PCI or PTCA
Diunggah oleh
Melissa KanggrianiHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
PCI or PTCA
Diunggah oleh
Melissa KanggrianiHak Cipta:
Format Tersedia
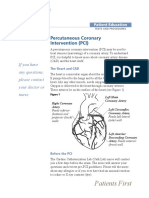
PCI/PTCA (Percutaneous Transluminal Coronary Angioplasty) Overview
Introduction to PCI Since the first human percutaneous transluminal coronary angioplasty (PTCA) procedure was performed in 1977, the use of percutaneous coronary intervention (PCI) has increased dramatically, becoming one of the most common medical interventions performed. The technique, originally developed in Switzerland by Andreas Gruentzig, has transformed the practice of revascularization for coronary artery disease (CAD). Initially used in the treatment of patients with stable angina and discrete lesions in a single coronary artery, coronary angioplasty has multiple indications today, including unstable angina, acute myocardial infarction (MI), and multivessel coronary artery disease. With the combination of sophisticated equipment, experienced operators, and modern drug therapy, PCI has evolved into an effective nonsurgical modality for treating patients with coronary artery disease. Recent advances in guidewires, stents, and devices to cross chronically occluded arteries are evolving, so that more patients with chronic total occlusions (CTOs) are now being successfully treated percutaneously. Improvements in catheter technique and the development of new devices, wires, stents, drug-eluting stents, and medications have occurred parallel to advances in the understanding of cardiovascular physiology, the pathogenesis of atherosclerosis, and the body's response to vascular injury. Intracoronary stents and atherectomy devices were developed to increase the success rate of, and decrease the complications associated with, conventional balloon dilation and to expand the indications for revascularization. These devices have enabled the interventionalist to safely treat more complex coronary lesions and restenosis. Stents have evolved to a level where the problems of restenosis seen with bare metal stents are a less frequent occurrence after drug-eluting stents are implanted. At the same time, advances in intravascular ultrasonography (IVUS) and fractional flow reserve evaluation have improved the understanding of coronary plaque morphology, plaque vulnerability, and coronary physiology. Furthermore, many of these technologies are able to help identify patients who will benefit most from PCI, coronary bypass surgery, or medical therapy. Adjunctive pharmacologic therapies aimed at preventing acute reocclusion have also improved the safety and efficacy of coronary angioplasty.
The growth of PCIs has been remarkable and will likely be sustained, as new technologies have resulted in improved outcomes. Since 1994, the use of intracoronary stents has risen dramatically, and now with drugeluting stents, stents are used in more than 80% of PCI cases in the United States. Innovations in PCIs over the past 2 decades have been paralleled by a dramatic reduction in 30-day death, myocardial infarction, and target-vessel revascularization rates. Go to Unstable Angina for complete information on this topic. Indications for PCI Clinical indications for PCI include the following: Acute ST elevation myocardial infarction (STEMI) Non ST elevation acute coronary syndrome Stable angina Anginal equivalent (eg, dyspnea, arrhythmia, dizziness/syncope) Asymptomatic or mildly symptomatic patients with objective evidence of a moderate to large area of viable myocardium or moderate to severe ischemia on noninvasive testing Angiographic indications include hemodynamically significant lesions in vessels serving viable myocardium (vessel diameter >1.5 mm). Contraindications to PCI Clinical contraindications include significant comorbidities (relative contraindication). Angiographic contraindications include the following: Left main stenosis in a patient who is a surgical candidate (coronary artery bypass graft [CABG] surgery is still the preferred treatment for left main stenosis; however, this area is rapidly evolving toward safe and feasible PCI options) Diffusely diseased small-caliber artery or vein graft Other coronary anatomy not amenable to percutaneous intervention
Preparation
Equipment for Percutaneous Coronary Intervention Balloon angioplasty The original description of angioplasty by Dotter and Judkins described enlargement of the vessel lumen through a mechanism of atheromatous plaque compression. This mechanism is also partially responsible for luminal enlargement with balloon angioplasty. In addition, however, improvement in luminal diameter following balloon angioplasty also results from stretching of the vessel wall by the balloon. Balloon inflation actually results in overstretching of the vessel wall and partial disruption
of not only the intimal plaque but also the media and adventitia, resulting in enlargement of the lumen and the outer diameter of the vessel. Axial redistribution of plaque material also contributes to improvements in lumen diameter. Atherectomy devices and, subsequently, intracoronary stents were developed, in part, to decrease the early and late loss in luminal diameter observed with conventional balloon angioplasty. Several different balloon catheter designs have existed (over-the-wire, monorail, fixed wire) with balloon materials that have different compliance characteristics allowing various degrees of expansion with increasing pressure. Irrespective of the balloon design, a steerable guidewire precedes the balloon into the artery and allows navigation through a considerable portion of the coronary tree. The development of balloon catheters that bend, allowing easy advancement through tortuous vascular segments (trackability), and that have increased shaft stiffness (pushability), allowing the catheter to be forced through stenotic lesions, has increased their versatility significantly. Another evolving feature of catheter design has been a reduction in the diameter of the deflated balloon, allowing easier passage through very stenotic lesions. Improvements in catheter design have been partially responsible for the improved success rates of PCIs. The balloon catheter also serves as an adjunctive device for many other interventional therapies, including atherectomy and coronary stents. Atherectomy devices and coronary stents As a result of technical challenges, suboptimal clinical outcomes, and the significant rates of restenosis following percutaneous coronary artery balloon angioplasty, 2 innovative types of devices were developed: those used for atherectomy and coronary stents. The idea behind atherectomy devices was to physically remove atheroma, calcium, and excess cellular material from the site of a coronary occlusion or stenosis. Both mechanical and laser-based approaches are used. An alternative approach developed at about the same time was intracoronary stent placement, based on the notion that permanent implantation of a scaffold to hold open the coronary artery at the site of an intervention would improve outcomes. Long-term outcomes from atherectomy alone have been disappointing and, in most cases, little better than balloon angioplasty. Stents, particularly stents coated with materials to reduce inflammatory and cell growth responses, have resulted in greatly improved outcomes.
Long-term outcomes from atherectomy alone have been disappointing and little better than balloon angioplasty in most cases. Stents, particularly stents coated with materials to reduce inflammatory and cell growth responses, have resulted in greatly improved outcomes. Atherectomy is still used for specific, niche indications, but the most common intracoronary device used today is a stent.[1] The rotational atherectomy catheter (Rotablator) is designed for the removal of plaque from coronary arteries. This device (see the image below), which has a diamond-studded burr at its tip, rotates at about 160,000 rpm and is particularly well suited for ablation of calcific or fibrotic plaque material.
Percutaneous transluminal coronary angioplasty (PTCA). The rotational atherectomy catheter (Rotablator) is a device designed for the removal of plaque from coronary arteries. This device, which has a diamond-studded burr at its tip, rotates at about 160,000 rpm and is particularly well suited for ablation of calcific or fibrotic plaque material.
Unlike other atherectomy devices that rely on tissue cutting, the rotational atherectomy device relies on plaque abrasion and pulverization. Rotational atherectomy is successful in 92-97% of these cases, with a low incidence of major complications. It causes dislodgement of particles into the microcirculation, which occasionally may lead to infarction and no reflow (impaired distal coronary flow). Currently, the use of rotational atherectomy is largely confined to fibrotic or heavily calcified lesions that can be wired but not crossed or dilated by a balloon catheter. The Excimer Laser, Rotational Atherectomy, and Balloon Angioplasty Comparison (ERBAC) Study showed that rotational atherectomy was associated with a higher short-term success rate than balloon angioplasty (90% vs 80%), but major ischemic complications and repeat revascularization were higher 6 months after treatment (46% vs 37%).[2,
3]
A meta-analysis failed to show any significant difference in mortality, major adverse cardiovascular events (MACE), or revascularization rates in patients treated with rotational atherectomy, laser, or cutting balloon angioplasty when compared with balloon angioplasty alone. In some cases, rotational atherectomy was actually associated with an increase in periprocedural myocardial infarction.[4] However, none of these trials compared stent-related outcomes. In fact, many of these devices may be used to facilitate stent delivery in complex lesions, especially when balloon angioplasty alone has failed. Since 1987, directional coronary atherectomy (DCA) has been used to debulk coronary plaques. A steel fenestrated cage housing a cupshaped blade is positioned against the coronary lesion by a lowpressure positioning balloon, allowing any protruding plaque to be removed. Atherectomy is typically followed by balloon dilation and stenting. The acute gain, therefore, is a combination of the removal of atheromatous plaque and radial displacement of plaque from dilation. Major complication rates associated with directional atherectomy are low and similar to those associated with conventional balloon angioplasty. Other complications (eg, distal embolization of plaque, transient side-branch occlusion, coronary vasospasm, the no-reflow phenomenon, nonQwave myocardial infarction) are greater with DCA than with balloon angioplasty. Because of the increased complication rates and the greater technical demands of DCA compared with balloon angioplasty or stenting, the use of DCAs has greatly decreased in recent years. A 2006 meta-analysis demonstrated that DCA is superior to stenting alone with regard to acute angiographic results and target-lesion revascularization with a similar prevalence of late MACEs. There was, however, a higher prevalence of early MACEs with DCA before stenting, which probably related to distal embolization.[5] Although initial excitement about the development of laser atherectomy was considerable, it is not used widely because of the technical demands of this device and no clear improvements in outcome over therapy with other devices. Intracoronary stents have been used widely since the early 1990s (the original FDA approval for intracoronary stents in the US was 1994). Many different stents are available and differ in composition (eg, stainless steel, cobalt chromium, nickel chromium), architectural design, and stent delivery system (ie, the balloon catheter that delivers it). The development of drug-eluting stents has revolutionized coronary intervention to the extent that balloon angioplasty and bare metal stents did in the 1980s and 1990s.
