Magic Milk Experiment
Diunggah oleh
api-210291577Deskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Magic Milk Experiment
Diunggah oleh
api-210291577Hak Cipta:
Format Tersedia
MAGIC MILK EXPERIMENT
Allison Cunningham
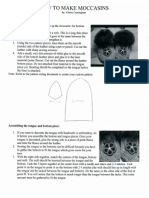
Materials Required: Shallow container (plate or petri dish works well) Milk (I used skim but any milk will work with a slightly different effect) Liquid food colouring (more colours = more fun!) Liquid dish soap Cotton swab (I used magic witch fingers for the entertainment value) Procedure: 1. Pour enough milk into the container to completely cover the bottom and allow it to settle (~1/4 inch). 2. Add a few drops of each of the colours of food coloring to the milk. 3. Ask students to try to make the colours move by only touching the milk in one spot with their finger (or cotton swab). Do they have the magic touch? (It's important not to stir the mix, just touch it with a finger or the tip of the cotton swab) 4. Demonstrate the magic by dipping your witch finger (or cotton swab) into soap (secretly) and then into the milk. Hold it for 10-15 seconds. 5. Notice that the food colouring streams away from the point where the soap touched the milk magic? Scientific Explanation: Liquids like water and milk have a property known as surface tension due to the cohesive forces of the liquid's molecules. This is why we see a meniscus along the edge of containers with liquid in them. The molecules in milk have a stronger attraction to each other than the container so the molecules pull away from the container into the center and create a concave meniscus. Since milk is mostly water, it has surface tension like water but milk is also made up of fat and protein molecules. Soaps are known as surfactants which means they reduce surface tension. Soap is a polar molecule with a hydrophilic head and a hydrophobic tail. The hydrophobic end is repelled by water but attracted to fat so when the soap is added to the milk mixture it moves around in search of fat molecules to attach to. As the soap molecules attach to the fat molecules they change the surface tension of the milk, lowing it in that area. Because the surrounding milk on the edge has a higher surface tension the surface is pulled to the edge away from the weaker soapy area. The food colouring moves with the surface, streaming away from the dish soap. Where This Fits Into the Ontario Science Curriculum: Gr. 8 Science Fluids Gr. 9 Applied Exploring Matter Gr. 10 Academic (SNC2D) - Chemical Reactions Gr. 10 Applied (SNC2P) - Chemical Reactions and Their Practical Application Chemistry Gr. 11 University (SCH3U) - Matter, Chemical Trends, and Chemical Bonding, Solutions and Solubility Sources: http://www.stevespanglerscience.com/experiment/milk-color-explosion http://www.nipissingu.ca/education/jeffs/4284Winter/PDFS/MagicMilk.pdf
Anda mungkin juga menyukai
- Observation Lesson Planning Record Keeping - C37Dokumen6 halamanObservation Lesson Planning Record Keeping - C37ramya100% (1)
- MS5002EDokumen8 halamanMS5002EMatt AgonyaBelum ada peringkat
- Preschool Daily Lesson Plan Word Free TemplateDokumen3 halamanPreschool Daily Lesson Plan Word Free TemplateArmie Yanga HernandezBelum ada peringkat
- Montessori MattersDokumen1 halamanMontessori MattersrahmearBelum ada peringkat
- Lesson Activity Plan Infant Toddler 1Dokumen2 halamanLesson Activity Plan Infant Toddler 1api-384820029Belum ada peringkat
- Dap For Infants & ToddlersDokumen7 halamanDap For Infants & Toddlersapi-264474189Belum ada peringkat
- Lesson Plan MoneyDokumen3 halamanLesson Plan Moneyapi-315049671100% (1)
- Water Conservation LessonDokumen4 halamanWater Conservation Lessonapi-269984318Belum ada peringkat
- Root Beer Float PlanDokumen9 halamanRoot Beer Float Planapi-282217475100% (1)
- Siop Insect Lesson Plan ADokumen8 halamanSiop Insect Lesson Plan Aapi-254452048Belum ada peringkat
- Classroom Management RationaleDokumen6 halamanClassroom Management Rationaleapi-300009409Belum ada peringkat
