Kinetics of Transalkylation
Diunggah oleh
chantran90Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Kinetics of Transalkylation
Diunggah oleh
chantran90Hak Cipta:
Format Tersedia
Sunil K. Maity is presently with NITRourkela, India. Sunil_maity2002@yahoo.co.in This paper is Archived in dspace@nitr http://dspace.nitrkl.ac.
in/dspace
Presented at CHEMCON, Ankleshwar, Gujarat, India, 2006.
Kinetics of Transalkylation of Diisopropylbenzenes with Benzene
Sunil K. Maitya, CH. Seetaramb, and Narayan C. Pradhanc,*
Department of Chemical Engineering, Indian Institute of Technology, Kharagpur -721302, India. Email: sunil_maity@che.iitkgp.ernet.in, b seetu009@rediffmail.com, c ncp@che.iitkgp.ernet.in
a
ABSTRACT
Transalkylation is an important class of reaction to utilize low value by-products of alkylation reactions. Diisopropylbenzenes (DIPBs) are such by-products formed during the production of cumene by isopropylation of benzene. In the present work, the DIPBs were utilized to produce value-added chemical like cumene by transalkylation with benzene. Transalkylation reactions were carried out in the vapor phase over commercial H-mordenite catalyst in a fixed-bed down-flow laboratory scale reactor. The influences of various process parameters such as temperature, space-time, and benzene-to-DIPB mole ratio on conversion were studied. The experiments were carried out to choose the zone in which the mass transfer effects were negligible. A detailed literature review was made establish a suitable mechanism to explain the course of the reaction. Key words: Transalkylation; Diisopropylbenzene; Cumene; H-mordenite; Kinetics.
INTRODUCTION
Transalkylation is an important class of reaction to convert low value polyalkylated by-products to commercially more important chemical intermediates or end products. Transalkylation is the reaction between two similar or dissimilar molecules involving transfer of an alkyl group. This reaction is commercially very interesting as some of the low valued polyalkylated byproducts can be converted to their monosubstituted or disubstituted homologues from dialkylated and trialkylated byproducts respectively, which are in higher demand and of higher value. For example, xylenes are the key raw materials for polyesters, plasticizers and engineering plastics. The major sources of these aromatic hydrocarbons, the reforming and pyrolysis gasoline, contains appreciable amount of toluene and trimethylbenzenes (TMB). A convenient way to increase xylenes production is to transform less valuable toluene and TMB by catalytic transalkylation. This transformation was carried out over various types of zeolite catalyst like Hbeta, H-mordenite, H-omega, Y, NaHY; molecular sieves like SAPO-5, transition metals Substituted aluminophosphate; and hybrid heteropoly acid/zeolite catalysts. [1-6] Ethyl benzene on the other hand is commercially important intermediate for the manufacture of styrene which is an important monomer in the production of synthetic rubber, plastics and resins. The worlds more than 90% of ethyl benzene demand is met through benzene ethylation. However, benzene ethylation is usually accompanied by the formation of large amount diethylbenzenes isomers. Many processes were developed to convert diethylbenzenes isomers byproduct to increase the yield of ethylbenzene by transalkylation with benzene over various types of zeolite catalyst like -zeolite, faujasite Y, beta, Y etc. [7-9] Yet another example of transalkylation reaction is the selective synthesis of 4-alkyl- or 4,4-dialkyl biphenyls or naphthalenes. These compounds find many applications as valuable industrial intermediates to produce thermotropic liquid crystals or heat-resistant polymers. The direct alkylation of biphenyls or naphthalenes with alcohols (methanol and ethanol) gives rise to undesirable product distribution. However, transalkylation of these compounds with polyalkylbenzenes (byproducts of alkylation reaction) was found to be highly shape selective as it proceeds through an extremely bulky transition state. Recently this approach was used for selective transmethylation [10], transethylation [11] and transisopropyalation [12] of biphenyl and naphthalene. Cumene is an important intermediate for the production of phenol and acetone. Conventionally it is produced by isopropylation of benzene. However, during the isopropylation of benzene to cumene, 5-10 wt% diisopropylbenzene (DIPB) isomers are produced as by-product. These isomers are diverted to the fuel pool in many commercial plants. This results in additional raw material consumption affecting the economy of the whole process. The one way of utilizing DIPBs is to produce valuable chemicals such as cymenes, by transalkylating with toluene [13-16] in a separate
CHEMCON, Ankleshwar, Gujarat, India, 2006.
transalkylation reactor. Cymenes are important starting materials for the production of a range of intermediates and end products, such as cresols, fragrances, pharmaceuticals, herbicides, heat transfer media, etc. Another way of utilizing DIPBs is to produce cumene by transalkylation with benzene [15-16]. In this case, the DIPBs (or heavy fractions) can be separated from alkylated product stream by conventional methods and recycled to produce cumene either in the same alkylation reactor or in a separate transalkylation reactor. Only a few studies were reported in open literature on transalkylation of DIPB with benzene. For example, Suresh et al. [15] studied this reaction under liquid phase conditions over sulfate-treated superacidic zirconia catalyst in the tepmperature range of 175-225 0C. It was also reported the use of large pore zeolites (H-mordenite, La-H-Y, and H-beta) for this reaction under liquid phase condition in the temperature range of 170-2100C both in atmospheric and high pressure condition (25 kg/cm2) [16]. Many processes were also developed using various types of zeolite catalyst for transformation of DIPB to cumene using pure isomers of DIPB or their mixture or cumene bottoms under liquid phase conditions and at high pressure [17-26]. However, detail kinetic study for such an important system is not reported in open literature under vapour phase conditions at atmospheric pressure using zeolite catalyst. Considering the importance of the system; the detail kinetic study was carried out under vapour phase conditions at atmospheric pressure over commercial H-mordenite catalyst using mixture of DIPB isomers (2:1: m-: p-) as the feed (since an industrial cumene bottom fraction consist of these isomers). It is well known to catalyze the alkylation of aromatics with a variety of conventional Friedel Crafts catalysts. These materials are highly corrosive to process equipment, create operational problems, and are often difficult to dispose of in an environmentally acceptable manner. Therefore, nowadays these catalysts are being replaced by Zeolites and zeolite-like materials which are shown to be promising catalysts for conversion of alkylaromatics due to their high activity, selectivity, environmental safety and avoidance of corrosion. Present study is thus carried out using protonic form of mordenite (H-M) catalyst.
