relatively shorter (5 h). The FTS of syngas with an Fe-based catalyst rst produces olen via the reduction of Fe2O3 to Fe and the production and hydrogenation of iron carbide as follows [16]: Catalyst reduction
Selectivity [%]
Fe2 O3 3H2 ! 2Fe 3H2 O
Iron carbide production
4 3.5 3
2CO 2H2 5Fe ! 2CFe2:5 2H2 O
H2:CO:N2 = 2:1:3
Iron carbide hydrogenation
2nCFe2:5 2nH2 ! 2CH2 n 5nFe
Selectivity [%]
Olen production (overall reaction)
2.5 2 1.5 1 0.5 0
nCO 2nH2 ! Cn H2n nH2 O
These reactions suggest that the use of CO in addition to H2 for catalyst reduction is better for the FTS of syngas using a Fe-based catalyst. This leads to a low conversion of CO during the FTS after the catalyst reduction in the absence of CO. Parafn is produced by the hydrogenation of olen as follows: Olen hydrogenation
Cn H2n H2 ! Cn H2n2
4 3.5 3
H2:CO:N2 = 1:1:2
On the other hand, olen reacts with H2 and CO to form aldehyde; this reaction is known as the oxo reaction. The oxo reaction is followed by the hydrogenation of aldehyde to form alcohol [51]: Oxo reaction
Cn H2n CO H2 ! Cn H2n1 CHO
Aldehyde hydrogenation
Selectivity [%]
2.5 2 1.5 1 0.5 0 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46
Cn H2n1 CHO H2 ! Cn1 H2n3 OH
Thus, we believe that these reactions caused the production of aldehyde and alcohol in addition to olen and parafn during the FTS. 3.2. Effect of reduction time of Fe-based catalyst on FTS The effect of the reduction time (208, 340 and 480 min) of the Fe-based catalyst on the FTS was investigated by the in situ reduction of the catalyst (Fe 100%) under the reduction gas H2:CO:N2 with a feed ratio of 2:1:3 in the FTS reaction at a temperature of 553 K. The change in CO conversion with the FTS time for each in situ reduction time is shown in Fig. 4. The CO conversion for the reduction time of 480 min became almost constant after approximately 1 h of FTS, while the CO conversion for the reduction times of 208 and 340 min did not. The CO conversion during the FTS after reduction for 480 min remained more than 95% for 4 h, while the CO conversions during the FTS after reductions for 208 and 340 min decreased with the FTS time. Finally, the overall CO conversions were 57%, 87% and 94% for the reduction times of 208, 340 and 480 min, respectively. This suggested that the catalyst activity progressively decreased due to the production of wax as the FTS reaction progressed; this can be explained by the fact that the production of CFe2.5, which is an active site in the
Carbon number [-]
Fig. 3. Changes in selectivity of CO with carbon number (greater than 6) for each in situ reduction gases.
The maximum carbon number detected in the liquid products for the reduction gases of H2:CO:N2 with feed ratios of 1:0:1 and 1:1:2 was 40, while the maximum carbon number corresponding to the reduction gas H2:CO:N2 with a feed ratio of 2:1:3 was 46. The selectivity to C1114 was higher in C6 + HCs. The selectivity to olen was higher than that to parafn for the feed ratio of 1:0:1. The selectivities to olen and others decreased with an increase in the carbon number for feed ratios of 2:1:3 and 1:1:2.
2092
K. Kumabe et al. / Fuel 89 (2010) 20882095
100 90
CO conversion [%] .