Today, 4 types of drug-eluting stents are available in the United States, the sirolimus-eluting stent (Cypher), the paclitaxel-eluting stent (Taxus), and the newer-generation zotarolimus-eluting stent (Endeavor) and everolimus-eluting stent (Xience V). These stents comprise a metal stent with a polymer that elutes a drug that reduces neointimal hyperplasia. Drug-eluting stents have been extensively tested in a wide spectrum of coronary lesions, all of which have demonstrated significant reductions in restenosis and target-lesion revascularization rates when compared with bare metal stents. The zotarolimus-eluting stent and everolimus-eluting stent have improved deliverability, thinner struts, and a thinner polymer layer, and they may have clinical advantages over sirolimus-eluting and paclitaxeleluting stents. Studies with drug-eluting stents are ongoing and include efforts to define safety and outcomes in every range of PCI setting, such as stable and unstable lesions, small vessels, vein grafts, chronic total occlusions, primary PCI, and comparing drug-eluting stent technologies with a coronary artery bypass graft (CABG) in left main coronary artery disease and in diabetes patients with multivessel coronary artery disease. Stone et al conducted an analysis of 6780 patients, 1869 (27.6%) of whom had diabetes mellitus, who were randomized to either everolimus-eluting stents or paclitaxel-eluting stents. Data were pooled from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions (SPIRIT) II, SPIRIT III, SPIRIT IV, and A Trial of Everolimus-Eluting Stents and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice (COMPARE) trials. Results showed that everolimus-eluting stents compared with paclitaxeleluting stents resulted in substantial 2-year reductions in death, myocardial infarction, stent thrombosis, and target lesion revascularization in patients without diabetes mellitus, whereas no significant differences in safety or efficacy outcomes were present in diabetic patients.[6] Although stents are conventionally placed after balloon predilation, a meta-analysis by Piscione et al suggests that, in selected coronary lesions, direct stenting may lead to better outcomes. Myocardial infarction rates were lower with direct stenting (3.16% vs 4.04% with conventional stenting), while rates of target vessel revascularization were similar.[7] See the images below showing stents used in percutaneous transluminal coronary angioplasty (PTCA).
Percutaneous transluminal coronary angioplasty (PTCA). TRISTAR stent.
Percutaneous transluminal coronary angioplasty (PTCA). NIR stent.
Percutaneous transluminal coronary angioplasty (PTCA). Wallstent.
Ancillary devices In addition to balloons, stents, and atherectomy devices, other devices such as thrombus extraction catheters and distal embolic protection devices have found roles in PCI. In the TAPAS trial (Thrombus Aspiration during Percutaneous Coronary Intervention in Acute Myocardial Infarction), which studied the use of thrombus aspiration during primary PCI for ST elevation myocardial infarction, thrombus aspiration with an Export catheter before stenting had a reduced allcause mortality (4.7% vs 7.6%) and reduced cardiac death (3.6% vs 6.7%) at 1 year compared with conventional PCI.[8] In a pooled analysis of data from 3 prospective randomized trials, De Vita et al found that although increasing time to treatment was associated with a decreased rate of optimal reperfusion in patients receiving standard PCI, this trend was not seen in patients treated with
thrombus aspiration. De Vita et al concluded that the use of thrombus aspiration limits the adverse effects that prolonged time to treatment has on myocardial reperfusion.[9] The use of distal embolic protection during saphenous vein graft intervention has become the standard of care. The SAFER trial (Saphenous Vein Graft Angioplasty Free of Emboli Randomized) initially proved the benefit of embolic protection in reducing 30-day rates of major adverse cardiac events (MACE) (9.6% vs 16.5%, P =0.004), myocardial infarction (8.6% vs 14.7%), and no reflow (3% vs 9%). A 15-month follow-up of the DEDICATION (Drug Elution and Distal Protection in ST Elevation Myocardial Infarction) trial found that in primary PCI for STEMI, the routine use of distal protection increased the incidence of stent thrombosis and clinically driven target lesion/vessel revascularization. Kaltoft et al reported that, in DEDICATION, patients with STEMI were assigned to distal protection (DP) (n =312) or conventional treatment (CT) (n =314) and the rate of definite stent thrombosis was significantly higher in the DP group than in the CT group, with 9 cases versus 1. Clinically driven target vessel revascularizations were more frequent in the DP group (37 patients vs 22 patients).[10]
Technique
Intravascular Ultrasonography Coronary angiography provides a display of luminal narrowing in multiple planes and is useful in guiding coronary interventions. However, angiography cannot provide information about the vessel wall, which is where the atherosclerotic process resides. Intravascular ultrasonography (IVUS) (see the image below) was developed to provide information about the plaque, the vessel wall, and the degree of luminal narrowing. It provides a tomographic cross-section of the vessel, allowing operators to gather significant qualitative and quantitative information that is potentially valuable in assessing stenosis severity and the true extent of atherosclerotic involvement.
Example of an intravascular ultrasound (IVUS) image in percutaneous transluminal coronary angioplasty (PTCA).
Identification of the lumen border and the media-adventitia interface form the key landmarks during interpretation. Plaque can be distinguished from the lumen by differences in echogenicity. In addition to providing information about the amount and distribution of plaque, IVUS can identify features of plaque composition, such as calcification and lipid collections, which may not be appreciated by angiography alone. Frequent uses of IVUS include the assessment of indeterminate lesions and the evaluation of adequate stent deployment. The latter has become increasingly important, since proper deployment of drug-eluting stents is critical to reduction in rates of thrombosis. Recent developments in ultrasonography (eg, virtual histology) and other technologies (eg, optical coherence tomography, plaque thermography) have led to ways of characterizing and identifying vulnerable segments of plaque that may pose a risk for future cardiac events. Coronary Physiologic Assessment Intracoronary Doppler pressure wires are able to characterize coronary lesion physiology and to estimate lesion severity. Comparison of pressure distal to a lesion with aortic pressure enables determination of fractional flow reserve (FFR) (see the image below). A measurement below 0.75-0.80 during maximal hyperemia (induced via administration of adenosine) is consistent with a hemodynamically significant lesion. This determination is useful in deciding whether to perform PCI in an angiographic intermediate lesion. Clinical data, namely the DEFER study, support using this approach, with a low event rate seen in medically managed patients with angina and an FFR measurement greater than 0.75.
Fractional flow ratio (FFR). Pressure wire is advanced across left anterior descending (LAD) stenosis and intracoronary adenosine is given. FFR ratio is recorded at baseline and then after adenosine push is given. Here, LAD lesion and FFR postadenosine is shown.
The Fractional Flow Reserve versus Angiography for Guiding PCI in Patients with Multivessel Coronary Artery Disease (FAME) trial showed that routine measurement of FFR during angioplasty reduced the risk of death, myocardial infarction, or repeat revascularization by 30% and death or myocardial infarction by 35%, compared with the current
practice of using angiography to guide stenting decisions. In this study, a cut-off of FFR greater than 0.80 was used to define a nonischemic lesion.[1] Two-year follow-up of the FAME trial showed continuing significant reductions in the combined endpoint of death and myocardial infarction with the use of FFR compared with standard angiographyguided PCI.[11] This form of physiologic lesion assessment is also useful for defining optimal stenting, assessing the angiographic severity of jailed side branch lesions, helping guide the decision for multivessel percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) in multiple intermediate lesions, and assessing the severity of instant restenosis. FFR measurements have excellent correlation with IVUS analysis, especially when determining lesion severity, such as in ambiguous left main coronary artery anatomy. Adjunctive Therapies in the Catheterization Laboratory Aspirin and heparin have been the traditional adjunctive medical therapies for patients undergoing coronary angioplasty and have been shown to decrease complications following the procedure. Since 1994, several new antithrombotic drugs have been developed that have advantages over standard heparin therapy. Although it is an effective anticoagulant, heparin has several limitations, including variable pharmacokinetics requiring careful monitoring, inhibition by substances released from activated platelets, and an inability to inhibit fibrin-bound thrombin. To address these limitations, several direct thrombin inhibitors have been developed. Hirudin and bivalirudin (Angiomax) were evaluated in 2 multicenter trials,[12, 13, 14, 15] and both agents were found to be slightly better than heparin in preventing ischemic complications during balloon angioplasty, but they had no effect on restenosis rates. Low-molecular-weight heparins are also being substituted for standard heparin in some centers in patients with acute coronary syndromes and during coronary interventions. Newer factor IX and factor Xa inhibitors are being evaluated as potential alternative anticoagulants. However, trials have failed to show a significant difference in efficacy of factor Xa inhibition compared with unfractionated heparin (UFH). In the HORIZONS-AMI trial, 3602 patients presenting with ST-segment elevation myocardial infarction (STEMI) and undergoing PCI who were treated with bivalirudin, a thrombin inhibitor, had substantially lower 30day rates of major hemorrhagic complications and reduced rates of net adverse clinical events (consisting of major bleeding or composite major adverse cardiovascular events [death, reinfarction, target vessel revascularization for ischemia, or stroke]) than did patients treated with