EXPERIMENTAL
Materials. Benzene (99.7%) of GR grade was procured from Merck (India) Ltd., Mumbai, India. Diisopropylbenzenes (98%), 2: 1 mixture of m- and p-isomer, was obtained from Acros Organics, New Jersey, USA. H-mordenite in the form of 1/16 inch extrudate was obtained from Sud-Chemie India Pvt. Ltd., Baroda, India. Experimental Setup. The catalytic experiments were carried out in a fixed-bed, continuous downflow cylindrical stainless steel (SS 316) tubular laboratory scale reactor (0.014m i.d. and 0.16m in length). The reactor was fitted with a preheater in the upstream and a condenser at its outlet. The reactor was heated electrically from outside and insulated to prevent heat loss. The temperature of the reactor and preheater was measured by a thermocouple placed in a thermowell placed at the centre of the respective parts of the setup. Procedure. In a typical run, about 0.0035 kg of catalyst was loaded into the reactor and supported by inert beads on either side of the bed. The catalyst was activated in situ for 2 h in an atmosphere of nitrogen before the experimental runs were started. The benzene-DIPB mixture was introduced with the help of a metering pump and vaporized in the pre-heater before contacting the catalyst. The reactant vapors along with nitrogen entered the reactor from the top. The product vapors, along with un-reacted reactants, were condensed in the condenser and the liquid samples collected. Products Analysis. The liquid samples collected from the condenser were analyzed in a gas liquid chromatography (GLC) using a 2 m3 mm stainless steel column packed with 10% OV-17 on Chromosorb W (80/100). A Chemito Model 8610 GC interfaced with a Shimadzu C-R6A Chromatopac data processor was used for the analysis. The column temperature was programmed with an initial temperature at 100 0C, increased at 10 0C/min to 250 0C. Nitrogen was used as the carrier gas with a flow rate of 15 cm3/min. An injector temperature of 150 0C was used during the analysis. An FID detector was used at the temperature of 3000C. The material balance was checked and it was >98%.
CHEMCON, Ankleshwar, Gujarat, India, 2006.
Transalkylation of DIPB with benzene: H C H CH C SN1 Mechanism
3 3
H3C H CH3 C + + H3C CH CH3
H3C H CH3 C
(1)
CH CH3
SN2 Mechanism
H3C
H3C H CH3 C
H3C H CH3 H3C H CH3 C C H3C
CH CH3 H3C
H3C CH3
CH3 CH H
(2)
Cumene-n-PB isomerisation:
CH3 CH + CH3
CH3 H C H CH3
H3C
C H2
H2 C
(3)
Scmeme 1.
RESULTS AND DISCUSSION
Tranalkylation of 2:1 mixture of m- and p-DIPB with benzene to produce cumene was carried out over H-mordenite catalyst. The n-propylbenzene (n-PB) was obtained as the byproduct. Reaction Mechanism. The DIPB undergo transalkylation with benzene to produce cumene. The The cumene formed in the reaction may also undergo isomerisation forming n-PB. Two mechanisms (Scheme 1) are generally used to describe tranalkylation/ disproportination types of reactions over zeolite catalysts: (1) SN1 mechanism in which alkyl carbenium ions were supposed as intermediate (2) SN2 mechanism involving bimolecular intermediates, in which two aromatic rings are bridged by a C-atom of the alkyl group. The various parameters can affect the relative proportion of the two mechanisms. As for example, disproportionation of n-PB over various zeolites (USY, beta, mordenite, ZSM-5) shows that reaction mechanism depends on the internal pore structures of zeolites [27]. The preference for n,n-propylbenzene (n,n-PB) formation decreases in the order of USY > beta > mordenite > ZSM-5. The ZSM-5 has inadequate internal pore space required for the formation of biphenylmethane intermediates as in the SN2 mechanism. Therefore reaction proceeds via SN1 mechanism that results statistical distribution of n,n-PB and n,i-PB. Sometimes stability of carbenium ions determines the mechanism of the reaction. For example, for xylene transformation over zeolite Y, transalkylation can not proceed through SN1 mechanism as it leads to the formation of unstable methenium ions [28]. Conversion of 1- and 2-methylnaphthalene over zeolites with different pore widths viz. HZSM-5 (0.54 0.56 nm), HZSM-12 (0.570.61 nm), and HY (0.74 nm) shows that transalkylation is the major reaction, besides isomerisation, on HY catalyst. On zeolite HZSM-12 and HZSM-5, there is virtually no transalkylation for both 1-methylnaphthalene and 2-methylnaphthalene although the zeolite is active in isomerization. This may be due to the fact that the voids inside HZSM-12 and HZSM-5 are not spacious enough to accommodate the very bulky transition states required for the bimolecular transalkylation reactions of naphthalene derivatives [29]. Table 2. Effect of Benzene-to-DIPB Mole Ratio on Conversion and Products Selectivity. a Benzene- Conversion Selectivity (%) to-DIPB (%) of cumene n-PB mole ratio DIPBs 1.16 0 4.0 100 2.25 0 5.0 100 6.5 4.69 94.9 5.1 8.6 4.73 95.3 4.7 12.2 6.59 96.3 3.7 19.3 9.24 95.9 4.1 a Conditions: pressure = 1atm; temperature = 493 K; space-time =19.7 h. kg catalyst/kmol; nitrogen-to-feed mole ratio = 0.5. Table 1. Effect of Space-Time on Conversion and Products Selectivity.a Space-time, Conversion (%) Selectivity (%) h. kg Benzene DIPB cumene ncatalyst/ PB kmol 11.26 0.12 1.44 100 0 14.07 0.33 3.98 100 0 18.76 0.54 6.59 99.1 0.9 28.15 0.77 9.39 98.4 1.6 2.67 15.50 3.7 56.30 96.3 a Conditions: pressure = 1 atm; temperature = 493 K; benzene-to-DIPB mole ratio = 12.2; nitrogen-tofeed mole ratio = 0.5.