80 70 60 50 40 30 20 10 0 0 50 100 150 200 250 300 350 208 min 340 min 480 min
the liquid products during the FTS for the reduction times of 208 and 340 min was 40, while that for 480 min was 46. This suggested that the chain growth was inhibited as a result of the reduction in the production of active sites on the surface of the catalyst [16,18]. The production of CFe2.5 on the surface of the catalyst during reduction was better for the FTS of syngas with an Fe-based catalyst, as discussed in the previous section. Thus, we believe that it takes more than 8 h to produce an adequate number of active sites on the surface of the catalyst under the conditions considered in the present study. 3.3. Effect of reaction temperature on FTS The effect of the reaction temperature (533, 553 and 573 K) on the FTS was investigated by the in situ reduction of the Fe catalyst (Fe 100%) using the reduction gas H2:CO:N2 with a feed ratio of 2:1:3 for 480 min. The change in CO conversion at each reaction temperature with the FTS time is shown in Fig. 5. The CO conversions for the reaction temperatures of 553 and 573 K became almost constant after an FTS time of approximately 1 h, while the CO conversion for the reaction temperature of 533 K did not. The CO conversions during the FTS at 553 and 573 K remained more than 90% for 4 h, while the CO conversion at 533 K decreased with the FTS time. This suggested that the catalyst activity progressively decreased because of a reduction in the production of active sites on the surface of the catalyst with a reduction in the FTS reaction temperature [52]. Finally, the overall CO conversions were 71%, 94% and 94% for the FTS reaction temperatures of 533, 553 and 573 K, respectively. This result was in good agreement with that of a previous study [15]. The selectivities of CO to CO2, HCs, and others during FTS for each reaction temperature are shown in Table 3. The selectivity to C1C4 was higher for the FTS reaction temperatures of 533 and 573 K, while that to C6+ was higher for the reaction temperature of 553 K. The a values were 0.88, 0.91 and 0.86 for the FTS reaction temperatures of 533, 553 and 573 K, respectively. The maximum carbon numbers detected in the liquid products during the FTS were 40, 46 and 41 for the FTS reaction temperatures of 533, 553 and 573 K, respectively. It has been suggested that the chain growth is inhibited at 533 K due to the low FTS reaction temperature [16,18]. It has also been suggested that the methanation reaction (Eq. (8)) proceeded at 573 K because the selectivities to CO2 and C1C4 were lower and higher, respectively, due to the high FTS reaction temperature [15]:
Time [min]
Fig. 4. Change in CO conversion with FTS time for different in situ reduction times.
FTS, on the surface of the catalyst with the progression of Eqs. (1) and (2) was low due to a short reduction time of the catalyst [16,18,52]. The selectivities of CO to CO2, HCs and others during the FTS for each in situ reduction time are shown in Table 2. The selectivity to C1C4 was higher for the reduction times of 208 and 340 min, while that to C6+ was higher for 480 min. The a values were 0.90, 0.88 and 0.91 for the reduction times of 208, 340 and 480 min, respectively. The maximum carbon number detected in
Table 2 Selectivities of CO to CO2, HCs and others during the FTS for each in situ reduction time. Total selectivity (%) 208 min CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18-C19 C20+ Total 340 min CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total 480 min CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total 22.5 24.5 23.4 4.5 3.7 5.0 3.0 1.9 8.8 97.3 37.1 25.3 17.3 3.3 5.7 5.3 2.5 1.3 6.8 104.7 36.0 6.7 4.9 1.7 14.3 11.1 5.7 2.6 10.6 93.6 HCs selectivity (%) Others selectivity (%)
24.5 23.3 4.4 2.1 3.3 2.4 1.5 7.5 69.1
0.0 0.1 0.1 1.7 1.7 0.6 0.3 1.3 5.7
100
25.3 17.2 0.7 3.1 3.5 2.1 1.1 5.5 58.5 0.0 0.1 2.7 2.6 1.7 0.4 0.3 1.3 9.1
90 . CO conversion [%] . 80 70 60 50 40 30 20 10 0 0 50 100 150 200 250 300 350 533 K 553 K 573 K
6.6 4.6 1.1 8.9 9.1 5.1 2.4 9.9 47.7
0.0 0.3 0.5 5.5 2.0 0.6 0.2 0.8 10.0
Time [min]
Fig. 5. Change in CO conversion with FTS time for different reaction temperatures.
K. Kumabe et al. / Fuel 89 (2010) 20882095 Table 3 Selectivities of CO to CO2, HCs and others during FTS for each reaction temperature. Total selectivity (%) 533 K CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total 553 K CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total 573 K CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total 38.2 17.7 13.7 2.5 5.1 6.4 3.9 2.4 9.4 99.2 36.0 6.7 4.9 1.7 14.3 11.1 5.7 2.6 10.6 93.6 35.4 23.5 16.1 2.1 6.8 5.7 2.9 1.4 5.0 99.0 HCs selectivity (%) Others selectivity (%)
2093
Reduced Fe Fe 100% 100 90
Fe/Cu Fe/Mg
Fe/Ca Fe/K
CO conversion [%] .
17.7 13.6 2.4 3.1 4.2 3.0 1.9 7.9 53.8
0.0 0.1 0.1 2.0 2.2 0.9 0.5 1.4 7.2
80 70 60 50 40 30 20 10 0 0 50 100 150 200 250 300 350
6.6 4.6 1.1 8.9 9.1 5.1 2.4 9.9 47.7
0.0 0.3 0.5 5.5 2.0 0.6 0.2 0.8 10.0
Time [min]
Fig. 6. Change in CO conversion with FTS time for different Fe-based catalysts.