- Install Medium Voltage Lines SafelyDokumen106 halamanInstall Medium Voltage Lines Safelyajayi micheal sunday100% (1)
- Connections and Relationships: Frame of ReferenceDokumen6 halamanConnections and Relationships: Frame of Referenceapi-512324035Belum ada peringkat
- Y1-Y2 Thematic SyllabiDokumen25 halamanY1-Y2 Thematic Syllabiसंजेश सुधेश शर्माBelum ada peringkat
- Learning To Learn Positive Dispositions As A Learning CurriculumDokumen5 halamanLearning To Learn Positive Dispositions As A Learning CurriculumjiyaskitchenBelum ada peringkat
- Module 5 Raeesa D-17230Dokumen21 halamanModule 5 Raeesa D-17230Raeesa ShoaibBelum ada peringkat
- EDUC 487 - Social Studies TPADokumen3 halamanEDUC 487 - Social Studies TPAashleyb12Belum ada peringkat
- The Role of a Montessori GuideDokumen9 halamanThe Role of a Montessori Guideparadise schoolBelum ada peringkat
- Q2 M 1requirements To Start House of ChildremDokumen3 halamanQ2 M 1requirements To Start House of ChildremNighat Nawaz100% (4)
- Children'S Learning in The Early Childhood PhaseDokumen5 halamanChildren'S Learning in The Early Childhood Phasealvin n. vedarozagaBelum ada peringkat
- Questions and Answers: Montessori EducationDokumen1 halamanQuestions and Answers: Montessori Educationaiman kazmiBelum ada peringkat
- Calamba Central Elementary School Grade 2 SY 2020-2021Dokumen4 halamanCalamba Central Elementary School Grade 2 SY 2020-2021CarmilleBelum ada peringkat
- Lesson Plans Final Week 5Dokumen3 halamanLesson Plans Final Week 5api-301740289Belum ada peringkat
- Names: Gulshan Shehzadi Roll# D17090 Topic: Exercise of Practical Life (EPL)Dokumen18 halamanNames: Gulshan Shehzadi Roll# D17090 Topic: Exercise of Practical Life (EPL)Gulshan ShehzadiBelum ada peringkat
- Assignment: Name: Shamaila Kiran Roll No:D16633Dokumen38 halamanAssignment: Name: Shamaila Kiran Roll No:D16633shamaila kiranBelum ada peringkat
- Educ 456 TpaDokumen4 halamanEduc 456 Tpaashleyb12Belum ada peringkat
- Classroom Manangement PlanDokumen6 halamanClassroom Manangement PlanAnonymous lNXj4qAUBelum ada peringkat
- Semi-Detailed Lesson Plan in Science & Health ViDokumen6 halamanSemi-Detailed Lesson Plan in Science & Health ViJoyce Ann Magayanes100% (1)
- Piaget'S Stages of Cognitive Development: - Maria Rita D. Lucas, Ph. DDokumen25 halamanPiaget'S Stages of Cognitive Development: - Maria Rita D. Lucas, Ph. DMarione John MarasiganBelum ada peringkat
- Science 4 Q4 W3Dokumen6 halamanScience 4 Q4 W3LARLEN MARIE T. ALVARADOBelum ada peringkat
- Introduction To Preliminary Activities C38Dokumen2 halamanIntroduction To Preliminary Activities C38Marinella PerezBelum ada peringkat
- Socio Emotional Development of The PreschoolerDokumen8 halamanSocio Emotional Development of The PreschoolerNathalie GetinoBelum ada peringkat
- Phil of EducationDokumen5 halamanPhil of Educationapi-317143084Belum ada peringkat
- Montessori Way of Teaching - Importance of Sensorial Exercises in MontessoriDokumen3 halamanMontessori Way of Teaching - Importance of Sensorial Exercises in MontessoriṦafia NazBelum ada peringkat
- Kindergarten Science - PopcornDokumen8 halamanKindergarten Science - Popcornapi-336329266Belum ada peringkat
- Kindergarten-DLL Week 1 (June 3-7, 2019)Dokumen7 halamanKindergarten-DLL Week 1 (June 3-7, 2019)Ma. Cristina DumallagBelum ada peringkat
- ECCO Observation ToolDokumen54 halamanECCO Observation Toolucanreachallison100% (2)
- Punctuation PPT Grade 1Dokumen16 halamanPunctuation PPT Grade 1suresh04Belum ada peringkat
- ECED History FoundationsDokumen7 halamanECED History Foundationsmariegold mortola fabelaBelum ada peringkat
- Mathematics Lesson Plan WK 3Dokumen3 halamanMathematics Lesson Plan WK 3api-235320425100% (1)
- ECE 13 Module1Dokumen5 halamanECE 13 Module1Silver VioletBelum ada peringkat
- The NAMTA Journal - Vol. 39, No. 2 - Spring 2014: Ginni SackettDokumen20 halamanThe NAMTA Journal - Vol. 39, No. 2 - Spring 2014: Ginni SackettSaeed Ahmed BhattiBelum ada peringkat