CHEMCON, Ankleshwar, Gujarat, India, 2006.
20
40
100
Cumene
80
16
Conversion of DIPB (%)
30
DIPB
60
Conversion (%)
12
20
40
n-PB
10
20
4
0
Benzene
490 500 510 520 530 540 550 560 570
100
200
300
400
500
600
Temperature (K)
Time-on-stream (min)
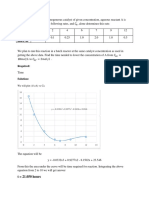
Fig 1. Effect of time-on-stream on conversion of DIPBs. Conditions: pressure = 1atm; space-time = 28.15 h. kg catalyst/kmol; benzene-to-DIPB mole ratio = 12.2; nitrogen-to-feed mole ratio = 0.5.
Fig. 2. Effect of temperature on conversion and products selectivity. Conditions: pressure = 1 atm; space-time = 11.26 h. kg catalyst/kmol; benzene-to-DIPB mole ratio = 12.2; nitrogen-to-feed mole ratio = 0.5.
During a study of cumene interaction with labeled benzene over medium pore H-ZSM-11 using 13C MAS NMR technique, the transalkylation and disproportionation reactions are not observed whereas this reaction proceeds over large pore mordenite. This can be explained by the space restrictions for the formation reaction intermediate in medium pore zeolites. The same experimental technique was also used to prove the existence of the intermolecular SN2 mechanism during the ethylbenzene transformations over mordenite catalysts [30]. In order to find out the cumene-n-PB isomerisation mechanism (intra or intermolecular) over HZSM-11; two experiments were carried out using in situ tracing MAS NMR techniques. (1) cumene labeled with 13C in the aromatic ring and (2) benzene labeled with 13C in the aromatic ring [30]. In both experiment, a benzeneNPB aromatic ring label transfer was observed. This confirmed unambiguously the intermolecular character of cumene isomerization. In order to prove whether the reaction proceeds through monomolecular SN1 or bimolecular SN2 mechanism, again two experiments were conducted using cumene labeled with 13C in the alkyl chain at (1) - (2) position [30]. The 13C label was observed to transfer into -position of n-PB in case of starting 13 C cumene and into - and -positions in the case of starting -13C cumene. This proves the bimolecular SN2 mechanism of cumene-n-PB isomerisation. Time-on-stream behavior of the catalyst. The stability of commercial H-mordenite was tested for about 10 hrs time-on-streams at 493 K under atmospheric pressure. The activity of the catalyst was found to decrease with time-on-stream as shown in the Fig. 1.The conversion of DIPB falls from about 20% to about 3% after 10 hrs time-on-stream as observed from the figure. The deactivation of the H-mordenite catalyst is largely due to the formation of coke on pores of the zeolite structure and its faster deactivation is attributed to its high acidity and monodimensional nature of pore structure [31]. Coke is formed preferentially on the strongest acid sites and causes their deactivation. The presence of one coke molecule in a channel makes all the acid sites of this channel inaccessible to the reactant from the end at which the molecule is located that results faster deactivation of the mordenite catalyst. However, the catalyst can be simply reactivated by hot benzene flush when necessary or the reactivation might also be accomplished by burn-off of carbonaceous deposits [32]. The conversion and selectivity of products reported in the subsequent experiments are therefore based on 30 min time-on-streams. Effect of space-time on conversion and products selectivity. The effect of space-time on conversion of benzene and DIPB and products distribution is shown in Table 1. As it is observed from the table, the conversion of both benzene and DIPB increases with increase in space-time as expected. However, the selectivity of cumene was observed to decrease with increase in spacetime whereas the selectivity of n-PB increases with space-time. With increase in space-time isomerisation of cumene to n-PB increases that result lower selectivity of cumene at higher spacetime.
Selectivity (%)
CHEMCON, Ankleshwar, Gujarat, India, 2006.
Table 3. Effect of External Diffusional Resistance on Conversion of DIPB a Space-time (h. Conversion (%) of DIPB kg b c catalyst/kmol) 11.26 1.40 1.44 18.76 6.62 6.59 28.15 9.45 9.39 a Conditions: pressure = 1 atm; temperature = 493 K; benzene-to-DIPB mole ratio = 12.2; nitrogen-to-feed mole ratio = 0.5. b = 0.0025 kg of catalyst. c= 0.0035 kg of catalyst.