23.5 16.0 1.9 3.6 3.6 2.1 1.0 3.7 55.4
0.0 0.1 0.2 3.2 2.1 0.8 0.4 1.3 8.1
Methanation
CO2 4H2 ! CH4 2H2 O;
DH573 177 kJ=mol
3.4. Effect of chemical species included in Fe-based catalyst on FTS The effect of the chemical species (reduced Fe, Fe 100%, Fe/Cu, Fe/Mg, Fe/Ca, and Fe/K) included in the Fe-based catalyst on the FTS was investigated by the in situ reduction of each catalyst using the reduction gas of H2:CO:N2 with a feed ratio of 2:1:3 for 480 min and at the FTS reaction temperature of 553 K. The change in CO conversion for each Fe-based catalyst with the FTS time is shown in Fig. 6. The CO conversions for reduced Fe, Fe 100% and Fe/Cu became almost constant after about 1 h of FTS, while that for Fe/Mg, Fe/Ca, and Fe/K did not. The CO conversions during FTS with reduced Fe, Fe 100% and Fe/Cu remained in almost constant for 4 h, while the CO conversion during FTS with Fe/Mg, Fe/Ca and Fe/K decreased with the FTS time. This suggested that the catalyst activities were maintained due to the decrease in the production of wax resulting from the very small surface area of 0.11 m2/g in the case of reduced Fe, the large surface area of 26.67 m2/g in the case of Fe 100% and the fact that Cu promoted the reduction of Fe despite the small surface area of Fe/Cu (6.76 m2/g) [15]. On the other hand, it appeared that the catalytic activities progressively decreased due to the smaller surface areas of Fe/Mg and Fe/Ca (10.65 and 6.81 m2/g respectively) and due to the fact that the production of wax was promoted by K in Fe/K [17,52]. Finally, the overall CO conversions in FTS were 24%, 94%, 95%, 57%, 90% and 94% for reduced Fe, Fe 100%, Fe/Cu, Fe/Mg, Fe/Ca and Fe/K, respectively. The CO conversion during FTS using reduced Fe was very low due to the very small surface area of the catalyst, while that
in the case where Fe 100% was used was high due to the large surface area of the catalyst. It was proved that the catalyst activity of Mg was low because the CO conversion during the FTS using Fe/Mg was low due to the small surface area of the catalyst. The CO conversions during the FTS were high for Fe/Cu, Fe/Ca and Fe/K despite the small surface areas of these catalysts because Cu promoted the reduction of Fe, Ca had a high catalyst activity, and K promoted the production of wax, respectively [15,17]. The selectivities of CO to CO2, HCs and other compounds during FTS for each Fe-based catalyst are shown in Table 4. The selectivity to C1C4 was high when reduced Fe was used, while that to C6+ was high when Fe 100% was used. The a values were 0.89 and 0.91 for reduced Fe and Fe 100%, respectively. The maximum carbon numbers detected in the liquid products during FTS were 40 and 46 for reduced Fe and Fe 100%, respectively. The selectivity to olen was higher than that to parafn in C6+ HCs in the case of reduced Fe. This suggested that the chain growth and parafn production due to olen hydrogenation were inhibited because the production of active sites on the surface of the catalyst was low due to the low surface area of the catalyst [16,18]. The selectivity to C1C4 was high in the cases of Fe/Cu, Fe/Mg and Fe/Ca, while that to C20+ was high in the case of Fe/K. The a values were 0.89, 0.89, 0.88 and 0.91 for Fe/Cu, Fe/Mg, Fe/Ca and Fe/K, respectively. The maximum carbon numbers detected in the liquid products during FTS were 42, 44, 46 and 46 for Fe/Cu, Fe/Mg, Fe/Ca and Fe/K, respectively. It was proved that the chain growth was inhibited due to the lower surface areas of the catalysts in the cases of Fe/Cu, Fe/Mg and Fe/Ca, while chain growth was promoted as a result of K contributing to an increase in the production of wax when Fe/K was used [17]. The results from the present study showed that the selectivity to kerosene during FTS was the second highest in C6 + HCs, the highest being the selectivity to wax; C6 + HCs was produced during FTS using an Fe-based catalyst. The high selectivity to wax is characteristic of FTS using an Fe-based catalyst [13]. The primary production of kerosene must be maximized to efciently produce alternative aviation fuel. However, it is difcult to produce only kerosene by FTS because the product distribution of HCs formed during FTS generally conforms to the AndersonSchultzFlory (ASF) distribution [49]. Thus, the hydrocracking of wax should be carried out as a part of the purication process after FTS in order to enhance the total kerosene yield.
