Chlorofluorocarbons: (CFCS)
Diunggah oleh
mansikakaniDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Chlorofluorocarbons: (CFCS)
Diunggah oleh
mansikakaniHak Cipta:
Format Tersedia
Chlorofluorocarbons (CFCs)
hemical compounds called chlorouorocarbons (CFCs), which are widely used today, have been linked to two environmental problems that are causing increasing concern: ozone depletion and global warming. A new federal law - Title VI of the Clean Air Act Amendments of 1990 - has been passed to reduce production of CFCs, leading to a total phaseout by the year 2000. Specific strategies to reduce CFC emissions include recycling, reclamation, and recovery of CFCs; early detection of refrigerant leaks and modification of repair procedures; and use of alternative compounds to replace CFCs.
c,
History of CFCs
Chlorofluorocarbons (CFCs) are chemical compounds made of chlorine, fluorine, and carbon. One member of the CFC family, called a halon, also contains bromine. CFCs were originally invented in the 1930s for the refrigerator market to replace the other common refrigerants, such as ammonia, carbon dioxide, ethyl chloride, isobutane, methyl chloride, and sulfur dioxide. Many of these coolants were flammable, toxic, or both. The new CFCs were hailed as the perfect compounds, because they are nontoxic, nonflammable, noncorrosive, and extremely stable. Furthermore, they have excellent thermodynamic properties and never wear out. CFCs are primarily used as refrigerants and solvents. Among the most useful chemicals ever invented, CFCs are found in many aspects of modern living. They are used to refrigerate food and drugs; cool homes, buildings, and cars; clean electronic components; insulate buildings and refrigerators; and create packaging. Halons are used as propellants in fire extinguishers. Products using CFCs account for $135 billion worth of products in the United States.
Ozone Depletion Theory
In the early 1970s, scientists discovered that CFCs were accumulating in the stratosphere, 7 to 28 miles above the earth, and destroying the ozone layer. The ozone layer absorbs and scatters ultraviolet rays from the sun, protecting the earth from excessive exposure to their harmful effects. A decrease in the ozone layer is suspected to have negative biological effects, including increased incidence of skin cancer and eye cataracts. The Environmental Protection Agency (EPA)estimates that each 1 percent depletion in stratospheric ozone could lead to a 2 percent increase in skin cancers among fair-skinned people. When CFCs and halons are released into the atmosphere they eventually reach the upper stratosphere. There they are broken down by ultraviolet radiation, releasing free chlorine or bromine atoms, which destroy the ozone. Each free chlorine atom is thought to be capable of destroying up to 100,000 ozone molecules. Bromine is considered even more harmful than chlorine; however, because halons are released in
Bonneville
Toll-free Hotline 1-800-872-3568
POWER A D M I N I STRAT ION
____
FAX 1-800-872-3882
Electronic Bulletin Board 1-800-762-3319
lower quantities than other CFCs, they account for less than 10 percent of the stratospheric ozone destruction. CFCs have long atmospheric lives of 40 to 380 years.
Ozone Depletion PotentiaI
Not all CFCs damage ozone to the same extent. A measure referred to as ozone depletion potential (ODP) describes the particular CFC compounds ability to destroy protective ozone. The ODP for a compound represents its destructive ability compared to that of CFC-11 and CFC-12, which have a baseline value of one. The larger the ODP value, the greater the ozone depletion potential. The ODPs for commonly-used compounds are shown in the table.
shorter atmospheric lifetimes than CFCs and only 2 to 5 percent of the ozone depletion potential. They are generally used in equipment such as residential, window, and commercial rooftop air-conditioning systems and heat pumps. HFCs, or hydrofluorocarbons, are compounds being developed to replace CFCs. These compounds contain no chlorine to harm the ozone layer. Some problems with HFCs include failure to pass longterm toxicity tests, lack of effective lubricants, and lack of production-level manufacturing techniques. HFC-134a would be a likely substitute for CFC-12. This refrigerant will be used in domestic refrigerators and automobile air-conditioning systems. Most manufacturers have solved the lubricant problem by using ester-based lubricants or polyalkylene glycols.
in causing global warming because CFCs are more efficient in absorbing infrared radiation. Studies indicate that CFCs account for about one-fifth of the global warming effect. The greenhouse effect of a particular compound is expressed as its global warming potential (GWP). The GWP of each compound is relative to that of CFC-11, which has a value of 1.O. The larger the GWP, the greater the contribution to the greenhouse effect. The GWPs for commonly-used refrigerants are shown in the table.
__
Government Regulat ion of CFCs
In 1978, the Food and Drug Administration banned the use of CFCs as aerosol propellants in all but a few essential applications. As a resuIt, the use of CFCs in aerosol propellants was reduced by 95 percent. However, even with this ban, between 1978and 1985, the global levels of stratospheric ozone dropped an average of 2.5 percent. In 1987,36 countries, including the United States, signed a treaty that seeks to protect the stratospheric ozone layer. This treaty, commonly known as the Montreal Protocol, calls for stepwise reductions of CFC and halon production over the next decade, ending in a total phase-out by the year 2000. The second Montreal Protocol, a follow-up agreement, was signed in London in June 1990 by 93 countries. It expands the treatys scope to other ozonedepleting chemicals, such as carbon tetrachloride and methyl chloroform, which will be phasedout by the years 2000 and 2005, respectively.