Table 4. Effect of Intraparticle Diffusion on Conversion of DIPB a Partical Conversion (%) of DIPB at spacesize, dp time (h. kg catalyst/kmol) of 11.26 18.76 28.15 0.5 1.35 6.63 9.37 1.0 1.41 6.55 9.35 1.5 1.44 6.59 9.39 a Conditions: pressure = 1 atm; temperature = 493 K; benzene-to-DIPB mole ratio = 12.2; nitrogen-to-feed mole ratio = 0.5.
Effect of benzene-to-DIPB mole ratio on conversion and products selectivity. The effect of benzene-to-DIPB mole ratio on DIPB conversion and products selectivity was studied by varying the benzene-to-DIPB mole ratio from 4 to 19.3 as shown in Table 2. The conversion of DIPB was found to increase with increase in benzene-to-DIPB mole ratio. The selectivity of cumene was however found to be higher at lower mole ratio. Effect of Temperature on Conversion and Products Selectivity. The effect of temperature on conversion of benzene and DIPB and product distribution was studied in the temperature range of 493-573 K under otherwise identical experimental conditions as shown in the Fig. 2. As it is observed from the figure, the conversion of benzene and DIPB increases with increase in temperature as expected. The selectivity of cumene was observed to decrease with increase in temperature whereas opposite trend was observed for n-PB. At higher temperatures, isomerisation of cumene to n-PB increases, thereby decreasing the selectivity of cumene and increasing the selectivity of n-PB. During the alkylation of benzene with propylene over HZSM-5 catalysts, n-PB becomes a major product (36% selectivity) at 3000C and at temperatures below 2500C; cumene isomerization is not a favorable reaction since there is a wide departure from the thermodynamic equilibrium ratios calculated for PBs [33]. The contamination of the n-PB isomer in cumene product purification has a profound impact on the economics of the cumene manufacturing process. The minimization of isomer byproduct yield to the lowest acceptable level is therefore growing need for this process. It is therefore desirable to carry out the reaction at temperature below 2400C. A small amount of C9-11 was observed to form above 533 K and found to increase with temperature. However, the total amount of these compounds remains less than 0.5wt% even at 3000C and therefore not included in the results of Fig. 2. Mass transfer considerations. For any kinetic study, it is important that the mass transfer resistances be negligible during the reaction. To estimate the external diffusional effects, experiments were carried out at constant space-time and catalyst size, but with varying feed rates. The results of Table 3 indicate that the conversion of DIPB for both the series at constant W/F are independent of feed rate. Therefore, the external mass transfer resistance is negligible. Experiments were also conducted to test the intraparticle diffusional limitations by varying the catalyst particle size while keeping space-time constant. The experimental data obtained are presented in Table 4. The results showed that there was no change in conversion of DIPB with catalyst size indicating negligible intraparticle mass transfer resistance in the particle size range studied. The particle sizes employed in the kinetic study were within the intraparticle diffusion free range. In zeolite-catalyzed reactions, two types of diffusion processes are involved: (i) micropore diffusion inside the zeolite crystal and (ii) macropore diffusion between the zeolite crystals within the catalyst pellets. The above experiments for mass transfer resistances confirms only the absence of diffusion in the macropores. The resistance due to micropore could not be evaluated, as it requires a modification of the synthesis conditions of the zeolite that affect the micropore size of the crystals, which would subsequently affect the diffusional characteristics. COLCLUSIONS The commercial H-mordenite catalyst was successfully used to transalkylate DIPB with benzene to cumene under vapour phase conditions at atmospheric pressure. Significant amount of n-PB was
CHEMCON, Ankleshwar, Gujarat, India, 2006.
observed to form above 513 K by isomerisation of cumene. The catalyst was found to deactivate with time-on-stream due to the formation of coke on pores of the zeolite structure. The conversion of DIPB increases with increase in space-time, benzene-DIPB mole ratio, and temperature. The selectivity of cumene increases with decrease in space-time and reactor temperature. The mass transfer effects were found to be negligible. A detailed review was reported on mechanism of transalkylation and isomerisation reaction.
ACKNOWLEDGMENT
Sunil K. Maity is thankful to the All India Council for Technical Education (AICTE), New Delhi, India, for the award of the National Doctoral Fellowship during the tenure of this work.