2094
K. Kumabe et al. / Fuel 89 (2010) 20882095
Table 4 Selectivities of CO to CO2, HCs and others during FTS for each Fe-based catalyst. Total selectivity (%) Reduced Fe CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total Fe 100% CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total Fe/Cu CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total Fe/Mg CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total Fe/Ca CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total Fe/K CO2 C1C2 C3C4 C5 C6C10 C11C14 C15C17 C18C19 C20+ Total 9.0 38.5 34.1 6.5 2.5 3.4 2.2 0.8 3.9 100.8 36.0 6.7 4.9 1.7 14.3 11.1 5.7 2.6 10.6 93.6 36.9 17.5 14.0 3.0 5.6 6.4 3.2 1.7 7.4 95.6 33.8 23.2 18.8 3.5 2.8 3.5 2.1 1.2 6.4 95.3 34.1 25.4 18.3 3.5 5.4 5.1 2.8 1.6 7.1 103.3 35.4 11.1 9.3 2.3 8.8 9.8 5.6 3.2 16.4 102.0 HCs selectivity (%) Others selectivity (%)
38.5 34.1 6.3 1.3 2.1 1.6 0.6 3.2 87.6
0.0 0.0 0.2 1.2 1.3 0.6 0.2 0.7 4.2
6.6 4.6 1.1 8.9 9.1 5.1 2.4 9.9 47.7
0.0 0.3 0.5 5.5 2.0 0.6 0.2 0.8 10.0
H2:CO:N2 with a feed ratio of 2:1:3 was used and the catalyst reduction time was 8 h at an FTS reaction temperature of 553 K. The results from the present study suggested that 1.0 and 1.8 L of kerosene were produced from 10 kgd.b of brown coal and woody biomass, respectively [53]. It is possible to compare this with the bench-scale production capacity of the BTL plant, which is 1.9 L of hydrocarbon liquid from 13 kg of woody biomass per day, in the National Institute of Advanced Industrial Science and Technology (AIST), Japan [54]. Kerosene for aviation fuel mainly consists of parafn (about 85%) [12]. An Fe-based catalyst is generally low cost and suitable for the CTL [13,14]. However, olen and CO2 are relatively much produced with an Fe-based catalyst, as shown in the present paper. In addition, it is believed that the GTL and BTL processes will be developed in the future because CO2 emissions from these processes are lower than that from CTL [55]. On the other hand, parafn is relatively much produced with a Co-based catalyst [13]. Thus, it will be an issue in the future to study the primary production of alternative aviation fuel by FTS with a Co-based catalyst suitable for the GTL and BTL.
4. Conclusions The FTS of syngas was carried out using an Fe-based catalyst in order to produce HCs equivalent to kerosene. The FTS reaction was conducted in a downdraft continuous-ow-type xed-bed reactor under temperatures of 533573 K and a pressure of 3.0 MPa. The effects of the reduction gases and time of Fe-based catalyst, FTS reaction temperature and chemical species included in the Febased catalyst on FTS were studied by focusing on primary kerosene yield and the carbon mass balances. The following conclusions were drawn: (1) The carbon mass balances were almost 100%. (2) The selectivity of CO to C1114 HCs was the second highest in C6 + HCs, the highest being the selectivity to C20 + HCs. (3) The activity of the Fe-based catalyst decreased when the reduction gas did not contain CO, resulting in the progressive enhancement of FTS. (4) The catalyst activity during FTS increased with the catalyst reduction time. (5) The catalyst activity during FTS increased with the reaction temperature. However, for 573 K, the selectivity of CO to C1C4 was higher. (6) Chain growth and parafn production were inhibited and promoted by the reduced Fe reagent and the prepared Fe catalyst, respectively. The chain growth was inhibited due to the small surface areas of Fe/Cu, Fe/Mg and Fe/Ca, while it was promoted due to the fact that K increased the production of wax when the Fe/K catalyst was used. (7) The production of kerosene was maximum when the prepared Fe catalyst was devoid of other chemical species and the catalyst reduction gas H2:CO:N2 with a feed ratio of 2:1:3 was used for a catalyst reduction time of 8 h at the FTS reaction temperature of 553 K. Acknowledgements This work was partially supported by a Grant-in-aid from the EcoTopia Science Institute, Nagoya University, Japan in 2008. The authors are deeply grateful to Dr. Koji Takahashi (Nagoya Municipal Industrial Research Institute, Japan) and Prof. Yukio Hirata (Toyohashi University of Technology, Japan) for their support in the characterization of Fe-based catalysts and the GCFID analysis, respectively.