heparin plus a glycoprotein IIb/IIIa inhibitor (GPI). Mehran et al continued to follow patients for 1 year. Data were available for 1696 patients in the bivalirudin group and for 1702 patients in the heparin/GPI group. Findings showed that the bivalirudin group continued to have reduced rates for major bleeding and adverse events at 1 year compared with the group treated with heparin plus a GPI. Death, reinfarction, target-vessel revascularization for ischemia, and stroke were similar between the 2 groups.[16] Other data that emerged from the HORIZONS-AMI study include evidence that a 600-mg dose of clopidogrel improved clinical outcomes compared with 300 mg in patients with STEMI undergoing PCI. In the 600-mg loading dose group, the 2158 patients had significantly lower unadjusted 30-day mortality rates than the 1153 patients in the 300-mg group (1.9% vs 3.1%). Additionally, the 600-mg group was superior to the 300-mg group for reinfarction (1.3% vs 2.3%) and stent thrombosis (1.7% vs 2.8%). Bleeding rates did not differ between the 600-mg and 300-mg loading doses. Similar differences were shown in patients who received either bivalirudin or unfractionated heparin plus a GPI.[17] The Gauging Responsiveness with A VerifyNow AssayImpact on Thrombosis And Safety (GRAVITAS) study enrolled 2214 patients with high on-treatment reactivity (defined as 230 P2Y12 reaction units [PRU] or higher) 12-24 hours after PCI. Patients were randomized to highdose clopidogrel (600-mg initial dose, 150 mg daily thereafter) or standard-dose clopidogrel (no additional loading dose, 75 mg daily) for 6 months. High-dose clopidogrel provided a 22% absolute reduction in the rate of high on-treatment reactivity at 30 days compared with standard treatment, but no difference was noted in the primary end point of 6-month incidence of death from cardiovascular causes, nonfatal myocardial infarction, or stent thrombosis (hazard ratio, 1.01; 95% confidence interval [CI], 0.58-1.76; P=.97). Severe or moderate bleeding according to the Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO) definition was lower in the standard group, but it did not reach statistical significance(HR, 0.59; 95% CI, 0.31-1.11; P=.10).[18] The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial studied the impact of age on outcomes in moderate- and high-risk non-ST-segment elevation acute coronary syndrome.[19] Patients aged 75 years or older treated with bivalirudin alone had similar ischemic outcomes but significantly lower rates of bleeding compared with those treated with heparin and glycoprotein IIb/IIIa inhibitors overall and in the PCI subset. Outcomes were analyzed at 30 days and at 1 year in 4 age groups, overall, and in those undergoing PCI. Of 13,819 patients in the ACUITY trial, 3655 (26.4%) were younger than 55 years of age, 3940 (28.5%) were aged 55-64 years, 3783
(27.4%) were aged 65-74 years, and 2441 (17.7%) were 75 years or older. Older patients had more cardiovascular risk factors and had a higher acuity at presentation. In the Novel Approaches for Preventing or Limiting Events (NAPLES) trial, Tavano et al compared bivalirudin with unfractionated heparin (UFH) plus a GPIIb/IIIa inhibitor (ie, tirofiban) during percutaneous coronary intervention (PCI) in 335 patients with diabetes mellitus and concluded that elective PCI with bivalirudin monotherapy is safe and feasible in patients with diabetes.[20] The bivalirudin group experienced significantly less in-hospital bleeding (8.4% vs 20.8%). Non-Q-wave myocardial infarction rates were similar in the 2 groups (bivalirudin 10.2% vs UFH/tirofiban 12.5%). In the early days of stenting, multiple antiplatelet agents and warfarin were used in an attempt to prevent stent thrombosis, but thrombosis continued to occur in approximately 6% of patients. With an improved understanding of how stents should be deployed, warfarin is no longer necessary. Patients receiving stents are now treated with a combination of aspirin and clopidogrel, and with this therapy, the incidence of subacute thrombosis is approximately 1%. Today, this combination is given to all stent patients for a minimum of 4 weeks after a bare metal stent and a minimum of 12 months when a drug-eluting stent is used. Issues remain as to whether the duration of aspirin and clopidogrel should be longer in drug-eluting stent patients. The authors advocate that a baby aspirin should be maintained for life and clopidogrel should be considered for life in drug-eluting stent patients, unless bleeding contraindications restrict its use. Other considerations with antiplatelet therapy during PCI include the cost of clopidogrel, the proper loading dose, and timing of the initial dose relative to cardiac catheterization. In elective situations, clopidogrel is most effective when given prior to PCI. In acute situations, this may not be practical, and clopidogrel is therefore often given after PCI. Concerns still exist regarding the risk of bleeding and platelet transfusion requirements in patients taking clopidogrel who require an urgent coronary artery bypass graft (CABG). However, as emergent CABG is rare, there may be time to risk-stratify patients and to give clopidogrel before cardiac catheterization. If CABG is required, the effect of clopidogrel usually diminishes within 5 days. Another important consideration is the dose of clopidogrel. If given 2 hours prior to PCI, 600 mg is recommended; if given more than 2 hours prior to PCI, then 300 mg is recommended. Some centers have even given 900 mg instead of 600 mg. At present, the ACC/AHA guidelines recommend giving 300 mg up to 6 hours prior to PCI. Development of newer intravenous antiplatelet therapies with shorter half-lives may help
overcome these issues. Aspirin, 325 mg, should be given prior to all PCIs and then maintained at 81 mg daily. Clopidogrel is a prodrug that is metabolized to the active form of the drug by the cytochrome (CYP) 450 enzyme system in the liver. Recent research has demonstrated that genetic variation at the CYP450 2C19 locus results in decreased metabolic activation of clopidogrel and increased risk of adverse cardiovascular events. This led to a recent update to the package insert for clopidogrel (Plavix) that includes a "black box" warning for use in patients who are "poor metabolizers" (individuals who have 2 abnormal alleles at the CYP 2C19 locus; approximately 2-4% of white patients). Individuals with one abnormal allele have intermediate metabolism of clopidogrel to the active metabolite. In a meta-analysis of 9 studies and almost 10,000 patients, Mega et al found that carriage of even one reduced-function CYP2C19 allele in patients treated with clopidogrel after percutaneous coronary intervention was associated with a significantly increased risk of major adverse cardiovascular events, particularly stent thrombosis.[21] The best treatment strategy for platelet inhibition in patients who are poor or intermediate metabolizers has not been established, but alternative antiplatelet strategies should be considered.[22] A consensus statement issued by the American College of Cardiology, American College of Gastroenterology, and American Heart Association in November 2010 addresses the issue of concomitant use of proton pump inhibitors (PPIs), which are CYP2C19 inhibitors, and thienopyridine antiplatelet drugs, such as clopidogrel.[23] The groups findings and recommendations are listed below. Clopidogrel reduces major CV events compared with placebo or aspirin Dual antiplatelet therapy with clopidogrel and aspirin, compared with aspirin alone, reduces major CV events in patients with established ischemic heart disease, and it reduces coronary stent thrombosis but is not routinely recommended for patients with prior ischemic stroke because of the risk of bleeding Clopidogrel alone, aspirin alone, and their combination are all associated with increased risk of GI bleeding Patients with prior GI bleeding are at highest risk for recurrent bleeding on antiplatelet therapy; other risk factors include advanced age, concurrent use of anticoagulants, steroids, or NSAIDs (including aspirin), and Helicobacter pylori infection; risk increases as the number of risk factors increases
Use of proton pump inhibitors (PPIs) or histamine (H2) receptor antagonists (H2RAs) reduces the risk of upper GI bleeding compared with no therapy; PPIs reduce upper GI bleeding to a greater degree than do H2RAs PPIs are recommended to reduce GI bleeding in patients with a history of upper GI bleeding; PPIs are appropriate in patients with multiple risk factors for GI bleeding who require antiplatelet therapy Routine use of either a PPI or an H2RA is not recommended for patients at lower risk of upper GI bleeding, who have much less potential to benefit from prophylactic therapy Clinical decisions regarding concomitant use of PPIs and thienopyridines must balance overall risks and benefits, considering both CV and GI complications Pharmacokinetic and pharmacodynamic studies, using platelet assays as surrogate endpoints, suggest that concomitant use of clopidogrel and a PPI reduces the antiplatelet effects of clopidogrel; the strongest evidence for an interaction is between omeprazole and clopidogrel; it is not established that changes in these surrogate endpoints translate into clinically meaningful differences Observational studies and a single randomized clinical trial have shown inconsistent effects on CV outcomes of concomitant use of thienopyridines and PPIs; a clinically important interaction cannot be excluded, particularly in certain subgroups, such as poor metabolizers of clopidogrel The role of either pharmacogenomic testing or platelet function testing in managing therapy with thienopyridines and PPIs has not yet been established Prasugrel is a thienopyridine adenosine diphosphate (ADP) receptor inhibitor that inhibits platelet aggregation. It has been shown to reduce new and recurrent myocardial infarctions.[24] The loading dose is 60 mg PO once, and maintenance is 10 mg PO qd (given with aspirin 75-325 mg/d). Prasugrel is indicated to reduce thrombotic cardiovascular events (including stent thrombosis) with acute coronary syndrome that is managed with PCI. It is used specifically for unstable angina or nonST-segment elevation myocardial infarction (NSTEMI) or with acute STsegment elevation myocardial infarction (STEMI) when managed with primary or delayed PCI. Additional information from the TRITON TIMI 38 trial analyzed whether the type, size, and timing of myocardial infarction affected prasugrels ability to reduce new or recurrent myocardial infarction. TRITON TIMI 38 included 13,608 patients with acute coronary syndrome randomized to either prasugrel or clopidogrel. Prasugrel significantly reduced the overall risk of myocardial infarction compared with clopidogrel for any