REFERENCES
1. 2. 3. 4. 5. 6. 7. 8. Lee, Y.; Park, S.; Rhee, H. Catalysis Today 1998, 44, 223-233., Dumitriu, E.; Hulea, V.; Kaliaguine, S.; Huang, M.M. Appl. Cata. A: Gen.1996, 135, 57-81., Chao, K.; Leu, L. Zeolites 1989, 9, 193-196. Hulea, V.; Bilba, N.; Lupascu, M.; Dumitriu, E.; Nibou, D.; Lebaili, S.; Kessler, H. Microporous Materials 1997, 8, 201-206. Dumitriu, E.; Guimon, C.; Hulea, V.; Lutic, D.; Fechete, I. Appl. Cata. A: Gen. 2002, 237, 211 221. Sulikowski, B.; Rachwalik, R. Appl. Cata. A: Gen. 2003, 256, 173182. Cavani, F.; Corazzari, M.; Bencini, E.; Goffredi, G. Appl. Cata. A: Gen. 2002, 226, 3140. Ngandjui, L.M.T.; Louhibi, D.; Thyrion, F.C. Chemical Engineering and Processing 1997, 36, 133-141. Forni, L; Cremona, G.; Missineo, F.; Bellussi, G.; Perego, C.; Pazzuconi, G. Appl. Cata. A: Gen. 1995, 121, 261-272. Brechtelsbauer, C.; Emig, G. Appl. Cata. A: Gen. 1997, 161, 79-92. Takeuchi, G.; Shimoura, Y.; Hara, T. Appl. Cata. A: Gen. 1996, 137, 87-91. Nesterenko, N.S.; Kuznetsov, A.S.; Timoshin, S.E.; Fajula, F.; Ivanova; I.I. Appl. Cata. A: Gen. 2006, 307, 7077. Reddy, K.S.N.; Rao, B.S.; Shiralkar, V.P. Appl. Cata. A: Gen.1995, 121, 191-201. Bandyopadhyay, R.; Sugi, Y.; Kubota, Y.; Rao, B.S. Catalysis Today 1998, 44, 245-252. Suresh, R.; Rajadhyaksha, R.A.; Kumbhar, P.S. J. Chem. Tech. Biotechnol. 1995, 62, 268271. A.R. Pradhan, A.R.; Rao, B.S. Appl. Cata. A: Gen. 1993, 106, 143-153. Van Der Aalst, M. J; Samson, M. S.; Meima, G. R. Patent No. WO03049857, 2003. Clark, M. C.; Cimini, R. J.; Cheng, J. C.; Stern, D. L.; Buchanan, J. S. Patent No. US 20050075523, 2005. Slaugh, L. H. Patent No. US 4375574, 1983. Amarilli, S.; Carluccio, L.; Perego, C.; Bellussi, G. Patent No. US6005152, 1999. Weber, W.A; Smith, M.C.; Bryan, F.S.; Brown, S.H.; Cheng, J.C. US Patent No. 6518471, 2003. Guo-Shuh, J. L.; Garces, J. M.; Meima, G. R.; Van Der Aalst, M. J. M. Patent No. EP0433932, 1991. Suciu, G. D.; Kwon, J. T. Patent No. WO8912613, 1989. Innes; R.A.; Zones; S. I.; Nacamuli; G. J. Patent No. US4891458, 1990. Gajda, G. J.; Gajek, R. T. Patent No. US5522984, 1996. Cheng, J. C.; Helton, T. E.; Mazzone, D. N.; Walsh; D. E. Patent No. US 6919491, 2005. Tsai, T.; Wang, I. Journal of Catalysis 1992, 133, 136-145. Corma, A.; Llopis, F.; Monton, J. B. Journal of Catalysis 1993, 140, 384-394. Neuber, M.; Karge, H. G.; Weitkamp, J. Catalysis Today 1988, 3, 11-22. Ivanova, I. I.; Nesterenko, N. S.; Fernandez, C. Catalysis Today 2006, 113, 115125. Guisnet, M.; Magnoux, P. Applied Catalysis 1989, 54, 1-27. Lee, G. J.; Garces Juan M, Meima Garmt R, Van Der Aalst Matheus J. M. Patent number EP0433932, 1991. Kaeding, W. W.; Holland, R. E. Journal of Catalysis 1988, 109, 212-216.
9.
10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33.
Anda mungkin juga menyukai
- Transalkylation and Isomerization of Ortho-Diethylbenzene With Benzene Using Trifluoromethanesulphonic Acid Catalyst: Kinetic AnalysisDokumen7 halamanTransalkylation and Isomerization of Ortho-Diethylbenzene With Benzene Using Trifluoromethanesulphonic Acid Catalyst: Kinetic AnalysisKok huiBelum ada peringkat
- Microporous and Mesoporous MaterialsDokumen6 halamanMicroporous and Mesoporous MaterialskristianBelum ada peringkat
- An Efficient Procedure For Protection of Carbonyls Catalyzed by Sulfamic AcidDokumen6 halamanAn Efficient Procedure For Protection of Carbonyls Catalyzed by Sulfamic AcidVina Octavia AzzahraBelum ada peringkat
- Rapid Approach To Biobased Telechelics Through Two One-Pot Thiol-Ene Click ReactionsDokumen8 halamanRapid Approach To Biobased Telechelics Through Two One-Pot Thiol-Ene Click ReactionsbelkhamasBelum ada peringkat
- Final Project Optimization CasiDokumen13 halamanFinal Project Optimization CasiAlfredo IllescasBelum ada peringkat
- Feedstock Recycling of Polyethylene in A Two-Step Thermo-Catalytic Reaction SystemDokumen9 halamanFeedstock Recycling of Polyethylene in A Two-Step Thermo-Catalytic Reaction SystemZahid FarooqBelum ada peringkat
- Selective Extraction of Benzene From Benzene-Cyclohexane Mixture Using 1-Ethyl-3-Methylimidazolium Tetrafluoroborate Ionic LiquidDokumen8 halamanSelective Extraction of Benzene From Benzene-Cyclohexane Mixture Using 1-Ethyl-3-Methylimidazolium Tetrafluoroborate Ionic LiquidBüşraBelum ada peringkat