17.5 13.8 2.8 3.3 4.6 2.5 1.4 5.9 51.7
0.0 0.1 0.2 2.3 1.9 0.6 0.3 1.5 6.9
23.2 18.7 3.4 1.5 2.3 1.7 1.0 5.6 57.6
0.0 0.0 0.1 1.3 1.2 0.4 0.2 0.8 3.9
25.4 18.3 3.3 3.1 3.4 2.2 1.3 5.6 62.5
0.0 0.1 0.2 2.3 1.7 0.6 0.4 1.4 6.7
11.0 9.2 2.0 5.6 7.5 4.8 2.7 13.3 56.1
0.1 0.1 0.3 3.2 2.3 0.9 0.5 3.1 10.5
The production of kerosene was the maximum under the following conditions in this study: when the prepared Fe catalyst was devoid of other chemical species, the catalyst reduction gas
K. Kumabe et al. / Fuel 89 (2010) 20882095
2095
References
[1] Boeing 2009. Available from: www.boeing.com/commercial/cmo. [2] BP 2009. Available from: www.bp.com/productlanding.do?categoryId= 6929&contentId=7044622. [3] Penner EJ, Lister HD, Griggs JD, Dokken JD, McFarland M. Aviation and the global atmosphere. Intergovernmental Panel on Climate Change; 1999. [4] Europa 2009. Available from: europa.eu/rapid/pressReleasesAction.do? reference=IP/06/1862. [5] IATA 2009. Available from: www.iata.org/whatwedo/cargo/environment.htm. [6] Brewer DG, Morris ER, Lange HR, Moore WJ. Study of the application of hydrogen fuel to long-range subsonic transport aircraft. Report NASA CR132559: National Aeronautics and Space Administration; 1975. [7] Escher DJW, Brewer DG. Hydrogen: make-sense fuel for an American supersonic transport. J Aircr 1975;12(1):310. [8] Reshotko E. Drag reduction by cooling in hydrogen-fueled aircraft. J Aircr 1979;16(9):58490. [9] Solash J, Hazlett NR, Hall MJ, Nowack JC. Relation between fuel properties and chemical composition. 1. Jet fuels from coal, oil shale and tar sands. Fuel 1978;57:5218. [10] Tsuchida K, Hayashi K, Kumabe K, Hasegawa T. Assessment on alternative aviation fuels from perspectives of petroleum depletion and environmental restrictions in the future. In: Proceedings of international symposium on eco topia science 2007 (ISETS07). Nagoya, Japan; 2007. [11] ASTM International 2009. Available from: www.astm.org. [12] Wikipedia 2009. Available from: en.wikipedia.org/wiki/Kerosene. [13] Bian G, Koizumi N, Yamada M. Recent development of FischerTropsch synthesis catalyst. J Jpn Inst Energ 2002;81:97480 (in Japanese). [14] Nacalai tesque for general catalog, vol. 29. Nacalai Tesque, Inc.; 2007 (in Japanese). [15] Yoneyama Y, He J, Morii Y, Azuma S, Tsubaki N. Direct synthesis of isoparafn by modied FischerTropsch synthesis using hybrid catalyst of iron catalyst and zeolite. Catal Today 2005;104:3740. [16] Hayakawa H, Tanaka H, Fujimoto K. Studies on precipitated iron catalysts for FischerTropsch synthesis. Appl Catal 2006;A310:2430. [17] Farias MEF, Sales GF, Fernandes NAF. Effect of operating conditions and potassium content on FischerTropsch liquid products produced by potassium promoted iron catalyst. J Nat Gas Chem 2008;17:1758. [18] Zhang Y, Bao J, Nagamori S, Tsubaki N. A new and direct preparation method of iron based bimodal catalyst and its application in FischerTropsch synthesis. Appl Catal 2009;A352:27781. [19] Dictor AR, Bell TA. FischerTropsch synthesis over reduced and