- M3 by Multisori Complete Curriculum Sample, Ages 2-6Dokumen161 halamanM3 by Multisori Complete Curriculum Sample, Ages 2-6katecoffman.4Belum ada peringkat
- Mrs. Robinson's Weekly Newsletter: Mark Your CalendarDokumen1 halamanMrs. Robinson's Weekly Newsletter: Mark Your CalendarHeather Curran RobinsonBelum ada peringkat
- 4 10 2020 Weekly Learning Activities Early ChildhoodDokumen5 halaman4 10 2020 Weekly Learning Activities Early ChildhoodF RengifoBelum ada peringkat
- The Importance of Play for Child DevelopmentDokumen11 halamanThe Importance of Play for Child DevelopmentMoon RequinaBelum ada peringkat
- School Experience Reflection Journal Generaled 250Dokumen6 halamanSchool Experience Reflection Journal Generaled 250api-284809101Belum ada peringkat
- Historical Timeline 201Dokumen6 halamanHistorical Timeline 201api-451601708Belum ada peringkat
- Prac Lesson Plan Primary EnglishDokumen6 halamanPrac Lesson Plan Primary Englishapi-293527475Belum ada peringkat
- In Casa de BambiniDokumen2 halamanIn Casa de Bambiniparadise schoolBelum ada peringkat
- Benefits of Problem-Based and Project-Based Learning ApproachesDokumen13 halamanBenefits of Problem-Based and Project-Based Learning ApproachesMikah de LeonBelum ada peringkat
- Preschool CurriculumDokumen4 halamanPreschool CurriculumLenny Rose LlidoBelum ada peringkat
- Reggio Emilia ApproachDokumen4 halamanReggio Emilia Approachmohanraj2purushothamBelum ada peringkat
- Self-Evaluation Lesson Plan 1Dokumen2 halamanSelf-Evaluation Lesson Plan 1api-350083105Belum ada peringkat
- Volcano Experiment at HomeDokumen4 halamanVolcano Experiment at HomeJessa Mae CasipongBelum ada peringkat
- Exploring dimensions with Montessori cylinder blocksDokumen17 halamanExploring dimensions with Montessori cylinder blocksparadise schoolBelum ada peringkat
- The Exceptional Child Observation ProjectDokumen10 halamanThe Exceptional Child Observation Projectapi-524668763100% (1)
- Introduction of Math in MontessoriDokumen4 halamanIntroduction of Math in MontessoriṦafia NazBelum ada peringkat
- Oral Communication Module 1 Nature and Process of Communication FinalDokumen27 halamanOral Communication Module 1 Nature and Process of Communication FinalAngelrose AbrisBelum ada peringkat
- Magic MilkDokumen3 halamanMagic Milklahsivlahsiv684Belum ada peringkat
- " A Color Symphony " (Color Changing Milk) : Mico Casile III-St. Ignatius de LoyolaDokumen2 halaman" A Color Symphony " (Color Changing Milk) : Mico Casile III-St. Ignatius de LoyolaROSELYN DAVIDBelum ada peringkat
- Middle School ExperimentsDokumen9 halamanMiddle School ExperimentsMelody ApapeBelum ada peringkat
- Letter-Essay RubricDokumen2 halamanLetter-Essay Rubricapi-210291577Belum ada peringkat
- 1 - Moccasin Making LPDokumen4 halaman1 - Moccasin Making LPapi-210291577Belum ada peringkat
- Weekly ScheduleDokumen2 halamanWeekly Scheduleapi-210291577Belum ada peringkat
- Grade 4 Daily ScheduleDokumen1 halamanGrade 4 Daily Scheduleapi-210291577Belum ada peringkat
- Quizquiztrade OrganellesDokumen2 halamanQuizquiztrade Organellesapi-210291577Belum ada peringkat
- 2 - Porcpine Caribou Case Study LPDokumen4 halaman2 - Porcpine Caribou Case Study LPapi-210291577Belum ada peringkat
- 5 - Migration LPDokumen3 halaman5 - Migration LPapi-210291577Belum ada peringkat
- Yt FN ResourcesDokumen5 halamanYt FN Resourcesapi-210291577Belum ada peringkat
- 4 - Bioaccumulation LPDokumen3 halaman4 - Bioaccumulation LPapi-210291577Belum ada peringkat
- Moccasin How ToDokumen4 halamanMoccasin How Toapi-210291577Belum ada peringkat
- 3 - Parasite LPDokumen4 halaman3 - Parasite LPapi-210291577Belum ada peringkat
- Heat and Codcution LabDokumen3 halamanHeat and Codcution Labapi-210291577Belum ada peringkat
- Grade 8 Cell Terminology QuizDokumen1 halamanGrade 8 Cell Terminology Quizapi-210291577Belum ada peringkat
- Cell Analogy Poster RubricDokumen1 halamanCell Analogy Poster Rubricapi-210291577Belum ada peringkat