- Bahar Meryemoglu, Burcak Kaya, Sibel Irmak, Arif Hesenov, Oktay ErbaturDokumen4 halamanBahar Meryemoglu, Burcak Kaya, Sibel Irmak, Arif Hesenov, Oktay ErbaturNatalie FloresBelum ada peringkat
- 10.2478 - PJCT 2018 0006Dokumen22 halaman10.2478 - PJCT 2018 0006م.احمد سالمBelum ada peringkat
- HPU/06 - Hydrogen Production from Fossil and Biomass FuelsDokumen4 halamanHPU/06 - Hydrogen Production from Fossil and Biomass FuelsMazen OthmanBelum ada peringkat
- Coking Kinetics On The Catalyst During Alkylation of FCC Off-Gas With Benzene To EthylbenzeneDokumen7 halamanCoking Kinetics On The Catalyst During Alkylation of FCC Off-Gas With Benzene To EthylbenzeneAndres PeñaBelum ada peringkat
- Chemical Recycling of Polystyrene: D.S. Achilias, I. Kanellopoulou, P. MegalokonomosDokumen8 halamanChemical Recycling of Polystyrene: D.S. Achilias, I. Kanellopoulou, P. MegalokonomosLuizaBelum ada peringkat
- Synthesis & Characterization of Quinoxalines Using ENPFSA CatalystDokumen29 halamanSynthesis & Characterization of Quinoxalines Using ENPFSA CatalystPrasada Rao Ch MMBelum ada peringkat
- Yang 2016Dokumen36 halamanYang 2016Febrian Dimas Adi NugrahaBelum ada peringkat
- Energies: Catalytic Pyrolysis of Wild Reed Over A Zeolite-Based Waste CatalystDokumen9 halamanEnergies: Catalytic Pyrolysis of Wild Reed Over A Zeolite-Based Waste CatalystSiddharth SinghBelum ada peringkat
- Detailed kinetic analysis of isobutane oxidationDokumen18 halamanDetailed kinetic analysis of isobutane oxidationJuan CamiloBelum ada peringkat
- Energy Conversion and Management: Seik Lih Lee, Yong Chen Wong, Yen Ping Tan, Sook Yan YewDokumen7 halamanEnergy Conversion and Management: Seik Lih Lee, Yong Chen Wong, Yen Ping Tan, Sook Yan YewRonaldo CMBelum ada peringkat
- Industrial Catalytic Processes for Phenol ProductionDokumen15 halamanIndustrial Catalytic Processes for Phenol ProductionUzair WahidBelum ada peringkat
- All Green Microwave Assisted 99 Depolymerisation of Polyethylene Terephthalate Into Value Added Products Via Glycerol Pretreatment and Hydrolysis ReactionJournal of Polymers and The EnvironmentDokumen13 halamanAll Green Microwave Assisted 99 Depolymerisation of Polyethylene Terephthalate Into Value Added Products Via Glycerol Pretreatment and Hydrolysis ReactionJournal of Polymers and The EnvironmentDana MateiBelum ada peringkat
- EPL-0009812 ArticleDokumen14 halamanEPL-0009812 Articlerajesh kothariBelum ada peringkat
- Non-Catalytic Liquefaction of Microalgae in Sub and Supercritical AcetoneDokumen28 halamanNon-Catalytic Liquefaction of Microalgae in Sub and Supercritical Acetonejosè CarhuapomaBelum ada peringkat
- Preparation of Carbon-Based Solid Acid Catalysts UDokumen19 halamanPreparation of Carbon-Based Solid Acid Catalysts UJustin Marc EstiponaBelum ada peringkat
- Ethyl Benzene Plant DesignDokumen45 halamanEthyl Benzene Plant DesignfaridzawiBelum ada peringkat
- Modeling and analysis of a methanol synthesis process using a mixed reforming reactorDokumen10 halamanModeling and analysis of a methanol synthesis process using a mixed reforming reactorVidal TxusBelum ada peringkat
- Bambang Veriansyah, Jae Young Han, Seok Ki Kim, Seung-Ah Hong, Young Jun Kim, Jong Sung Lim, Young-Wong Shu, Seong-Geun Oh, Jaehoon KimDokumen8 halamanBambang Veriansyah, Jae Young Han, Seok Ki Kim, Seung-Ah Hong, Young Jun Kim, Jong Sung Lim, Young-Wong Shu, Seong-Geun Oh, Jaehoon Kimscorpion2001glaBelum ada peringkat
- Benzene XyleneChemicals 30052012Dokumen50 halamanBenzene XyleneChemicals 30052012Chakravarthy Bharath100% (1)
- Aspen HYSYS simulation of biomass pyrolysis for methanol productionDokumen6 halamanAspen HYSYS simulation of biomass pyrolysis for methanol productionAmna ShahbazBelum ada peringkat
- Design of EthylbenzeneDokumen5 halamanDesign of Ethylbenzenesahar vahdatifarBelum ada peringkat
- GRGGFDokumen4 halamanGRGGFGraciaVelitarioBelum ada peringkat
- Inui 2002Dokumen9 halamanInui 2002Rohit BabelBelum ada peringkat
- Bokade2008 PDFDokumen7 halamanBokade2008 PDFJiana NasirBelum ada peringkat
- Rheological Studies of MDPE with 1% MAPEDokumen11 halamanRheological Studies of MDPE with 1% MAPEiaydn1810Belum ada peringkat
- MetododologiaDokumen16 halamanMetododologiaLuis Lozano SBelum ada peringkat
- 14Dokumen14 halaman14Demetrius WoodardBelum ada peringkat
- Reference For R-101Dokumen9 halamanReference For R-101aibbycatalanBelum ada peringkat
