WPI Modeling Batch Distillation
Diunggah oleh
Walter MiguelDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
WPI Modeling Batch Distillation
Diunggah oleh
Walter MiguelHak Cipta:
Format Tersedia
Modeling Batch Distillation W. M.
Clark, WPI, March 2008 This report documents my efforts to model a batch distillation process in an attempt to support an educational collaboration between Professor Jim Henry at the University of Tennessee at Chattanooga, Professor Marina Miletic at the University of Illinois UrbanaChampaign, and Professor David DiBiasio at Worcester Polytechnic Institute. The main goal of this collaboration is to investigate the advantages and disadvantages of remote operation of a distillation column for a chemical engineering laboratory component via distance learning. The learning experiences of students doing hands-on batch distillation experiments at WPI and Urbana-Champaign are being compared to those of other students at these same locations who are conducting batch distillation experiments at Chattanooga by remote control over the web. Adding the capability of modeling a batch distillation process could potentially be useful for either the remote learners or the handson learners or both. In addition, it might be interesting to compare students who study only a simulated batch distillation process to those who do hands-on or remote experiments. The initial thought was to try to develop a model of batch distillation similar in concept to models we have recently developed for a membrane process and a heat exchanger using Comsol Multiphysics finite element software. The goal was to have a model that provides a visual representation of the temperature and concentration profiles that result from solving the differential equations representing the process going on within the distillation unit. I still believe that such a model would be better for promoting conceptual understanding than a black box model where students provide input and get the expected results based on behind-the-scene calculations that are not revealed to the students. Modeling the complex and dynamic behavior of a multistage batch distillation column proved to be overwhelming, however, and this attempt was abandoned in favor of evaluating existing models such as ChemSep, MultiBatchDS, and Aspen BatchSep. ChemSep was created by Professors Hendrik Kooijman and Ross Taylor at Clarkson University as a teaching tool for courses in thermodynamics and separation processes. It can perform calculations and plot result profiles for a variety of separation processes including multistage, multicomponent distillation. Among the key features are a wide range of built-in thermodynamic models for calculating physical properties and plotting phase diagrams and the automatic generation of McCabe Thiele diagrams complete with stepped stages. ChemSep can be purchased from the Computer Aided Chemical Engineering (CACHE) Corporation for a $100 initial license and a $60 fee for annual license renewal after the first year. There is also a free ChemSep-Lite version. ChemSep does not have a built-in batch distillation simulator at present, but according to the ChemSep book, batch distillation is another area which would be possible to investigate if we were able to specify an initial amount of each component present in the reboiler. Startup would begin with a total reflux simulation [then a dynamic simulation could be run where] the perturbation consists then of starting to draw a distillate
product. ChemSep was purchased from CACHE and this process was attempted without success. The ChemSep book indicates that there are four modes of operation available: flash, equilibrium column, non-equilibrium column, and dynamic column. The ChemSep-Lite version that I downloaded for free only had flash and equilibrium column available. Version 6.06 of ChemSep that I purchased along with the ChemSep Book from CACHE in fall 2007 included non-equilibrium column and dynamic column but dynamic column was grayed out and apparently still under development or unavailable through CACHE. Noting that the ChemSep book I was sold is not consistent with Version 6.06 of the software, that a new edition of the ChemSep book is under development with proofs available on the web, and that batch distillation is still not addressed in the new edition, attempts to model batch distillation with ChemSep were abandoned. ChemSep did prove useful and easy to use for modeling steady state distillation processes including total reflux operation. Figure 1 shows a McCabe Thiele diagram that was generated with ChemSep for ethanol-water distillation at 1 atm operating pressure with a 60% stage efficiency, 12 stage (including a condenser and a reboiler) column operating at total reflux. In addition to the number of stages and stage efficiency, required input included the boilup rate (1e-4 kmol/s), the amount of subcooling in the condenser (40 K), and the composition of the liquid in the condenser (0.78 mole fraction ethanol). Figures 2 and 3 show the corresponding temperature and composition profiles generated by ChemSep for this total reflux condition.
Figure 1. McCabe Thiele diagram drawn by ChemSep for ethanol-water at total reflux with operating conditions as specified in the text.
Figure 2. Column temperature profile for ethanol-water at total reflux for operating conditions given in the text.