type of myocardial infarction (eg, procedure-related, nonprocedural, and consistently across myocardial infarction size). Significant, sometimes fatal, bleeding occurred more frequently with prasugrel than with clopidogrel.[24] All types of percutaneous coronary interventions result in disruption of the coronary endothelium, which leads to platelet activation. Activated platelets bind to the vessel wall (adhesion) and to each other (aggregation) and release numerous vasoactive compounds. Aspirin blocks the cyclooxygenase pathway and reduces thrombotic complications after balloon angioplasty. However, despite heparin and aspirin therapy, thrombotic complications are not eliminated. Further studies identified the importance of the GP IIb/IIIa receptor, which binds fibrinogen and mediates the cross-linking of platelets and platelet aggregation. The introduction of GP IIb/IIIa receptor inhibitors has had a major influence on current treatment strategies in the catheterization laboratory, such as the following: Abciximab, tirofiban, and eptifibatide have all been shown to reduce ischemic complications in patients undergoing balloon angioplasty and coronary stenting In primary PCI, GP IIb/IIIa receptor inhibitors have also been shown to improve flow and perfusion and to reduce adverse events Abciximab may improve outcomes in patients when given prior to their arrival in the catheterization lab for primary PCI[25] A meta-analysis of GP IIb/IIIa inhibitor trials showed a significant reduction in early mortality rates when these agents are used during coronary intervention[26] ; the combined end point of death or myocardial infarction was also reduced significantly at 30 days The EVA-AMI trial (Eptifibatide vs Abciximab in Primary PCI for Acute ST Elevation Myocardial Infarction) compared the efficacy of 2 glycoprotein (GP) IIb/IIIa inhibitors as adjunct to PCI in 427 patients with STEMI; results showed double-bolus eptifibatide followed by a 24-hour infusion was as effective as single-bolus abciximab followed by a 12-hour infusion for ST-segment resolution[27] These agents are effective at reducing ischemic complications of PCIs; however, they have not been shown to improve outcome in saphenous vein graft PCI One meta-analysis of 22 studies including 10,123 patients evaluated the use of GP IIb/IIIa inhibitors during elective PCI in patients pretreated with clopidogrel. The analysis found that use of GPIIb/IIIa inhibitors had no effect on mortality or major bleeding but were associated with a decrease in the incidence of nonfatal myocardial infarction and an increase in the rate of minor
bleeding.[28]
Post-Procedure
Outcome of PCI A major 2009 task force report viewed favorably, in general, the use of coronary revascularization for patients with acute coronary syndromes and combinations of significant symptoms and/or ischemia. However, revascularization of asymptomatic patients or patients with low-risk findings on noninvasive testing and minimal medical therapy were viewed less favorably.[29] Stable Angina (PCI vs Medical Therapy) Early trials demonstrated the benefit of percutaneous coronary intervention (PCI) over medical therapy for symptomatic angina in single and multivessel coronary artery disease, with improvements in symptoms, reduction in need to take antianginal medications, improvement in exercise duration, and similar survival rates to medical therapy.[30, 31, 32] The Randomized Intervention in the Treatment of Angina (RITA-II) study demonstrated that balloon angioplasty results in better control of ischemic symptoms and greater improvement in exercise capacity than does medical therapy, but balloon angioplasty is associated with an increased incidence of the combined endpoint of death and myocardial infarction. In RITA-II, in which 1018 patients with stable angina were randomized to balloon angioplasty or medical therapy, death or definite myocardial infarction occurred in 6.3% of the balloon angioplasty patients and in 3.3% of the medical patients; only 44% of the deaths were actually due to heart disease.[33] Angina improved in both groups, but a 16.5% absolute excess of grade 2 or worse angina occurred in the medical group 3 months following randomization. In the RITA-II study, 23% of the medical group required revascularization during follow-up. During follow-up, 7.9% of the angioplasty patients required bypass surgery, compared with 5.8% of the medically treated patients. Although the patients in RITA-II were asymptomatic or mildly symptomatic, it is important to emphasize that most had severe anatomic coronary artery disease; 62% had multivessel coronary artery disease, and 34% had important disease of the proximal left anterior descending (LAD) artery. In the Atorvastatin Versus Revascularization Treatment (AVERT) trial, after 18 months of follow-up, 13% of the medically treated group had ischemic events, compared with 21% of the angioplasty group, suggesting that in low-risk patients with stable coronary artery disease,
aggressive lipid-lowering therapy may be as effective as balloon angioplasty in reducing ischemic events. In the study, 341 patients with stable coronary artery disease symptoms, normal left ventricle (LV) function, and class I or II angina were assigned randomly to balloon angioplasty or medical therapy with atorvastatin.[34] Based on the limited data available from randomized trials comparing medical therapy with balloon angioplasty, considering medical therapy seems prudent for the initial management of most patients with Canadian Cardiovascular Society Classification Class I and II symptoms, and reserving percutaneous or surgical revascularization is appropriate for patients with more severe symptoms and ischemia. The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial demonstrated that in patients with stable angina symptoms and coronary artery stenosis, medical therapy alone may be an appropriate strategy if medical therapy can control the angina symptoms. The trial randomized the addition of percutaneous coronary intervention (PCI) to intensive pharmacological therapy, with the endpoints of death from any cause and nonfatal myocardial infarction during a median follow-up period of 4.6 years. Inclusion criteria for the study included patients with a 70% or greater lesion in 1 or more proximal epicardial arteries, AHA/ACC Class I or II indications for PCI, and objective evidence of myocardial ischemia on stress testing. Both primary endpoints demonstrated no statistical benefit between those patients who received PCI with medical therapy and those who received only medical therapy (19.0% vs 18.5%). Teo et al found that in older patients with stable coronary artery disease, optimal medical treatment without percutaneous coronary intervention (PCI) remains an appropriate initial management strategy. Analysis of 904 patients aged 65 years or older showed that, during a median 4.6-year follow-up, clinical outcome in patients randomized to optimal medical treatment plus PCI was no better or worse than in patients who received optimal medical treatment alone. Compared with 1381 patients younger than 65 years with coronary artery disease (CAD), older patients had similar success in achieving treatment targets and similar rates of myocardial infarction, stroke, and major cardiac events, although the death rate was 2- to 3-fold higher in the older patients. This analysis was done from patients enrolled in the COURAGE trial, and thus the limitations of that study have to be kept in mind, including the following[35] : All patients had angiography prior to enrollment Only 1 in 12 patients who were screened were actually enrolled
At the time of enrollment, most patients were either asymptomatic or had minimal symptoms Overall, medical therapy is recommended as first-line therapy in patients with stable angina unless the following occur: a change in symptom severity, failed medical therapy, high-risk coronary anatomy, or worsening LV dysfunction, which provide an indication for cardiac catheterization and PCI of coronary artery bypass graft (CABG). Stable Angina (PCI vs Surgical Revascularization) Two prospective clinical trials have evaluated balloon angioplasty versus surgery for revascularization of isolated left anterior descending (LAD) coronary artery disease.[36, 37] Using a combined endpoint (cardiac death, myocardial infarction, or refractory angina requiring revascularization by surgery), the Medicine, Angioplasty, or Surgery Study (MASS) showed, after 3 years of follow-up, that endpoint events occurred in 24% of angioplasty patients, 17% of medical patients, and 3% of surgical patients. However, overall survival rates between the 3 groups were similar. The other trial compared balloon angioplasty and bypass surgery with an internal mammary artery graft to the LAD artery and also showed no difference in survival during follow-up. Although 94% of the angioplasty patients and 95% of the bypass patients were free of limiting symptoms, those treated by angioplasty required more antianginal drugs. At median follow-up of 2.5 years, 86% of the surgery patients were free from late events, compared with 43% of angioplasty patients. This difference was primarily due to restenosis requiring a second revascularization procedure. Emphasizing that balloon angioplasty, rather than stent placement, was used in these trials is important; thus, current rates of restenosis with stenting should be lower. Five large (>300 patients) randomized trials comparing balloon angioplasty with bypass surgery in patients with multivessel coronary artery disease (see Table, below, summarizing 3 of the trials) all showed that in appropriately selected patients, the incidence rates of death or of myocardial infarction were similar regardless of whether balloon angioplasty or bypass surgery was used. However, more patients treated with angioplasty required a second revascularization procedure.[38, 39, 40, 41, 42] In the Bypass Angioplasty Revascularization Investigation (BARI), 5year survival was 86.3% for those assigned to angioplasty versus 89.3% for those assigned to surgery, and 5-year freedom from Q-wave myocardial infarction was 78.7% and 80.4%, respectively. However, after 5 years of follow-up, 54% of those assigned to angioplasty required an additional revascularization procedure, compared with only 8% of those assigned to surgery.
Table. Comparison of Surgical Therapy and Coronary Angioplasty (Open Table in a new window) Pocock et al* CAB PTCA G Pocock et al CABG PTCA BARI Study CAB PTCA G
End Point (N=3 58)
(N=374 )
(N=13 03)
(N=1336 ) (N=9 14)
(N=915)
Death (%) 0.3 1.9 2.8 3.1 10.7 13.7 Death or MI 4.5 7.2 8.5 8.1 11.7 10.9 II II Repeat 1.4 16.0 0.8 18.3 0.7 20.5II CABG Repeat 3.6 30.5II 3.2 34.5II 8.0 54.0II CABG or PTCA More than 6.5 14.6II 12.1 17.8II mild angina *Meta-analysis of the results of 3 trials at 1 year: Patients with singlevessel disease were studied.[42]
Meta-analysis of the results of 3 trials at 1 year: Patients with multivessel disease were studied.[42]
Reported results are for the 5-year follow-up. Patients with multivessel disease were studied.