- Computers and Chemical EngineeringDokumen11 halamanComputers and Chemical EngineeringEla syBelum ada peringkat
- Manufacturing Proceses for Styrene ProductionDokumen9 halamanManufacturing Proceses for Styrene ProductionMohd Zulazreen50% (2)
- Vinicius Rossa, Production of Solketal Using Acid Zeolites As CatalystsDokumen18 halamanVinicius Rossa, Production of Solketal Using Acid Zeolites As Catalystsnysa arientikaBelum ada peringkat
- Project: Design of A Reactor For The Aniline ProductionDokumen19 halamanProject: Design of A Reactor For The Aniline ProductionLUIS ESTEBAN VÁSQUEZ CASTANEDABelum ada peringkat
- Application of Sulfonated Carbon-Based Catalyst For The Furfuralproduction From D-Xylose and Xylan in A Microwave-Assisted BiphasicreactionDokumen6 halamanApplication of Sulfonated Carbon-Based Catalyst For The Furfuralproduction From D-Xylose and Xylan in A Microwave-Assisted BiphasicreactionRokhain VilBelum ada peringkat
- Plant DesignDokumen42 halamanPlant Designmuhammad ilyasBelum ada peringkat
- Gas: China: Volume One-Scienceand TechnologyDokumen4 halamanGas: China: Volume One-Scienceand TechnologyAnurita GhoshBelum ada peringkat
- Develop Diesel Fuel AdditivesDokumen9 halamanDevelop Diesel Fuel AdditivesidownloadbooksforstuBelum ada peringkat
- Methanol Production by CO Hydrogenation: Analysis and Simulation of Reactor PerformanceDokumen19 halamanMethanol Production by CO Hydrogenation: Analysis and Simulation of Reactor PerformancehelloBelum ada peringkat
- CiclohexanoDokumen6 halamanCiclohexanoSebastian BelloBelum ada peringkat
- Non Thermal Plasma, Libo Yao Et AlDokumen7 halamanNon Thermal Plasma, Libo Yao Et AlDaniel Emmanuel AyomideBelum ada peringkat
- CMC-g-PAN SynthesisDokumen8 halamanCMC-g-PAN SynthesisRisqi Putri Nur PriatmaBelum ada peringkat
- Process simulation of hydrogen production from producer gas using HTS catalysisDokumen33 halamanProcess simulation of hydrogen production from producer gas using HTS catalysisKaren GomezBelum ada peringkat
- Catalytic Conversion of Glycerol To Oxygenated Fuel Additive in A Continuos Flow Reactor - Process OptimizationDokumen7 halamanCatalytic Conversion of Glycerol To Oxygenated Fuel Additive in A Continuos Flow Reactor - Process OptimizationEduardo CarmineBelum ada peringkat
- Ethyl-Acetate Synthesis in Gas Phase by Immobilised LipaseDokumen6 halamanEthyl-Acetate Synthesis in Gas Phase by Immobilised LipaseMuhammad Abdur RokhimBelum ada peringkat
- Ethyl Benzene Plant DesignDokumen31 halamanEthyl Benzene Plant DesignRohit Kakkar100% (13)
- 2010 - Energy - Paper With Cover Page v2Dokumen8 halaman2010 - Energy - Paper With Cover Page v2Elhissin ElhissinnBelum ada peringkat
- Latest Edited KineticsDokumen14 halamanLatest Edited KineticsMasterTopupBelum ada peringkat
- 2016good and ImportantDokumen4 halaman2016good and ImportantMahdi koolivandBelum ada peringkat
- A Comparative Study of Physical and Chemical Method For Separation Ofbenzoic Acid From Industrial Waste Stream 2090 4568 1000161Dokumen11 halamanA Comparative Study of Physical and Chemical Method For Separation Ofbenzoic Acid From Industrial Waste Stream 2090 4568 1000161Zubair AslamBelum ada peringkat
- 4 Catalytic Performances ofDokumen5 halaman4 Catalytic Performances ofAchyut Kumar PandaBelum ada peringkat
- Aqueous-Phase Hydrodeoxygenation of Sorbitol With PT SiO2 Al2O3Dokumen12 halamanAqueous-Phase Hydrodeoxygenation of Sorbitol With PT SiO2 Al2O3Paulo CésarBelum ada peringkat
- List Project Topics For Final Year Chemical Engg. StudentsDokumen4 halamanList Project Topics For Final Year Chemical Engg. StudentssumitBelum ada peringkat
- Sustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeDari EverandSustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeBelum ada peringkat
- Catalytic Cracking of Cumene in Fluidized Bed Reactor With ZeoliteDokumen3 halamanCatalytic Cracking of Cumene in Fluidized Bed Reactor With Zeolitechantran90Belum ada peringkat
- PBE 1 Flyer Lug09 PDFDokumen8 halamanPBE 1 Flyer Lug09 PDFchantran90Belum ada peringkat
- P425-Modified Beta Zeolites For Alkylation of Benzene PDFDokumen2 halamanP425-Modified Beta Zeolites For Alkylation of Benzene PDFchantran90Belum ada peringkat
- Cumene Aspen PDFDokumen50 halamanCumene Aspen PDFUlises ValienteBelum ada peringkat