Figure 3. Column compostion profiles for ethanol-water at total reflux for operating conditions given in the text.
Other results are also readily available in tabular or graphical form. With this software, students can readily see the effect that changes in the operating conditions have on the column at total reflux. One significant limitation is that the composition of the distillate (or bottoms) at total reflux must be specified instead of that of the initial charge. This software appears to be quite useful for illustrating steady state distillation operation with known feed and product flow rates. MultiBatchDS is a commercial software package developed by Urmila Diwekar at Carnegie Mellon University for simulating batch distillation processes. An educational version of the software is available from CACHE Corp. for a $90 initial license and a $50 fee for annual license renewal after the first year. Several factors including (1) my experience with the limitations of the educational version of ChemSep purchased from CACHE, (2) the statement that the software comes on two diskettes for the PC on the current CACHE order form, (3) a quick web search indicating that the software is apparently not widely used, and (4) acquisition of ASPEN BatchSep software, caused me to postpone, perhaps forever, any study of the MultiBatchDS software. Aspen BatchSepTM is a core element of AspenTechs aspenONETM Process Engineering applications specifically for simulation of batch distillation. We have been using Aspen Plus for teaching design at WPI for a number of years but until now, we were unaware that this additional program was available to us. Aspen BatchSep runs separately and needs to be installed separately from Aspen Plus but it appears that our site license for Aspen Plus also allows us to install and use Aspen BatchSep. The BatchSep program is similar to Aspen Plus but different enough and complex enough that it presents a significant learning curve even for someone familiar with Aspen Plus. The expense of Aspen software might be a deterrent for students at locations that dont currently have a license. We can probably make results from a limited set of simulations done under our license available to students over the web, but we cannot allow students at other locations to use the software under our license. An undergraduate student working with Professor DiBiasio has obtained data on our distillation column working in batch mode to recover an ethanol-rich overhead product from an initial charge of 1 weight percent (0.0039 mole fraction) ethanol in water. This low concentration was selected because it allowed a batch run to be completed within a three hour time limit. The column has 12 stages including the condenser and reboiler and was operated at 1 atm pressure at total reflux before distillate was removed at a reflux ratio of 7.5. Experimental results led to the conclusion that the murphree stage efficiency was approximately 60 % for this experiment. Figure 4 shows the distillate concentration versus time as measured by the student. Figure 5 shows the corresponding temperatures at the top and bottom stages versus time.
0.8 Ethanol in Distillate 0.7 0.6 0.5 0.4 0.3 0.2 0 0.5 Time (hr) 1 1.5
Figure 4. Experimental results for mole fraction ethanol in distillate as a function of time.
105 100 Temperature 95 90 85 80 75 70 0 0.5 Time (hr) 1 1.5
Figure 5. Experimental results for temperature at the top, stage 2, (blue diamonds) and bottom, stage 11, (red squares) of the column as a function of time. Note that the initial conditions at total reflux are similar to those modeled above using ChemSep. The Chemsep values were 0.78 mole fraction ethanol and T = 78 oC at the top of the column. Although it required some guesswork and trial and error to estimate some of the required input, I was able to simulate, at least qualitatively, the batch process described above using Aspen BatchSep. This comprehensive software can incorporate many details like
holdup volumes, efficiencies, and heat losses on trays, but if these are not known, they must be estimated appropriately. Figure 6 shows the simulation results for distillate composition as a function of time beginning at total reflux. Note that the composition of the initial charge of 0.0039 mole fraction ethanol was specified for the simulation and the total reflux results were calculated. Figure 7 presents the simulation results for temperature as a function of time at 4 locations (stages 2,5,8, and 11) including the ones at the top and the bottom of the column. A wide range of other results in either tabular or graphical form can be readily obtained from the simulation output.
Figure 6. Aspen BatchSep simulation results for distillate composition as a function of time.
Figure 7. Aspen BatchSep simulation results for temperature at 4 locations as a function of time.