Coronary artery bypass graft
II
P < .05
In a similar manner, the 3-year follow-up of the Argentine Randomized Trial of Percutaneous Transluminal Coronary Angioplasty Versus Coronary Artery Bypass Surgery in Multivessel Disease (ERACI) showed that freedom from combined cardiac events was significantly better for bypass surgery (77% vs 47%) than for angioplasty. However, no differences occurred in overall and cardiac mortality rates or in the frequency of myocardial infarction between the 2 groups. Patients who had bypass surgery were free of angina more frequently (79% vs 57%) and had fewer additional revascularization procedures (6% vs 37%) than patients treated with angioplasty.[43] Patients with diabetes mellitus are an exception regarding equivalent mortality rate results of balloon angioplasty and bypass surgery in multivessel disease. Among diabetic patients in the BARI trial, 5-year survival was 65.5% in those treated by balloon angioplasty and 80.6% for those having bypass surgery.[41] The improved survival with surgery was due to a reduced cardiac mortality rate (5.8% vs 20.6%) and was confined to those receiving at least 1 internal mammary artery graft. Better survival among diabetic patients with multivessel disease treated with bypass surgery rather than angioplasty also was observed in a large retrospective study. The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial randomized 2364 men and women with type 2 diabetes mellitus, documented coronary artery disease, stable symptoms, and myocardial ischemia treated with optimal medical therapy to an initial strategy of coronary revascularization (REV) or watchful waiting with the option of subsequent revascularization (MED). At 5 years, rates of survival or the composite of cardiovascular death, myocardial infarction, and stroke did not differ significantly between the groups.[44] A substudy of the BARI 2D trial reports that the REV strategy improved outcomes at the 3-year follow-up, with patients experiencing a lower rate of worsening angina, new angina, and subsequent coronary revascularizations, and a higher rate of angina-free status.[45] Long-term mortality rates are similar after CABG and PCI in most patient subgroups with multivessel coronary artery disease; therefore, the choice of treatment should depend on patient preferences for other outcomes. In a collaborative analysis of individual patient data from 10 randomized trials, Hlatky and colleagues found CABG to be a superior option for patients with diabetes and patients aged 65 years or older because mortality was lower in these subgroups.[12, 46] Bare Metal Stents and Coronary Artery Bypass Grafting The major limitations of balloon angioplasty have been acute vessel closure and restenosis. Early studies with intracoronary stents showed that these devices were highly effective for treating or preventing acute
or threatened vessel closure and, thus, avoiding emergency bypass surgery. Two randomized trials, STRESS and BENESTENT, demonstrated that coronary stenting of de novo lesions in native vessels reduced angiographic restenosis by approximately 30%, compared with conventional balloon angioplasty. Stenting produces a larger lumen diameter than conventional balloon angioplasty immediately following the procedure (acute gain) and at follow-up (net gain), resulting in less restenosis.[47, 48] The use of stenting, instead of balloon angioplasty, was compared with bypass surgery for the treatment of multivessel coronary artery disease in the Arterial Revascularization Therapies Study (ARTS), and after 1 year of follow-up, no difference was noted between the groups in the rate of death, stroke, or myocardial infarction. Event-free survival was better in the surgery group than in the stent group (87.8% vs 73.8%), and only 3.5% in the surgery group required a second revascularization procedure,[49] versus 16.8% in the stent group. In the ERACI and BARI trials, 37% and 54%, respectively, needed a second revascularization when treated by balloon angioplasty. Overall, patients with diabetes and those who received incomplete surgical revascularization did worse. The cost of the initial revascularization procedure was $4212 less for those treated by stent placement, but because of the need for more repeat revascularization procedures in the stent group, the cost advantage for stenting was reduced to $2973 after 1 year. The Stent or Surgery (SoS) trial compared base metal stents (BMS) with CABG in similar patients and reported a 21% 2-year target vessel revascularization rate in stent patients, versus 6% in CABG patients, with similar death and myocardial infarction rates in the 2 groups. However, the SoS trial had a higher noncardiac death rate in the PCI arm, thought to be attributed to a type II error that may have affected the study results. Few stent patients in the SoS trial received glycoprotein (GP) IIb/IIIa receptor inhibitors. Still, this and the ARTS study do point to the safety of PCI treatment in multivessel disease. Mortality risk is low (discounting the noncardiac deaths), and the rates for repeat target vessel revascularization have been halved.[50] According to the New York Cardiac Registry, as with the prior trials, those patients who received PCI as the initial therapy had a higher incidence of target vessel revascularization (35.1%) versus CABG (4.9%). The registry identified 59,314 patients with multivessel disease who either underwent CABG (37,212) or had PCI with bare metal stents (22,102) with reported endpoints of repeat revascularization and survival rates within 3 years. The registry demonstrated, by unadjusted survival curves, that for patients with 2-vessel disease without LAD
involvement, PCI offered a small survival benefit. For patients with 2vessel disease with proximal LAD disease, the 2 procedures had similar mortality rates (91.4% for CABG, 91.2% for PCI). The registry reported a statistically significant survival benefit of CABG over PCI in patients with 3-vessel disease with proximal LAD disease.[51] Drug-Eluting Stents and Coronary Artery Bypass Grafting Sirolimus-eluting stents were associated with an 8% MACE rate (13% for CABG in ARTS I) and an 8.5% target vessel revascularization rate (4% for CABG and 21% for PTCA in ARTS I), according to the ARTS II trial. The use of drug-eluting stents was compared with CABG in stable angina populations in the ARTS II trial, which was a registry comparing sirolimus-eluting stent (Cypher) with the PTCA and CABG arms of the ARTS I trial. The 1-year MACE rate was 10.5% for sirolimus-eluting stent patients.[52] The New York Cardiac Registry, as previously reported with data from bare metal stents, also found that patients who underwent PCI had a higher rate of target vessel revascularization than those who underwent CABG (30.6% vs 5.2%). They analyzed 17,400 patients who either received a drug-eluting stent (9,963) or CABG (7,437) and observed them for 18 months. Unadjusted survival curves did not demonstrate a statistical significance in survival for 2- or 3-vessel disease. However, when adjusted for several factors (age; sex; ejection fraction; hemodynamic state; history or no history of myocardial infarction before the procedure; the presence or absence of cerebrovascular disease, peripheral arterial disease, congestive heart failure, chronic obstructive pulmonary disease, diabetes, and renal failure; and involvement of the proximal LAD artery), there was statistically significant 18-month survival benefit of CABG over PCI with drug-eluting stents. The SYNTAX study assessed differences in quality of life and angina score in 1,800 patients undergoing bypass surgery or PCI. Patients with 3-vessel or left main coronary artery disease were randomized to CABG or PCI with paclitaxel-eluting stents. Quality of life and angina scores were improved in both groups at 6 and 12 months. Angina score improved more in the CABG group, but the between-group differences were small.[53] The ongoing FREEDOM trial will compare drug-eluting stents and CABG in patients with diabetes and multivessel coronary artery disease. Acute Coronary Syndromes (Unstable Angina and NSTEMI) The management of patients with nonQ-wave myocardial infarction and unstable angina has changed considerably over the past 5 years. Before the widespread use of stents and GP IIb/IIIa receptor inhibitors,
conventional balloon angioplasty in this subgroup of patients was associated with substantial risks, including myocardial infarction (as much as 9%), restenosis (as much as 50%), need for emergency coronary bypass surgery (as much as 12%), and death (as much as 5%). The optimal strategy in patients presenting with acute coronary syndromes remains a controversial issue in contemporary cardiology. Several studies have investigated the use of a conservative strategy versus an early invasive strategy of revascularization for patients with unstable coronary syndromes. To better assess the natural history of coronary artery disease, 697 patients with an acute coronary syndrome treated with PCI were evaluated at the time of the procedure with multivessel intravascular ultrasonography (grey scale and radiofrequency) in the PROSPECT study.[54] The major findings included the following: In patients with ACS treated with PCI, adverse events during a median follow-up of 3.4 years were divided evenly between recurrence at the site of culprit lesions and to nonculprit lesions. Intravascular ultrasonography predictors of nonculprit lesions that will progress include a large plaque burden, small luminal area, and thin-cap fibroatheroma. The Veterans Affairs NonQ-Wave Infarction Strategies in Hospital (VANQWISH) trial compared an invasive strategy with conservative medical treatment in patients with nonQ-wave myocardial infarction and found that the rates of death or nonfatal myocardial infarction were higher in the invasive strategy group than in the conservative strategy group before hospital discharge, at 1 month, and at 1 year. Criticisms of this study include the exclusion of patients at very high risk; the lack of current aggressive medical therapies; a high rate of crossover to angiography in the conservative arm; a higher surgical mortality rate than expected compared with contemporary standards; and the observation that most of the complications at 30 days occurred in patients who underwent coronary artery bypass surgery and very few occurred in patients who underwent balloon angioplasty.[55] In contrast to the VANQWISH trial, 3 randomized studies[56, 57, 58] found that an early invasive approach in patients with acute coronary syndromes was associated with improved outcomes. The Thrombolysis in Myocardial Infarction (TIMI) IIIb study[56] showed less ischemia, shorter hospital stays, fewer readmissions, and fewer symptoms in patients treated by an early invasive approach. The Fragmin and Fast Revascularization during Instability in Coronary Artery Disease (FRISC) II trial[57] prospectively randomized 2457 patients to an early invasive treatment versus a noninvasive treatment strategy and used intracoronary stenting; they found that at 6 months,
the composite endpoint of death or myocardial infarction was higher in the noninvasive treatment group than in patients undergoing an early invasive approach to management. Additionally, symptoms of angina and hospital readmissions in the noninvasive arm were twice that observed using the invasive treatment strategy. The Randomized Intervention in the Treatment of Angina (RITA-III) study reported improved outcomes with early invasive therapy in 1810 patients at 5 years of follow-up.[58] There was a statistically significant difference for all-cause mortality (15.1% vs 12.1%) and cardiac death/myocardial infarction (15.9% vs 12.2%) when comparing an interventional strategy and conservative medical therapy. Data from the Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis in Myocardial Infarction (TACTICS-TIMI) 18 trial showed that the primary end point of death, myocardial infarction, or rehospitalization at 6 months occurred in 19.4% of the conservative group and 15.9% of the invasive group, with the incidence of death and/or myocardial infarction occurring in 9.5% versus 7.3%, respectively. Patients who had a positive troponin test, who had ST segment changes, who were older than 65 years, and, especially, who were women with elevated brain natriuretic peptide (BNP) and C-reactive protein (CRP) levels did particularly better from an early invasive strategy.[59] An analysis of 1200 Dutch patients comparing an early invasive strategy (angiography and revascularization within 48 hours) with a selective invasive strategy (medical stabilization with angiography and revascularization in refractory cases)the Early Invasive versus Selectively Invasive Management for Acute Coronary Syndromes (ICTUS) trialdemonstrated no statistical difference in mortality or the composite endpoint (death, nonfatal myocardial infarction, or rehospitalization for anginal symptoms within 1 year).[60] At 3 years of follow up, there was a trend toward significance favoring the selective invasive strategy for the combined endpoints (30% early invasive vs 26% selective invasive) and no difference in all-cause mortality and cardiac death. Based on these results, the American Heart Association/American College of Cardiology (AHA/ACC) guidelines[61] recommended that an early (within 48 h) invasive approach should be used to treat patients presenting with the following higher-risk features: PCI or CABG in the past 6 months, new ST-segment depression, elevated cardiac biomarkers, recurrent angina at rest or low level of activity, left ventricular systolic function of less than 40%, or TIMI score greater than 2. In lower-risk patients (TIMI score of 2), evidence has shown that invasive therapy and medical therapy provide similar outcomes.