- Cumene Aspen PDFDokumen50 halamanCumene Aspen PDFUlises ValienteBelum ada peringkat
- Emmision STD Petrochemplants IndiaDokumen189 halamanEmmision STD Petrochemplants IndiaFinigan JoyceBelum ada peringkat
- Mono101 009 PDFDokumen24 halamanMono101 009 PDFchantran90Belum ada peringkat
- Kinetics of TransalkylationDokumen6 halamanKinetics of Transalkylationchantran90Belum ada peringkat
- Ind Eng - Chem.res - Dec.2001Dokumen23 halamanInd Eng - Chem.res - Dec.2001chantran90Belum ada peringkat
- Jpvariko 2003Dokumen191 halamanJpvariko 2003chantran90Belum ada peringkat
- Kim 30 3 4 0501 17Dokumen9 halamanKim 30 3 4 0501 17chantran90Belum ada peringkat
- Dynamics Split 1Dokumen100 halamanDynamics Split 1chantran90Belum ada peringkat
- 50 6th Sem-C13-Kinetics Oct Substitution - Dr. Sunirban DasDokumen10 halaman50 6th Sem-C13-Kinetics Oct Substitution - Dr. Sunirban DasSIMARAN JAISWAL 41 M3SBelum ada peringkat
- Kinetics (Gjjkkkgty)Dokumen5 halamanKinetics (Gjjkkkgty)Chrysler Kane DepnagBelum ada peringkat
- CHM 112 Kinetics Practice Problems Answers - Reader ViewDokumen19 halamanCHM 112 Kinetics Practice Problems Answers - Reader ViewSyasya FaqihahBelum ada peringkat
- Ch14 TestbankDokumen53 halamanCh14 TestbankJeremy Martin100% (11)
- Soal LajuDokumen3 halamanSoal Lajufhandayani8Belum ada peringkat
- AMIT PPT Sem 1Dokumen21 halamanAMIT PPT Sem 1Uma VedulaBelum ada peringkat
- Biginille Reaction Mechanism PDFDokumen4 halamanBiginille Reaction Mechanism PDFshenn0Belum ada peringkat
- Tutorial Sheet 4 - CHEMICAL KINETICSDokumen4 halamanTutorial Sheet 4 - CHEMICAL KINETICSSaviour SichizyaBelum ada peringkat
- Methanol To Ole Fins (MTO) : From Fundamentals To CommercializationDokumen17 halamanMethanol To Ole Fins (MTO) : From Fundamentals To Commercializationsrijangupta1996Belum ada peringkat
- Introduction To Chemical Kinetics: CHEM 102 T. HughbanksDokumen16 halamanIntroduction To Chemical Kinetics: CHEM 102 T. HughbanksKarthikBelum ada peringkat
- Name Reactions: Detailed Reaction MechanismsDokumen4 halamanName Reactions: Detailed Reaction MechanismsvanbanbinhdinhBelum ada peringkat
- Chemical Reactor Theory: Unit 1Dokumen3 halamanChemical Reactor Theory: Unit 1rajaraghuramvarmaBelum ada peringkat
- Chemical Reduction of Methylene BlueDokumen9 halamanChemical Reduction of Methylene BluefredBelum ada peringkat
- 11 Chapter Reaction Kinetics Text Book Exercise PDFDokumen14 halaman11 Chapter Reaction Kinetics Text Book Exercise PDFBilal KhanBelum ada peringkat
- Vinyl Chloride from Acetylene and Hydrogen ChlorideDokumen6 halamanVinyl Chloride from Acetylene and Hydrogen ChlorideEvan Afrista Wiokartina PurbaBelum ada peringkat
- Elimination Reactions: Alkenes, Alkynes: Dr. Muntari, M. PhilDokumen64 halamanElimination Reactions: Alkenes, Alkynes: Dr. Muntari, M. PhilharyBelum ada peringkat
- Chemical Kinetics: Practice ExamplesDokumen31 halamanChemical Kinetics: Practice ExamplesJudith Del Valle MorejonBelum ada peringkat
- Acids QuizDokumen462 halamanAcids Quizwondimu0% (1)
- Inorganic Chemistry I (100 Items)Dokumen10 halamanInorganic Chemistry I (100 Items)maria jeusa matiasBelum ada peringkat
- Chemical Kinetics and Catalysis: Richard I: MaselDokumen7 halamanChemical Kinetics and Catalysis: Richard I: MaselShiv KumarBelum ada peringkat
- CHEM102 GuideDokumen16 halamanCHEM102 GuidenadyahginiceBelum ada peringkat
- A Molecular Approach ch13Dokumen117 halamanA Molecular Approach ch13Stephen100% (1)
- Organic Chemistry I Extra Synthesis Practice ProblemsDokumen6 halamanOrganic Chemistry I Extra Synthesis Practice ProblemsVinh HoangBelum ada peringkat
- Chemical Kinetics FactorsDokumen16 halamanChemical Kinetics FactorsjoaseBelum ada peringkat
- Essentials of Chemical KineticsDokumen49 halamanEssentials of Chemical KineticsJohn KanteBelum ada peringkat
- Gavhane Chemical EngineeringDokumen670 halamanGavhane Chemical EngineeringPriyal VargisBelum ada peringkat
- Review Questions - Solutions: Multiple ChoiceDokumen10 halamanReview Questions - Solutions: Multiple ChoiceMarikBelum ada peringkat
- ASR 2020 J2Prelim H2Chem P4 QP PDFDokumen20 halamanASR 2020 J2Prelim H2Chem P4 QP PDFchuasioklengBelum ada peringkat
- Zumdahl Chemical Kinetics NotesDokumen6 halamanZumdahl Chemical Kinetics NotesMalletNjonkemBelum ada peringkat
- Reaction Rate FactorsDokumen10 halamanReaction Rate Factorssb7204jBelum ada peringkat