unreduced iron oxide catalyst. J Catal 1986;97:12136. [20] Raje PA, Davis HB. FischerTropsch synthesis over iron-based catalysts in a slurry reactor. Reaction rates, selectivities and implications for improving hydrocarbon productivity. Catal Today 1997;36:33545. [21] Li S, OBrien JR, Meitzner DG, Hamdeh H, Davis HB, Iglesia E. Structural analysis of unpromoted Fe-based FischerTropsch catalysts using X-ray absorption spectroscopy. Appl Catal 2001;A219:21522. [22] Li S, Krishnamoorthy S, Li A, Meitzner DG, Iglesia E. Promoted iron-based catalysts for the FischerTropsch synthesis: design, synthesis, site densities, and catalytic properties. J Catal 2002;206:20217. [23] Wang Y-N, Ma W-P, Lu Y-J, Yang J, Xu Y-Y, Xiang H-W, et al. Kinetics modelling of FischerTropsch synthesis over an industrial FeCuK catalyst. Fuel 2003;82:195213. [24] Zhang C-H, Yang Y, Teng B-T, Li T-Z, Zheng H-Y, Xiang H-W, et al. Study of an ironmanganese FischerTropsch synthesis catalyst promoted with copper. J Catal 2006;237:40515. [25] Yang J, Sun Y, Tang Y, Liu Y, Wang H, Tian L, et al. Effect of magnesium promoter on iron-based catalyst for FischerTropsch synthesis. J Mol Catal 2006;A 245:2636. [26] Zhang C-H, Wan H-J, Yang Y, Xiang H-W, Li Y-W. Study on the ironsilica interaction of a co-precipitated Fe/SiO2 FischerTropsch synthesis catalyst. Catal Commun 2006;7:7338. [27] Wan H-J, Wu B-S, Tao Z-C, Li T-Z, An X, Xiang H-W, et al. Study of an iron-based FischerTropsch synthesis catalyst incorporated with SiO2. J Mol Catal 2006;A 260:25563. [28] An X, Wu B, Hou W, Wan H, Tao Z, Li T, et al. The negative effect of residual sodium on iron-based catalyst for FischerTropsch synthesis. J Mol Catal 2007;A 263:26672. [29] Li T, Yang Y, Zhang C, An X, Wan H, Tao Z, et al. Effect of manganese on an ironbased FischerTropsch synthesis catalyst prepared from ferrous sulfate. Fuel 2007;86:9218.
[30] Wan H-J, Wu B-S, An X, Li T-Z, Tao Z-C, Xiang H-W, et al. Effect of Al2O3 binder on the precipitated iron-based catalysts for FischerTropsch synthesis. J Nat Gas Chem 2007;16:1308. [31] Tao Z, Yang Y, Zhang C, Li T, Ding M, Xiang H, et al. Study of manganese promoter on a precipitated iron-based catalyst for FischerTropsch synthesis. J Nat Gas Chem 2007;16:27885. [32] Chang J, Bai L, Teng B-T, Zhang R-L, Yang J, Xu Y-Y, et al. Kinetic modeling of FischerTropsch synthesis over FeCuKSiO2 catalyst in slurry phase reactor. Chem Eng Sci 2007;62:498391. [33] Wan H-J, Wu B-S, Li T-Z, Tao Z-C, An X, Xiang H-W, et al. Effects of SiO2 and Al2O3 on performances of iron-based catalysts for slurry FischerTropsch synthesis. J Fuel Chem Technol 2007;35(5):58994. [34] Li T, Yang Y, Tao Z, Wan H, An X, Zhang C, et al. Effect of sulfate on an iron manganese catalyst for FischerTropsch synthesis. J Nat Gas Chem 2007;16:35462. [35] Hou W, Wu B, Yang Y, Hao Q, Tian L, Xiang H, et al. Effect of SiO2 content on iron-based catalysts for slurry FischerTropsch synthesis. Fuel Process Technol 2008;89:28491. [36] Lohitharn N, Goodwin Jr GJ, Lotero E. Fe-based