Comparing Figures 4 and 5 to Figures 6 and 7 indicates that the current simulation results are qualitatively similar to the experimental results. Discrepancies between the two can be attributed to a number of factors including experimental error and uncertainties in estimated parameters for the model. Part of the problem is likely to be the fact that the low amount of ethanol in the system makes the composition of ethanol at the lower stages very small and makes the simulation (and the experiment) sensitive to small changes in process parameters. Simulation of a column with more ethanol would likely be easier. After several failed attempts to adjust the model so that it would agree with the experiment, it was concluded that an exact match between model and experiment may not be necessary. Conceptual understanding of the process can probably be obtained from a model that is not in complete agreement with the experiment. In fact, it might be better if simulation results are provided to the students at conditions that are different from the experimental conditions. Students could study the simulation results for conceptual understanding and expected trends but not be tempted to dry lab the experiment because they know the expected results. What shall we do next? Is Aspen Plus and/or Aspen BatchSep available at the other schools? Is there any merit to having students run simulations or look at simulated results either before, during, or after they run the lab? Are there other results that we should focus on besides temperature and distillate composition? Do we need to have a simulation that gives good agreement with experiment to make it credible to the students? Do we want to include some modeling results in the upcoming talk? Do we want to try for some add-on funding for our current grant aimed at simulating laboratory experiments? Do we want to write a separate proposal that includes the distance versus hands-on experimentation and the modeling?
Anda mungkin juga menyukai
- FlowsheetingWithCOCOandChemsep NotesDokumen104 halamanFlowsheetingWithCOCOandChemsep NotesShoaib Jadoon100% (1)
- Chem Sep Release 8 V 1Dokumen8 halamanChem Sep Release 8 V 1randi martaBelum ada peringkat
- Mass Tr. Process SimulationDokumen38 halamanMass Tr. Process SimulationMohammed SalehBelum ada peringkat
- Why Is Rate-Based Distillation Better Than Using Equilibrium Stages With EfficienciesDokumen5 halamanWhy Is Rate-Based Distillation Better Than Using Equilibrium Stages With Efficienciessushant0261Belum ada peringkat
- T2004E (21e) Step-Test Free APC Implementation Using Dynamic SimulationDokumen17 halamanT2004E (21e) Step-Test Free APC Implementation Using Dynamic SimulationAbderrahman MahiddiniBelum ada peringkat
- BD Theory HandoutDokumen4 halamanBD Theory HandoutJunaid AhmadBelum ada peringkat
- 5 PDFDokumen18 halaman5 PDFEr Mayur PatilBelum ada peringkat
- Why Is Rate-Based Distillation Better Than Using Equilibrium PDFDokumen6 halamanWhy Is Rate-Based Distillation Better Than Using Equilibrium PDFRajendraBelum ada peringkat
- Coco SoftwareDokumen22 halamanCoco SoftwareMisael RamírezBelum ada peringkat
- Problem StatementDokumen5 halamanProblem StatementMreza JafariBelum ada peringkat
- Tutorial 13 ASPEN PLUS Aspen DistillationDokumen18 halamanTutorial 13 ASPEN PLUS Aspen DistillationramsrivatsanBelum ada peringkat
- Lab Report 1 PDF FormatDokumen14 halamanLab Report 1 PDF FormatSapna RanaBelum ada peringkat
- Aspen Dynamics Simulation of A Middle-Vessel BatchDokumen11 halamanAspen Dynamics Simulation of A Middle-Vessel BatchabramsBelum ada peringkat
- Design Guidelines For Proplyene Splitters - Rev inDokumen10 halamanDesign Guidelines For Proplyene Splitters - Rev intotongopBelum ada peringkat