Acute Myocardial Infarction (STEMI) The recognition that intracoronary thrombosis is the primary mechanism of vessel occlusion in acute myocardial infarction and that prompt restoration of vessel patency provides significant clinical benefit has led to the development of aggressive new treatments for this disorder. Thrombolytic therapies, such as front-loaded tissue plasminogen activator (tPA), reteplase (r-PA), and tenecteplase (TNK), open approximately 80% of infarct-related vessels within 90 minutes, but only 50% will have normal (TIMI grade 3) flow. In addition, 10% of vessels opened by thrombolysis either reocclude or are the source for recurrent symptoms of angina. Because of these limitations to thrombolytic therapy, several randomized trials have evaluated mechanical revascularization, so-called primary angioplasty, in the setting of acute myocardial infarction. An analysis of 23 trials confirms the superiority of primary angioplasty over fibrinolytic therapy in terms of adverse events and mortality reduction in both the short term and long term. Overall, primary PCI was associated with significant reductions in death, recurrent myocardial infarction, reinfarction, and the combined endpoint of death, myocardial infarction, and stroke. In the situation where patients are transferred from outside hospitals, primary angioplasty is often preferred to onsite fibrinolytic therapy for patients with the following: expected door-to-balloon time less than 90 minutes and symptom duration less than 3 hours, symptom duration more than 3 hours, cardiogenic shock, contraindications to fibrinolytic therapy, and age older than 75 years. The use of thrombolytic therapy and then referral for intentional PCI (facilitated PCI) has not been shown to be superior to primary PCI and may actually worsen outcomes with increased risk of stroke and bleeding (ASSENT 4). Recent data suggest that early use of GP IIb/IIIa inhibitors may help to achieve earlier infarct vessel patency and better outcomes during PCI. Whether this is so for all of these agents is being assessed in several studies. One meta-analysis has shown that abciximab is associated with a 46% reduction in death and reinfarction in primary PCI patients and the AHA/ACC STEMI guidelines currently recommend early use of abciximab in these patients. When fibrinolytic therapy is given but fails to produce ST resolution, then immediate PCI (rescue PCI) is recommended.[62, 63] Some of the most important considerations in providing effective primary PCI relate to the logistical issues and barriers that are known to exist. The PCI system or network, ambiguity of leadership and organization, protocols/care, pathways/interfacility transfer, and
reimbursement issues are the main areas of contention. Studies of the US primary PCI sites that are considered the best (those sites that deliver door-to-balloon times consistently within 90 minutes, which is currently about 5% of the US myocardial infarction population) have identified the key determinants of shorter door-to-balloon times as the following: ECG being performed within 10 minutes, the emergency department independently making the decision to engage the catheterization laboratory team, and interdisciplinary teamwork. The key factor for effective primary PCI is timely reperfusion therapy. Studies from the National Registry of Myocardial Infarction (NRMI) data have shown that shortening door-to-balloon time to less than 90 minutes is associated with a reduction in mortality. In certain situations, timely reperfusion may be best achieved with fibrinolytic therapy if delays are likely in accessing primary PCI. Rathore and colleagues found that any delay in primary PCI after a patient with STEMI arrives at the hospital is associated with higher mortality.[64] In a prospective cohort study of 43,801 patients enrolled in the American College of Cardiology National Cardiovascular Data Registry, 2005-2006, longer door-to-balloon times were associated with a higher adjusted risk of in-hospital mortality, in a continuous nonlinear fashion (30 min = 3%, 60 min = 3.5%, 90 min = 4.3%, 120 min = 5.6%, 150 min = 7%, 180 min = 8.4%). A reduction in door-to-balloon time from 90 minutes to 60 minutes was associated with 0.8% lower mortality, and a reduction from 60 minutes to 30 minutes was associated with a 0.5% lower mortality.[64] In an analysis of the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial and the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, Brodie et al found that door-to-balloon time of less than 90 minutes was associated with a lower mortality rate in patients with ST-elevation myocardial infarction[65] ; however, the benefit was primarily noted in patients with who presented with less than 90 minutes of symptoms. Door-to-balloon time of less than 90 minutes was associated with similar relative risk reductions in high-risk and low-risk patients, although the absolute benefit was greatest in high-risk patients. From a procedural perspective, because primary PCI involves a thrombotic plaque, the potential risk of more complications exists, especially no reflow and distal embolization. These patients should achieve final TIMI 3 flow. Stenting plus GP IIb/IIIa inhibition has been shown to improve outcomes, reducing target vessel revascularization and myocardial infarction rates in comparison with balloon angioplasty. The use of adjunctive antithrombotic approaches, including early GP
IIb/IIIa inhibition use and mechanical thrombectomy, has shown benefit. Important issues remain as to which type of stent to use (drug-eluting or bare metal), and timing of antiplatelet therapy (both IV and oral) might provide better outcomes for certain patients. Stone and colleagues studied the safety and efficacy of drug-eluting stents and bare-metal stents in patients with STEMI undergoing primary PCI. Patients (n =3006) were assigned in a 3:1 ratio to receive paclitaxel-eluting stents or otherwise identical bare-metal stents. Paclitaxel-eluting stents significantly reduced angiographic evidence of restenosis and recurrent ischemia necessitating repeat revascularization, compared with bare-metal stents, at 12-month followup. Rates of death and stent thrombosis were similar for the 2 groups.[38] General Comparison of PCI and CABG A 2007 research review by the Agency for Healthcare Research and Quality (AHRQ) examined 23 randomized controlled trials of PCI versus CABG (9963 patients) and found that short-term survival was high for both procedures and did not differ significantly.[66] However, freedom from procedural stroke was significantly higher after PCI than after CABG. In the AHRQ study, there was no significant difference in long-term survival (1-5 years of follow-up) between the 2 procedures. However, survival was significantly better after PCI in patients with single-vessel disease that did not involve the proximal LAD and was significantly better after CABG in patients with extensive triple-vessel or left main artery disease. Long-term freedom from angina was significantly greater after CABG than after PCI, as was the need for repeat coronary revascularization procedures. The use of coronary artery stents narrowed, but did not close, the gap between PCI and CABG in repeat revascularization procedures. Although PCI initially was less costly than CABG, the cost difference narrowed substantially over time, to roughly 5%. Complications Associated with PCI Early registries of balloon angioplasty results showed complication rates that were much higher than those typically observed today. With advancements in technique, devices, and adjuvant medical therapy, percutaneous transluminal coronary intervention is now associated with mortality and emergency bypass rates of less than 1%. The rate of nonfatal myocardial infarction following coronary angioplasty ranges from 5-15%, whereas the rate following stent placement is 2-5%. Restenosis after balloon angioplasty requiring a second revascularization procedure is a major limitation occurring in about 15-
30% of patients, depending on the definition of restenosis applied. However, with drug-eluting stents, restenosis rates are now less than 10%. Reduction in the complications of balloon angioplasty has been complemented by improvements in the acute success rate. Registries, such as the National Heart, Lung, and Blood Institute (NHLBI) Coronary Angioplasty Registry from the early 1980s, reported primary success rates of 61%. Today, success rates are 95-99% with the use of stents and adjunctive pharmacotherapy. The mechanism by which balloon angioplasty or stenting improves luminal diameter is associated with significant local trauma to the vessel wall, which can, in turn, lead to occlusive complications in a minority of patients. Coronary artery dissection typically results from the vessel injury secondary to balloon expansion. Animal and postmortem studies have shown that localized dissection at the site of balloon expansion is a common occurrence, detected angiographically in as many as 50% of patients immediately following the procedure. Such small dissections probably are necessary to obtain adequate lumen expansion, rarely interfere with antegrade blood flow, or are important. Angiographic follow-up typically shows no residual evidence of a dissection as early as 6 weeks after angioplasty in most of the cases studied. However, larger dissections can lead to complications. Abrupt vessel closure may occur in as many as 5% of balloon angioplasty cases and typically develops when there is compression of the true lumen by a large dissection flap, thrombus formation, superimposed coronary vasospasm, or a combination of these processes. The presence of large coronary dissections immediately after balloon angioplasty is associated with a 5-fold increase in the risk of abrupt closure. This underscores the importance of a good postprocedure angiographic result on clinical outcomes. Since the introduction and use of intracoronary stents and newer antiplatelet drugs, the incidence of abrupt closure has significantly decreased (to < 1%). Microembolization of plaque debris or adherent thrombus may also cause acute complications during angioplasty and may contribute to postprocedure cardiac enzyme elevation and chest pain in some patients. In less than 1% of patients undergoing angioplasty, microembolization of the platelet-rich thrombus may cause diffuse distal arteriolar vasospasm secondary to the release of vasoactive agents, resulting in the phenomenon of no-reflow. This complication is difficult to treat but may respond to intracoronary calcium channel antagonists, adenosine, or nitroprusside. Patients undergoing balloon angioplasty of saphenous
vein graft lesions and primary angioplasty in the setting of acute myocardial infarction with a large amount of adherent thrombus are at greatest risk of distal embolization. Coronary perforation or rupture following balloon angioplasty is very rare (< 1%) and is typically associated with the use of ablative devices or oversized balloons. Restenosis Following balloon angioplasty or stent implantation, the vessel wall undergoes a number of changes. Platelets and fibrin adhere to the site within minutes of vessel injury. Within hours to days, inflammatory cells infiltrate the site and vascular smooth muscle cells begin to migrate toward the lumen. The vascular smooth muscle cells then hypertrophy and excrete an extensive extracellular matrix. During this period of vascular smooth muscle cell proliferation, endothelial cells colonize the surface of the lumen and regain their normal function. Over the course of several weeks to months, multiple forces interact to cause remodeling of the vessel wall with either a decrease in lumen diameter (negative remodeling) or an increase in lumen diameter (positive remodeling). The amount of late loss in lumen diameter is dependent on the amount of neointimal proliferation and the degree of remodeling following intervention. After 6 months, the repair process stabilizes and the risk of restenosis decreases significantly. See the restenosis image below.