FischerTropsch synthesis catalysts containing carbide-forming transition metal promoters. J Catal 2008;255:10413. [37] Zhao G, Zhang C, Qin S, Xiang H, Li Y. Effect of interaction between potassium and structural promoters on FischerTropsch performance in iron-based catalysts. J Mol Catal 2008;A286:13742. [38] Lohitharn N, Goodwin Jr GJ. Impact of Cr, Mn and Zr addition on Fe Fischer Tropsch synthesis catalysis: Investigation at the active site level using SSITKA. J Catal 2008;257:14251. [39] Pour NA, Shahri KMS, Bozorgzadeh RH, Zamani Y, Tavasoli A, Marvast AM. Effect of Mg, La and Ca promoters on the structure and catalytic behavior of iron-based catalysts in FischerTropsch synthesis. Appl Catal 2008;A 348:2018. [40] Lohitharn N, Goodwin Jr GJ. Effect of K promotion of Fe and FeMn Fischer Tropsch synthesis catalysts: Analysis at the site level using SSITKA. J Catal 2008;260:716. [41] Lohitharn N, Goodwin Jr GJ. An investigation using SSITKA of chain growth on Fe and FeMnK FischerTropsch synthesis catalysts. Catal Commun 2009;10:75862. [42] Smit de E, Beale MA, Nikitenko S, Weckhuysen MB. Local and long range order in promoted iron-based FischerTropsch catalysts: a combined in situ X-ray absorption spectroscopy/wide angle X-ray scattering study. J Catal 2009;262:24456. [43] Qin S, Zhang C, Xu J, Wu B, Xiang H, Li Y. Effect of Mo addition on precipitated Fe catalysts for FischerTropsch synthesis. J Mol Catal 2009; A304:12834. [44] Li T, Yang Y, Tao Z, Zhang C, Xiang H, Li Y. Study on an ironmanganese FischerTropsch synthesis catalyst prepared from ferrous sulfate. Fuel Process Technol 2009;90:124751. [45] Abbaslou MMR, Tavassoli A, Soltan J, Dalai KA. Iron catalysts supported on carbon nanotubes for FischerTropsch synthesis: effect of catalytic site position. Appl Catal 2009;A367:4752. [46] Graaf de J, Beuzekom van M. Clean energy and gasication technology. J Jpn Inst Energ 2002;81:104755 (in Japanese). [47] Wikimedia Foundation, Inc., USA; 2009. Available from: en.wikipedia.org/ wiki/Alkane. [48] Sekiyumodo, 2009. Available from: www.geocities.jp/yanakennjj/ sekiyumodo.htm [in Japanese]. [49] Suehiro Y. Leading-edge trend and vision of GTL technologies. J Combust Soc Jpn 2007;49:927 [in Japanese]. [50] Fan L, Yokota K, Fujimoto K. Supercritical phase FischerTropsch synthesis: catalyst pore-size effect. AIChE J 1992;38:163948. [51] Jpn Inst Energ, editor. Annual energy reviews-2007. J Jpn Inst Energ 2008;87:541697 [in Japanese]. [52] Anderson BR. The FischerTropsch synthesis. New York: Academic Press; 1984. [53] Kumabe K, Hanaoka T, Fujimoto S, Minowa T, Sakanishi K. Co-gasication of woody biomass and coal with air and steam. Fuel 2007;86:6849. [54] Hanaoka T, Liu Y, Matsunaga K, Hirata S, Sakanishi K. Bench-scale production of hydrocarbon liquid fuel from woody biomass via gasication. J Jpn Inst Energ 2007;86:73743. [55] Tsuchida K, Hayashi K, Kumabe K, Hasegawa T. Comparative study on CTL, GTL and BTL as alternative aviation fuels. Kankyo Joho Kagaku Ronbunshu 2009;23:22732 [in Japanese].