- Solving Mass Transfer Problems On The Computer Using MathcadDokumen8 halamanSolving Mass Transfer Problems On The Computer Using MathcadCarlos Alfonzo Calderón RiveroBelum ada peringkat
- Crude DistillationDokumen6 halamanCrude DistillationNag RajBelum ada peringkat
- Distillation Dynamics and Control Workbook 2006 PDFDokumen18 halamanDistillation Dynamics and Control Workbook 2006 PDFEr Mayur PatilBelum ada peringkat
- 2009 McCabe Thiele DiagramDokumen9 halaman2009 McCabe Thiele DiagramSyazwan WanBelum ada peringkat
- pl4 PDFDokumen23 halamanpl4 PDFDhairyashil SantreBelum ada peringkat
- McCabe Thiele MethodDokumen3 halamanMcCabe Thiele MethodniezajanepatnaBelum ada peringkat
- Visualizing The Mccabe-Thiele Diagram: Reactions and SeparationsDokumen9 halamanVisualizing The Mccabe-Thiele Diagram: Reactions and SeparationsMicheal BrooksBelum ada peringkat
- Richard Nakka's Experimental Rocketry Web Site: Solid Rocket Motor Theory - GUIPEPDokumen8 halamanRichard Nakka's Experimental Rocketry Web Site: Solid Rocket Motor Theory - GUIPEPSharat ChandraBelum ada peringkat
- Uncovering The Realities of Simulation, Part 1 (Of 1)Dokumen11 halamanUncovering The Realities of Simulation, Part 1 (Of 1)bjsatola100% (2)
- Model Based Control of A Four-Tank SystemDokumen6 halamanModel Based Control of A Four-Tank SystemJuan Manuel MauroBelum ada peringkat
- Applied Thermodynamics For Process ModelingDokumen7 halamanApplied Thermodynamics For Process ModelingdhavalmpBelum ada peringkat
- Lectura 3 1 10Dokumen10 halamanLectura 3 1 10ANDRESBelum ada peringkat
- Process Simulation and Control Using AspenDokumen331 halamanProcess Simulation and Control Using Aspenfarshidian96% (27)
- Distillation Handbook 10004 01-08-2008 USDokumen52 halamanDistillation Handbook 10004 01-08-2008 USzuhalelbarqiBelum ada peringkat
- Aspen Vs HYSYSDokumen8 halamanAspen Vs HYSYSdanyjwBelum ada peringkat
- Aspen Tutoria1lDokumen72 halamanAspen Tutoria1lMohammed Hassan B KorainaBelum ada peringkat
- Process Modeling Using HYSYS With Chemical Industry Focus (TQN)Dokumen5 halamanProcess Modeling Using HYSYS With Chemical Industry Focus (TQN)Cesar Augusto Vanegas MurilloBelum ada peringkat
- McCabe-Thiele Distillation Column Design For A Methanol-Propanol SystemDokumen12 halamanMcCabe-Thiele Distillation Column Design For A Methanol-Propanol SystemPeyton EllenBelum ada peringkat
- Acetone and Water DistillationDokumen17 halamanAcetone and Water Distillationinvincible111100% (1)
- Co-Chemsep: Nonequilibrium Modelling: The Cape Open WayDokumen19 halamanCo-Chemsep: Nonequilibrium Modelling: The Cape Open Wayvsraochemical1979100% (1)
- Two Examples of Steady State Simulation With HYSYS atDokumen6 halamanTwo Examples of Steady State Simulation With HYSYS atRolando Enrique Zelada MuñozBelum ada peringkat
- Modeling Separation Systems With Aspen PlusDokumen23 halamanModeling Separation Systems With Aspen PlusparykoochakBelum ada peringkat
- HDA Simulation Laboratory - 054330Dokumen18 halamanHDA Simulation Laboratory - 054330Fussy Taksn100% (1)
- Simulation of Ethanol Production Process Using Aspen Plus and Optimization Based On Response Surface MethodologyDokumen10 halamanSimulation of Ethanol Production Process Using Aspen Plus and Optimization Based On Response Surface MethodologyMaria Camila Ortiz SarmientoBelum ada peringkat
- Mordechai Shacham, Ben-Gurion University, Beer-Sheva, Israel Michael B. Cutlip, University of Connecticut, Storrs, CT 06269, USADokumen9 halamanMordechai Shacham, Ben-Gurion University, Beer-Sheva, Israel Michael B. Cutlip, University of Connecticut, Storrs, CT 06269, USAingbarragan87Belum ada peringkat
- Distillation Handbook 10004 01-08-2008 USDokumen52 halamanDistillation Handbook 10004 01-08-2008 USSumit SinghBelum ada peringkat