Mechanism of restenosis following percutaneous transluminal coronary angioplasty (PTCA).
Several studies have shown that the lumen diameter or area after
treatment is one of the major predictors of restenosis. The use of coronary artery stents has decreased the rate of restenosis by improving the acute gain achieved and by minimizing negative remodeling. Depending on the definition used, angiographic restenosis has been reported in as many as 50% of patients within 6 months after balloon angioplasty, necessitating repeat target vessel revascularization (TVR) in approximately 20-30% of patients. Drug-eluting stents have reduced restenosis rates to less than 10%. Poststent lumen diameter and lesion complexity are still the major predictors of restenosis with these newer stents. Stent thrombosis While drug-eluting stents have significantly reduced restenosis events, concerns of stent thrombosis with these newer stents still exist. In fact, the rate of thrombosis with a drug-eluting stent is virtually identical to that for a bare metal stent at 1 year (0.5-0.7%). However, late stent thrombosis (>1 y) continues to occur with a drug-eluting stent, while it is exceedingly rare for a bare metal stent. The biggest factor contributing to stent thrombosis is interruption of antiplatelet therapy. Current guidelines recommend a minimum of 1 year of dual antiplatelet therapy for drug-eluting stents and a month for bare metal stents.[29] Drug-eluting stents take longer to endothelialize on the coronary vessel wall than bare metal stents, and discontinuing dual antiplatelet therapy may expose patients to an increased risk for stent thrombosis over time. In some clinical situations (such as before urgent noncardiac surgery where antiplatelet therapy may have to be discontinued and in patients with known or potential medicine compliance issues), implanting a bare metal stent may be preferred during PCI rather than using a drug-eluting stent. Another important factor is final stent diameter and area. Underdeployment or incomplete apposition of the drug-eluting stent may also increase the risk for stent thrombosis. This is not to say that drug-eluting stents are unsafe. In fact, there is no difference in longterm rates of death and myocardial infarction between drug-eluting and bare metal stents; however, there is a striking reduction in restenosis. Stone and colleagues found that although stent thrombosis is infrequent, it results in higher rates of myocardial infarction and death. The more frequent target lesion revascularization results in a lower rate of myocardial infarction and death. Although late stent thrombosis is a risk with drug-eluting stents, the noticeable reduction in restenosis may offset the risk.[67]
Anda mungkin juga menyukai
- Dialysis Treatment Options and Techniques for Kidney FailureDokumen11 halamanDialysis Treatment Options and Techniques for Kidney FailureGail Leslie HernandezBelum ada peringkat
- Percutaneous Coronary Intervention 10 05Dokumen8 halamanPercutaneous Coronary Intervention 10 05benypermadiBelum ada peringkat
- 2012 Cath Lab Consensus DocumentDokumen85 halaman2012 Cath Lab Consensus DocumentDorin DocBelum ada peringkat
- Drugs Given During Cardiac Arres For CPRDokumen5 halamanDrugs Given During Cardiac Arres For CPREevyaj MimiBelum ada peringkat
- Drug Study (Print3copiesDokumen8 halamanDrug Study (Print3copiesPhylum ChordataBelum ada peringkat
- Clinical Examination of Genitourinary SystemDokumen43 halamanClinical Examination of Genitourinary SystemKeamina .aBelum ada peringkat
- Nursing Care PlanDokumen5 halamanNursing Care PlanNicolne LorraineBelum ada peringkat
- Cardiac BiomarkersDokumen7 halamanCardiac BiomarkersAnand VeerananBelum ada peringkat
- Ivc FilterDokumen15 halamanIvc FilterashishBelum ada peringkat
- Prepared By, Gayathri R 2 Yr MSC (N) UconDokumen41 halamanPrepared By, Gayathri R 2 Yr MSC (N) UconGayathri RBelum ada peringkat
- Arterial Line Arterial LineDokumen13 halamanArterial Line Arterial LineLinamaria Lozano100% (1)
- Ventricular Septal DefectsDokumen7 halamanVentricular Septal DefectsMuhammadAldoGiansyahBelum ada peringkat
- Types of Anesthesia and Their Purpose and RisksDokumen8 halamanTypes of Anesthesia and Their Purpose and RisksAshlene Kate BagsiyaoBelum ada peringkat
- Water Seal DrainageDokumen9 halamanWater Seal DrainagenikaaraaaBelum ada peringkat
- Fulminant Hepatic FailureDokumen12 halamanFulminant Hepatic Failureafghansyah arfiantoBelum ada peringkat
- Mic Cabg Procedure PDFDokumen12 halamanMic Cabg Procedure PDFprofarmah6150Belum ada peringkat
- DefibrillationDokumen9 halamanDefibrillationJara Maris Moreno BudionganBelum ada peringkat
- SP42 Thoracentesis (Adult)Dokumen7 halamanSP42 Thoracentesis (Adult)Adam HuzaibyBelum ada peringkat
- Congestive Cardiac FailureDokumen20 halamanCongestive Cardiac FailureAnand VaghasiyaBelum ada peringkat
- What is a gastrectomy surgeryDokumen4 halamanWhat is a gastrectomy surgeryPriyanka JangraBelum ada peringkat
- Thoracic Surgery Secrets Book PDFDokumen4 halamanThoracic Surgery Secrets Book PDFRishi MangalBelum ada peringkat
- CABG Case Study ReportDokumen83 halamanCABG Case Study ReportSherena NicolasBelum ada peringkat
- IntroductionDokumen13 halamanIntroductionSiyara AntonyBelum ada peringkat
- Acute Pancreatitis NOTESDokumen17 halamanAcute Pancreatitis NOTESsameeha semiBelum ada peringkat
- Angiotensin I Angiotensin II, Angiotensin-Converting Enzyme (ACE) - Vasoconstrictor Angiotensin II Stimulates Aldosterone SecretionDokumen11 halamanAngiotensin I Angiotensin II, Angiotensin-Converting Enzyme (ACE) - Vasoconstrictor Angiotensin II Stimulates Aldosterone SecretionAbdullah asadBelum ada peringkat
- Kidney TransplantDokumen11 halamanKidney TransplantPrincess Xzmae RamirezBelum ada peringkat
- Advanced Airway Care: Intensive Care Unit PerspectiveDokumen42 halamanAdvanced Airway Care: Intensive Care Unit PerspectiveJeffery Samuel100% (1)
- Hypertensive Emergencies (ESC 2019)Dokumen10 halamanHypertensive Emergencies (ESC 2019)Glen LazarusBelum ada peringkat
- Modes of VentilatorDokumen17 halamanModes of VentilatorFahrizal MuhammadBelum ada peringkat
- Blood Gas Analysis For Bedside DiagnosisDokumen6 halamanBlood Gas Analysis For Bedside DiagnosisMuhamad Wirawan AdityoBelum ada peringkat
- Cardiac SurgeryDokumen27 halamanCardiac SurgeryReeti Singh100% (1)
- CardioversionDokumen48 halamanCardioversionDeeksha RajputBelum ada peringkat
- Chest Tube, Urinary Catheter, Ryles Tube InsertionDokumen60 halamanChest Tube, Urinary Catheter, Ryles Tube InsertionMohd Johari Mohd ShafuwanBelum ada peringkat
- Drugs Used For Conscious SedationDokumen1 halamanDrugs Used For Conscious SedationonslowmoBelum ada peringkat
- Renal TransplantationDokumen50 halamanRenal Transplantationregie cuaresmaBelum ada peringkat
- Assisting For Application of POPDokumen7 halamanAssisting For Application of POPINSERVICE EDUCATIONBelum ada peringkat
- Disorders of AortaDokumen25 halamanDisorders of Aortavani reddyBelum ada peringkat
- Hepatic Failure & Hepatic EncephalopathyDokumen37 halamanHepatic Failure & Hepatic Encephalopathyapi-19916399Belum ada peringkat
- Pneumonia Management ProtocolDokumen2 halamanPneumonia Management Protocolsky nuts100% (1)
- Endotracheal IntubationDokumen11 halamanEndotracheal Intubationanon_784834955100% (1)
- LESSON 9 Organ Donation ActDokumen56 halamanLESSON 9 Organ Donation ActnullBelum ada peringkat
- ECG MonitoringDokumen28 halamanECG MonitoringGlaiza Mae Olivar-ArguillesBelum ada peringkat
- Care of Patient With TPM Slide PresentationDokumen16 halamanCare of Patient With TPM Slide PresentationirzehronBelum ada peringkat
- Ards PDFDokumen2 halamanArds PDFgireeshsachinBelum ada peringkat
- Mobile Coronary Care UnitDokumen16 halamanMobile Coronary Care UnitArcha100% (2)
- Cardiac TamponadeDokumen29 halamanCardiac Tamponadeanimesh pandaBelum ada peringkat
- Blood Transfusion ReactionDokumen9 halamanBlood Transfusion ReactionReema Akberali nooraniBelum ada peringkat
- Cardiac EmergenciesDokumen21 halamanCardiac Emergenciesgus_lionsBelum ada peringkat