- Demo Day LPDokumen4 halamanDemo Day LPapi-210291577Belum ada peringkat
- Photosynthesis Macro Model InstructionsDokumen1 halamanPhotosynthesis Macro Model Instructionsapi-210291577Belum ada peringkat
- Conduction Lab LPDokumen4 halamanConduction Lab LPapi-210291577Belum ada peringkat
- Photosynthesis Lesson PlanDokumen5 halamanPhotosynthesis Lesson Planapi-210291577100% (2)
- Yeast Vs Sand ExperimentDokumen2 halamanYeast Vs Sand Experimentapi-210291577Belum ada peringkat
- LP Cells Day 1Dokumen5 halamanLP Cells Day 1api-210291577Belum ada peringkat
- 2004catalog PDFDokumen30 halaman2004catalog PDFPH "Pete" PetersBelum ada peringkat
- Application of DryingDokumen31 halamanApplication of Dryinguzzal ahmedBelum ada peringkat
- Asme CCase N-432Dokumen4 halamanAsme CCase N-432julianmorantesBelum ada peringkat
- Premier R' Series Generator: Operator'S Manual For YourDokumen37 halamanPremier R' Series Generator: Operator'S Manual For YourRon SchmittBelum ada peringkat
- Term Paper OF: Online Automation SystemsDokumen20 halamanTerm Paper OF: Online Automation SystemsAditya MalhotraBelum ada peringkat
- Robotics and Vision SystemDokumen64 halamanRobotics and Vision Systemasuras1234Belum ada peringkat
- 11 CR-CB Data SheetDokumen2 halaman11 CR-CB Data SheetJLZ972Belum ada peringkat
- Biostone C20 Safety Data SheetDokumen10 halamanBiostone C20 Safety Data Sheetchoton_iiiBelum ada peringkat
- I S 613 - 2000Dokumen11 halamanI S 613 - 2000Hariprasad gantyalaBelum ada peringkat
- Dynalene HC Engineering GuideDokumen38 halamanDynalene HC Engineering GuideSH1961Belum ada peringkat
- Cutting RoomDokumen45 halamanCutting RoomSuchismitaShauryaBelum ada peringkat
- Specific Gravity and Water Absorption of Coarse Aggregate PDFDokumen9 halamanSpecific Gravity and Water Absorption of Coarse Aggregate PDFHonggo KuncoroBelum ada peringkat
- Epoxy Grout for ConstructionDokumen3 halamanEpoxy Grout for Constructionarunjacobn100% (2)
- Sigma mixer description and specificationsDokumen1 halamanSigma mixer description and specificationsBalRam DhimanBelum ada peringkat
- EX22CT (RHFE-559FTA2) : FeaturesDokumen4 halamanEX22CT (RHFE-559FTA2) : Featuresrafiullah353Belum ada peringkat
- Cabluri Monofilare Fara Manta Unsheathed Single-Core Cables: Test Voltage: 3 KV, 50 HZ, 5 Minutes in WaterDokumen2 halamanCabluri Monofilare Fara Manta Unsheathed Single-Core Cables: Test Voltage: 3 KV, 50 HZ, 5 Minutes in WaterCirtiu SandaBelum ada peringkat
- Mouse monoclonal OC4-30 to Osteocalcin SDSDokumen6 halamanMouse monoclonal OC4-30 to Osteocalcin SDSIna MarsomBelum ada peringkat
- Assignment: Q.N.1. in A Turning Operation, It Was Observed That The Tool Life Was 150 Minutes When The CuttingDokumen7 halamanAssignment: Q.N.1. in A Turning Operation, It Was Observed That The Tool Life Was 150 Minutes When The CuttingAnkesh KapilBelum ada peringkat
- Functionally Graded MaterialsDokumen16 halamanFunctionally Graded MaterialsPradeepkumar Chikkamath100% (1)
- Job Safety Assessment FormDokumen161 halamanJob Safety Assessment FormFadhlan Rasyid RBelum ada peringkat
- ME-6401 Kinematics of Machines: Basic ConceptsDokumen81 halamanME-6401 Kinematics of Machines: Basic ConceptsMugilBelum ada peringkat
- Corod SWR High Strength 29feb12Dokumen3 halamanCorod SWR High Strength 29feb12Ronald LlerenaBelum ada peringkat
- Sikadur 30 PDFDokumen5 halamanSikadur 30 PDFDarwin SyahputraBelum ada peringkat
- Copper Grades %: Value Per BlockDokumen7 halamanCopper Grades %: Value Per BlockJhon Jayro Lavado FernándezBelum ada peringkat
- Essie Waste ManagementDokumen2 halamanEssie Waste ManagementrupenderBelum ada peringkat
- GTDMC ReportDokumen11 halamanGTDMC ReportOwais AhmedBelum ada peringkat
- Advanced m52umCT2014Dokumen79 halamanAdvanced m52umCT2014Anonymous ys297cBxBelum ada peringkat
- Các loại đầu nối cho ống- fittings for high pressure PDFDokumen45 halamanCác loại đầu nối cho ống- fittings for high pressure PDFKiên MaiBelum ada peringkat