- Acid Gas Cleaning Demo: Working InstructionsDokumen13 halamanAcid Gas Cleaning Demo: Working Instructionsreclatis14Belum ada peringkat
- Chemical Engineering Process SimulationDari EverandChemical Engineering Process SimulationPenilaian: 4 dari 5 bintang4/5 (13)
- Petroleum Production Engineering, A Computer-Assisted ApproachDari EverandPetroleum Production Engineering, A Computer-Assisted ApproachPenilaian: 4.5 dari 5 bintang4.5/5 (11)
- Dimensional Analysis: Practical Guides in Chemical EngineeringDari EverandDimensional Analysis: Practical Guides in Chemical EngineeringBelum ada peringkat
- Computer-Controlled Systems: Theory and Design, Third EditionDari EverandComputer-Controlled Systems: Theory and Design, Third EditionPenilaian: 3 dari 5 bintang3/5 (4)
- Preparative Chromatography for Separation of ProteinsDari EverandPreparative Chromatography for Separation of ProteinsArne StabyBelum ada peringkat
- The Partition Method for a Power Series Expansion: Theory and ApplicationsDari EverandThe Partition Method for a Power Series Expansion: Theory and ApplicationsBelum ada peringkat
- Experiments and Modeling in Cognitive Science: MATLAB, SPSS, Excel and E-PrimeDari EverandExperiments and Modeling in Cognitive Science: MATLAB, SPSS, Excel and E-PrimeBelum ada peringkat
- Computational Fluid Dynamics Applied to Waste-to-Energy Processes: A Hands-On ApproachDari EverandComputational Fluid Dynamics Applied to Waste-to-Energy Processes: A Hands-On ApproachPenilaian: 4 dari 5 bintang4/5 (1)
- Development and Application of Classical Capillary Number Curve TheoryDari EverandDevelopment and Application of Classical Capillary Number Curve TheoryBelum ada peringkat
- Process Design Strategies for Biomass Conversion SystemsDari EverandProcess Design Strategies for Biomass Conversion SystemsBelum ada peringkat
- Computational Methods for Process SimulationDari EverandComputational Methods for Process SimulationPenilaian: 3 dari 5 bintang3/5 (1)
- Applied Drilling Circulation Systems: Hydraulics, Calculations and ModelsDari EverandApplied Drilling Circulation Systems: Hydraulics, Calculations and ModelsPenilaian: 5 dari 5 bintang5/5 (4)
- Diagnosis and Robust Control of Complex Building Central Chilling Systems for Enhanced Energy PerformanceDari EverandDiagnosis and Robust Control of Complex Building Central Chilling Systems for Enhanced Energy PerformanceBelum ada peringkat
- Survival Analysis Using SAS: A Practical Guide, Second EditionDari EverandSurvival Analysis Using SAS: A Practical Guide, Second EditionBelum ada peringkat
- 11 3323 PDFDokumen16 halaman11 3323 PDFmohammedelamenBelum ada peringkat
- Auto Plant EstructuralDokumen108 halamanAuto Plant EstructuralpedroBelum ada peringkat
- Column DiscriptionDokumen2 halamanColumn DiscriptionWalter MiguelBelum ada peringkat
- 11-5606 JS Plus RefreshDokumen26 halaman11-5606 JS Plus RefreshHENRY LAPACABelum ada peringkat
- Jack Paper1 2006Dokumen10 halamanJack Paper1 2006Walter MiguelBelum ada peringkat
- Process FlowsheetDokumen1 halamanProcess FlowsheetWalter MiguelBelum ada peringkat
- Practical ThermoDokumen41 halamanPractical ThermoWalter MiguelBelum ada peringkat
- SetupDokumen1 halamanSetupWalter MiguelBelum ada peringkat
- Flow and Level Measurement HandbookDokumen100 halamanFlow and Level Measurement Handbookmtayyab_786Belum ada peringkat
- High Rate Gravity Sand Filtration of Water To Remove Fish Eggs A N D LarvaeDokumen27 halamanHigh Rate Gravity Sand Filtration of Water To Remove Fish Eggs A N D LarvaeWalter MiguelBelum ada peringkat
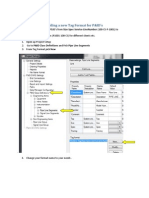
- Adding A New Tag Format For PidDokumen6 halamanAdding A New Tag Format For PidWalter MiguelBelum ada peringkat