- Cardiac SurgeryDokumen35 halamanCardiac SurgeryxxxBelum ada peringkat
- Baska MaskDokumen8 halamanBaska MaskAnish H DaveBelum ada peringkat
- Nursing Management of Common Skin and Parasitic DiseasesDokumen14 halamanNursing Management of Common Skin and Parasitic Diseasesyer tagalajBelum ada peringkat
- Management of Vacuum Assisted Closure TherapyDokumen14 halamanManagement of Vacuum Assisted Closure TherapyVoiculescu MihaelaBelum ada peringkat
- Lessonplan FinDokumen14 halamanLessonplan Finx483xDBelum ada peringkat
- HAI bUNDLESDokumen54 halamanHAI bUNDLESNurhayati100% (1)
- Acute Pyelonephritis Treatment GuidelinesDokumen4 halamanAcute Pyelonephritis Treatment GuidelinesPeter InocandoBelum ada peringkat
- Endotracheal Intubation GuideDokumen4 halamanEndotracheal Intubation Guiderupali gahalianBelum ada peringkat
- Cyanotic Congenital Heart DiseasesDokumen25 halamanCyanotic Congenital Heart DiseasesAlvin OmondiBelum ada peringkat
- Laryngeal Mask Airway GuideDokumen25 halamanLaryngeal Mask Airway GuideCiptadi IqbalBelum ada peringkat
- HOSPITAL DEPTS GUIDEDokumen16 halamanHOSPITAL DEPTS GUIDEfictoriaBelum ada peringkat
- Oral Cancer in Young Adults Report ofDokumen4 halamanOral Cancer in Young Adults Report ofMelissa KanggrianiBelum ada peringkat
- Formato Denver IIDokumen1 halamanFormato Denver IIMelly AnidaBelum ada peringkat
- Jnma00288 0098Dokumen3 halamanJnma00288 0098Melissa KanggrianiBelum ada peringkat
- Chest XDokumen2 halamanChest XMelissa KanggrianiBelum ada peringkat
- Indian JOurnal of PediatricsDokumen8 halamanIndian JOurnal of Pediatricsliska ramdanawatiBelum ada peringkat
- Diagnosis, Treatment, and Prevention of Typhoid Fever (WHO)Dokumen48 halamanDiagnosis, Treatment, and Prevention of Typhoid Fever (WHO)Nina KharimaBelum ada peringkat
- CDC Chapter IIIDokumen20 halamanCDC Chapter IIIMelissa KanggrianiBelum ada peringkat
- CDC Chapter VDokumen17 halamanCDC Chapter VMelissa KanggrianiBelum ada peringkat
- STABLE Program Module Objectives SummaryDokumen2 halamanSTABLE Program Module Objectives SummaryMelissa Kanggriani100% (1)
- Daily Multivitamins With Iron To Prevent Anemia in High-Risk Infants - MelDokumen10 halamanDaily Multivitamins With Iron To Prevent Anemia in High-Risk Infants - MelMelissa KanggrianiBelum ada peringkat
- Sepsis Neonatorum NICE 2012Dokumen40 halamanSepsis Neonatorum NICE 2012Melissa KanggrianiBelum ada peringkat
- Burning Mouth Syndrome-EtiologyDokumen5 halamanBurning Mouth Syndrome-EtiologyMelissa KanggrianiBelum ada peringkat
- Fever in Child Apa Peds.2010-3852v1Dokumen10 halamanFever in Child Apa Peds.2010-3852v1Ibrahim AlmoosaBelum ada peringkat
- Nephrotic SyndromeDokumen8 halamanNephrotic SyndromeAde Gustina SiahaanBelum ada peringkat
- Faring It IsDokumen34 halamanFaring It IsMelissa KanggrianiBelum ada peringkat
- Sepsis - Fanarof N AveryDokumen56 halamanSepsis - Fanarof N AveryMelissa KanggrianiBelum ada peringkat
- The Host Response To Sepsis and Developmental ImpactDokumen13 halamanThe Host Response To Sepsis and Developmental ImpactMelissa KanggrianiBelum ada peringkat
- Korti Ko SteroidDokumen5 halamanKorti Ko SteroidMelissa KanggrianiBelum ada peringkat
- Korti Ko SteroidDokumen5 halamanKorti Ko SteroidMelissa KanggrianiBelum ada peringkat
- Nephrotic Syndrome PDFDokumen16 halamanNephrotic Syndrome PDFSulabh ShresthaBelum ada peringkat
- Rekomendasi TTG TLSDokumen9 halamanRekomendasi TTG TLSMelissa KanggrianiBelum ada peringkat
- Kista GanglionDokumen7 halamanKista GanglionMelissa KanggrianiBelum ada peringkat
- HaematomasDokumen6 halamanHaematomasMelissa KanggrianiBelum ada peringkat
- GI TRACT GabunganDokumen7 halamanGI TRACT GabunganMelissa KanggrianiBelum ada peringkat
- Chest XDokumen2 halamanChest XMelissa KanggrianiBelum ada peringkat
- Urinary Incontinence in WomenDokumen10 halamanUrinary Incontinence in WomenMelissa KanggrianiBelum ada peringkat
- Chronic Gastritis - Emedicine - 2 Des 2010Dokumen15 halamanChronic Gastritis - Emedicine - 2 Des 2010Melissa KanggrianiBelum ada peringkat
- Hipertensi Dan KehamilanDokumen15 halamanHipertensi Dan KehamilanMelissa KanggrianiBelum ada peringkat
- Reduced Renal Sodium Excretion: Forced Through A Narrow LumenDokumen5 halamanReduced Renal Sodium Excretion: Forced Through A Narrow LumenFlowerBelum ada peringkat
- Presentation BP MonitorDokumen7 halamanPresentation BP MonitorImwaniki21Belum ada peringkat
- Normal Hemodynamic Parameters and Laboratory Values PDFDokumen3 halamanNormal Hemodynamic Parameters and Laboratory Values PDFKenNiyaruBelum ada peringkat
- Stroke: Hypertensive UrgencyDokumen4 halamanStroke: Hypertensive UrgencyMaricar TaboraBelum ada peringkat
- Biology DLP KSSM Form 4 Chapter 10Dokumen37 halamanBiology DLP KSSM Form 4 Chapter 10Dewi SallehBelum ada peringkat
- Syllabus ANP1105Dokumen10 halamanSyllabus ANP1105Nusrat TazkiaBelum ada peringkat
- Circulation: Campbell Biology: Concepts & ConnectionsDokumen88 halamanCirculation: Campbell Biology: Concepts & ConnectionsBehshxbxBelum ada peringkat
- Coronary Perfusion Pressure - Wikipedia, The Free EncyclopediaDokumen1 halamanCoronary Perfusion Pressure - Wikipedia, The Free EncyclopediaAniket MittalBelum ada peringkat
- Pharmacology Lecture - 20 Antianginal DrugsDokumen3 halamanPharmacology Lecture - 20 Antianginal DrugsChris QueiklinBelum ada peringkat
- Dental Management in Medically Compromised Patients Hypertens IonDokumen17 halamanDental Management in Medically Compromised Patients Hypertens Ionآلقہيہسہيہ مہجہآهہدBelum ada peringkat
- Biophysical Profile& Color Doppler Ultrasound in The High Risk PregnancyDokumen56 halamanBiophysical Profile& Color Doppler Ultrasound in The High Risk Pregnancykhadzx100% (4)
- Cardiovascular TSDokumen22 halamanCardiovascular TSAsimaJulianaSiregarBelum ada peringkat
- Decreased Cardiac OutputDokumen3 halamanDecreased Cardiac OutputCristina L. JaysonBelum ada peringkat
- Crochet Anatomical Human Heart: by Kyla RaaymakersDokumen7 halamanCrochet Anatomical Human Heart: by Kyla RaaymakersJavier BejaranoBelum ada peringkat
- CMR - Guide - 2nd - Edition - 148x105mm - 03may2017 - Last VersionDokumen124 halamanCMR - Guide - 2nd - Edition - 148x105mm - 03may2017 - Last VersionCristián Martínez BocazBelum ada peringkat
- ANAPHY Lec Session #16 - SAS (Agdana, Nicole Ken)Dokumen10 halamanANAPHY Lec Session #16 - SAS (Agdana, Nicole Ken)Nicole Ken AgdanaBelum ada peringkat
- Activity 7 The Cardiovascular SystemDokumen4 halamanActivity 7 The Cardiovascular SystemEllen Mynelle MabulacBelum ada peringkat
- 2° Consenso Mexicano de AQEDokumen11 halaman2° Consenso Mexicano de AQEFernando Vega RiverosBelum ada peringkat
- Interpreting The Coronary-Artery Calcium ScoreDokumen3 halamanInterpreting The Coronary-Artery Calcium ScoreGaby Alejandra Ordonez AndradeBelum ada peringkat
- What causes a strokeDokumen5 halamanWhat causes a strokeAfia TawiahBelum ada peringkat
- % Chapter 3: Regulation of Gas Content in BloodDokumen33 halaman% Chapter 3: Regulation of Gas Content in BloodK CBelum ada peringkat
- Coronary Artery Disease PathophysiologyDokumen3 halamanCoronary Artery Disease Pathophysiologynursing concept maps67% (3)
- Physical Inactivity: Aging Men Hypertension Smoker ObesityDokumen1 halamanPhysical Inactivity: Aging Men Hypertension Smoker ObesityKEn PilapilBelum ada peringkat
- Pathway Stroke Non Hemoragik EngDokumen1 halamanPathway Stroke Non Hemoragik EngFitria NorkhalidaBelum ada peringkat
- Fetal CirculationDokumen18 halamanFetal CirculationDr_ibk100% (1)
- Prognosis of Patients With Complete Heart BlockDokumen7 halamanPrognosis of Patients With Complete Heart BlockRaul OrtegaBelum ada peringkat
- Treatment of Heart attack-हृदय घात का इलाज-by Shri Rajiv DixitDokumen5 halamanTreatment of Heart attack-हृदय घात का इलाज-by Shri Rajiv Dixitvirendrak11100% (1)
- Blood Supply of The GITDokumen66 halamanBlood Supply of The GITgtaha80Belum ada peringkat
- Laboratory Worksheet 12 Heart Structure and FunctionDokumen4 halamanLaboratory Worksheet 12 Heart Structure and FunctionAndrea SaldivarBelum ada peringkat
- Cardiac Emergencies (Autosaved)Dokumen30 halamanCardiac Emergencies (Autosaved)Sakshi RaturiBelum ada peringkat