Anda mungkin juga menyukai
- Citric Acid ProductionDokumen20 halamanCitric Acid ProductionDiana MuñozBelum ada peringkat
- Constants For DaubertDokumen1 halamanConstants For DaubertsaadiisBelum ada peringkat
- Kmol/m xl0 M /S, What Fractional Conversion Will Be Obtained If Each of M ?Dokumen2 halamanKmol/m xl0 M /S, What Fractional Conversion Will Be Obtained If Each of M ?saadiisBelum ada peringkat
- Furnace FiguresDokumen1 halamanFurnace FiguressaadiisBelum ada peringkat
- CP302 Example 02 OKDokumen4 halamanCP302 Example 02 OKsaadiis100% (1)
- Chapter 9 Mass TransferDokumen64 halamanChapter 9 Mass TransfersaadiisBelum ada peringkat
- HAZOP Case Studies For Workshop SessionDokumen4 halamanHAZOP Case Studies For Workshop Sessionsaadiis100% (2)
- 3200 Types of Waste Heat RecoveryDokumen30 halaman3200 Types of Waste Heat Recoverysaadiis100% (2)
- 413 Topic v-3 (Run-Around Coil Systems, Regenerative Heat Ex Changers and Pinch Technology)Dokumen41 halaman413 Topic v-3 (Run-Around Coil Systems, Regenerative Heat Ex Changers and Pinch Technology)Preethish PrakashBelum ada peringkat
- Matlab and Gams DifferenceDokumen20 halamanMatlab and Gams DifferencesaadiisBelum ada peringkat
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (345)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- OH-6A Misshap Experience ReportDokumen21 halamanOH-6A Misshap Experience ReportrobbertmdBelum ada peringkat
- Guide To Out of Phase Tasks and MaintenaceDokumen1 halamanGuide To Out of Phase Tasks and MaintenacesaradeepsBelum ada peringkat
- Service Bulletin: Teledyne Continental Aircraft Engine Category 3Dokumen4 halamanService Bulletin: Teledyne Continental Aircraft Engine Category 3Kevin ArteagaBelum ada peringkat
- FS V2N1Dokumen9 halamanFS V2N1JMMPdosBelum ada peringkat
- FT Guinea (Mini)Dokumen32 halamanFT Guinea (Mini)Kamran AhmadBelum ada peringkat
- Airport Information: Details For SHAH MOKHDUMDokumen6 halamanAirport Information: Details For SHAH MOKHDUMTanvir HasanBelum ada peringkat
- Book BindDokumen4 halamanBook BindnathanBelum ada peringkat
- Epwaefhk PDF 1698772419Dokumen20 halamanEpwaefhk PDF 1698772419Antoni GarciaBelum ada peringkat
- 8 S 20Dokumen4 halaman8 S 20wielkiromanBelum ada peringkat
- Clio II Super 1600 enDokumen104 halamanClio II Super 1600 enAlexandru TerciuBelum ada peringkat
- Renewables Made in Germany 2010 2011Dokumen255 halamanRenewables Made in Germany 2010 2011Franz OrtegaBelum ada peringkat
- Pre ATPL Maths and Physics Revision Course-1Dokumen23 halamanPre ATPL Maths and Physics Revision Course-1filzovoc90% (10)
- Idoc - Pub Embraer-145 PDFDokumen9 halamanIdoc - Pub Embraer-145 PDFRina PngBelum ada peringkat
- APW Airplane MaintenanceDokumen64 halamanAPW Airplane Maintenancenovoair.techservicesBelum ada peringkat
- Gyrobee PlansDokumen131 halamanGyrobee PlansRichard Neville100% (10)
- J Fluids Engineering 2009 Vol 131 N7Dokumen132 halamanJ Fluids Engineering 2009 Vol 131 N7Нильва Александр100% (1)
- Radar DecoyDokumen2 halamanRadar DecoyAnonymous OeXzBaNyBBelum ada peringkat
- SunExpress 2023 BroschuereDokumen17 halamanSunExpress 2023 BroschuereMateo Rofman100% (1)
- Bo 105Dokumen60 halamanBo 105Chan Sin Loon100% (4)
- Lgav VFR Routes LgpaDokumen9 halamanLgav VFR Routes LgpaHubert LindenthalerBelum ada peringkat
- Citation CJ4 Specification & DescriptionDokumen30 halamanCitation CJ4 Specification & DescriptionLas Ukcu100% (4)
- Critical SpeedDokumen3 halamanCritical SpeedbamarppBelum ada peringkat
- Laboratory Experiment 3: Mohd Ashraf Mohd IsmailDokumen29 halamanLaboratory Experiment 3: Mohd Ashraf Mohd IsmailMohd Ashraf Mohd IsmailBelum ada peringkat
- Aviation MeteorologyDokumen5 halamanAviation MeteorologyrsisinternationalBelum ada peringkat
- 2151903Dokumen14 halaman2151903hunnyBelum ada peringkat
- Rto 41422 - Cricos: 03590DDokumen16 halamanRto 41422 - Cricos: 03590Dtesfaye awelBelum ada peringkat
- Airline Safety Assessment Mechanism: Safe TravelDokumen64 halamanAirline Safety Assessment Mechanism: Safe TravelFazrulBelum ada peringkat
- The Gyroplane Its Principles and Its Possibilities by Louis BreguetDokumen56 halamanThe Gyroplane Its Principles and Its Possibilities by Louis BreguetHerba4100% (1)
- Programs Hp35sDokumen16 halamanPrograms Hp35sRodolfo Sergio Cruz FuentesBelum ada peringkat