- Period 4 Elements - OdtDokumen343 halamanPeriod 4 Elements - OdtAl GongBelum ada peringkat
- Automation and Robotic Lec 2Dokumen9 halamanAutomation and Robotic Lec 2Josh Sam Rindai MhlangaBelum ada peringkat
- Light NcertDokumen55 halamanLight NcertDani MathewBelum ada peringkat
- Microfluidics Fluid Physics at The Nanoliter Scale PDFDokumen50 halamanMicrofluidics Fluid Physics at The Nanoliter Scale PDFDavid CoralBelum ada peringkat
- BSC Combined SO (8 Banks & FIs) - 2018 (Written Math Solution) by Ajgar Ali - PDF Version 1Dokumen10 halamanBSC Combined SO (8 Banks & FIs) - 2018 (Written Math Solution) by Ajgar Ali - PDF Version 1Zia UddinBelum ada peringkat
- Sko0061283 R2Dokumen52 halamanSko0061283 R2ciwidop390Belum ada peringkat
- Thickening Agent - Wikipedia, The Free EncyclopediaDokumen5 halamanThickening Agent - Wikipedia, The Free EncyclopediaJohnBelum ada peringkat
- A Comparison of IEC 479-1 and IEEE STD 80 On Grounding Safety CriteriaDokumen10 halamanA Comparison of IEC 479-1 and IEEE STD 80 On Grounding Safety Criteriaperijoy100% (1)
- Exemplo 9.5, Tipler e MoscaDokumen1 halamanExemplo 9.5, Tipler e MoscaLaís VelameBelum ada peringkat
- On Hidden Projection of Plackett Burman Design by Yashi PalDokumen26 halamanOn Hidden Projection of Plackett Burman Design by Yashi PalyashiBelum ada peringkat
- CPT Brochure 1Dokumen12 halamanCPT Brochure 1jose antonio becerra mosqueraBelum ada peringkat
- D.C.WDokumen150 halamanD.C.Wapi-3808225Belum ada peringkat
- Biophy-Lec (Mod1 - The-Basics) PDFDokumen18 halamanBiophy-Lec (Mod1 - The-Basics) PDFShekinah LeynesBelum ada peringkat
- 2017 Tubing InformationDokumen28 halaman2017 Tubing InformationSatyabrat GaanBelum ada peringkat
- p1k Pde2577tcuk 052009 PDFDokumen20 halamanp1k Pde2577tcuk 052009 PDFwalid8311Belum ada peringkat
- Jouf University: Department of Information Systems Dr. Abd El-Aziz Ahmed Java Programming Fall 2018 Practice Lab 1Dokumen2 halamanJouf University: Department of Information Systems Dr. Abd El-Aziz Ahmed Java Programming Fall 2018 Practice Lab 1wiemBelum ada peringkat
- Ionic Equilibrium - 1Dokumen18 halamanIonic Equilibrium - 1Aditya BajajBelum ada peringkat
- Tolerances and FitsDokumen12 halamanTolerances and FitsnikitaBelum ada peringkat
- Lab Report On AdsorptionDokumen12 halamanLab Report On AdsorptionElizabeth Polancos BruaBelum ada peringkat
- M. Tech. Computer Aided DesignDokumen42 halamanM. Tech. Computer Aided DesignHamid MojiryBelum ada peringkat
- Applied III, Worksheet 1Dokumen4 halamanApplied III, Worksheet 1mintuwondeBelum ada peringkat
- 1004 Winkler Spring Models PDFDokumen7 halaman1004 Winkler Spring Models PDFkukuh kurniawan dwi sungkonoBelum ada peringkat
- Simarine Deck Paint: Product Data SheetDokumen2 halamanSimarine Deck Paint: Product Data SheetPerseroan MustikaBelum ada peringkat
- A Foster Network Thermal Model For HEV/EV Battery ModelingDokumen8 halamanA Foster Network Thermal Model For HEV/EV Battery Modelingal-masriBelum ada peringkat
- Lighting ModuleDokumen133 halamanLighting ModulePrashanth KochuveetilBelum ada peringkat
- Forces and Motion ActivityDokumen5 halamanForces and Motion Activityjanice alquizarBelum ada peringkat
- Chemistry of PhosphorusDokumen7 halamanChemistry of PhosphorusChandra ReddyBelum ada peringkat
- Astronomy - November 2013 (Gnv64)Dokumen80 halamanAstronomy - November 2013 (Gnv64)edies50% (2)
- Filetype PDF Photoconduction SemiconductorDokumen2 halamanFiletype PDF Photoconduction SemiconductorGregBelum ada peringkat
- MEF University Math 115 Calculus Fall 2018-19 Midterm Exam 1 2 3 4 PDokumen4 halamanMEF University Math 115 Calculus Fall 2018-19 Midterm Exam 1 2 3 4 PMELİHA KOÇBelum ada peringkat