It is important to understand the differences between the various compounds: CFCs, HCFCs, and HFCs. A simple hydrocarbon molecule is the basic structure from which all three are derived.
CFCs are fully-halogenated hydrocarbon molecules in which all the hydrogen atoms have been replaced with chlorine or fluorine atoms. CFCs are considered to cause the most ozone depletion; it is theorized that the high ODPs are due to chlorine. Examples of fully-halogenated CFCs are CFC11, CFC-12, CFC-113, CFC-114, and CFC-115. HCFCs, or h ydrofluorocarbons, are hydrocarbon molecules in which some, but not all, of the hydrogen atoms have been replaced with bromine, chlorine, or fluorine atoms. HCFCs are less harmful to the ozone because most of the molecules break down at elevations below the ozone layer. These compounds have much
Global Warming Potential
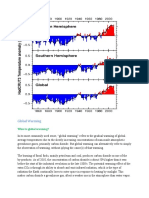
Not long after their effect on the ozone layer was discovered, scientists discovered that CFCs were also contributing to the greenhouse effect. The greenhouse effect results when infrared radiation emitted from the earth is trapped by the atmosphere, causing the earths climate to increase in temperature. The greenhouse effect is caused by certain gases that collect in the atmosphere and radiate the earths infrared energy back to the earth. The following gases contribute to this effect: carbon dioxide, methane, nitrous oxide, and CFCs. The most abundant of the greenhouse gases is carbon dioxide. Although CFCs are not as abundant as carbon dioxide, pound for pound they are thousands of times as potent as carbon dioxide
V Electric Ideas Clearinghouse is a
comprehensive information source for commercial and industrial energy users. It is operated by the Washington State Energy Office and is part of the Electric Ideas technology transfer program sponsored by participating utilities and the Bonneville Power Administration. V Neither the United States nor the Bon nevi11e Power Ad ministration, the state of Washington, the Washington State Energy Office, nor any of their contractors, subcontractors, or their employees make any warranty, expressed or implied, or assume any legal responsibility for the accuracy, completeness, or usefulness of any informa t ion, apparatus, product, or process disclosed within this publica tion.
V Technology Update CH - 9
The Future for CFCs
As restrictions become enacted into law, reduction strategies will become much more prevalent. Because of decreasing supply and increasing tax, the price of refrigerants will rise, creating an economic incentive for reducing their use. Chemical companies are now working on potential replacements for ozone-destroying CFCs. Air-conditioning and refrigeration manufacturers must redesign equipment to accommodate new refrigerants. It will cost the U.S. economy billions of dollars to make the transition to safer refrigerants. However, the time, effort, and money spent on this transition will help to secure human health and environmental soundness for future generations.
Bibliography
ASHRAE. 1989 ASHRAE Handbook of Fundamentals. 1989. ASHRAE. Reducing Emission of Fully Halogenated Chlovofluorocarbon (CFC)Refrigerants in Refrigeration and Air-conditioning Equipment and Applications. Guideline ANSI/ASHRAE 3-1990. Atwood, T. CFCs in Transition. CFCs: Time of Transition. ASHRAE. Pp. 6065. 1989. Cale, P.S. and K. H. Heiting. The Wonder Chemicals Must Go. Iowa Energy Bulletin. Volume 16, No. 1.Iowa Department of Natural Resources. January/February 1991. Clarke, E.M., G. G. Anderson, W. D. Wells, and R.L. Bates. Retrofitting Existing Chillers with Alternative Refrigerants. ASHRAE Journal. Pp. 41. April 1991. Denny, R.J. The CFC Footprint. CFCs: Time of Transition. ASHRAE. Pp. 2328. 1989. Fischer, S. Energy Use Impact of CFC Alternatives. Energy Engineering. Volume 88, No.3, Pp. 8.1991. Frank, A. L. CFC, Halon Phaseouts Set for 2000 in Protocol Signed by 93 Countries. Energy and Housing Report. ALFA Publishing. June 1990. Gilkey, H.T. The Coming Refrigerant Shortage. HeatinglPipinglAir Conditioning. Pp. 41-46. April 1991. McLinden, M.O. and D.A. Didion. Quest for Alternatives. CFCs: Time of Transition. ASHRAE. Pp. 69-78. 1989. Miller, R. Refrigeration and Aiv Conditioning Technology. Peoria, Illinois: Bennett & McKnight Publishing Company. 1983. Moore, T. Global Response to CFC Refrigerants. EPRI Journal. September 1989.
V Toll-, -e Hotline:
1-800-872-3568 Fax: 1-800-872-3882 Elect ronic Bullet in Board: 1-800-762-331 9
Other Sources
Refrigeration Service Engineers Society
1666 Rand Rd.
Des Plaines, IL 60016-3552
DOE/BP-39833-17 December 1991 ISM
Anda mungkin juga menyukai
- International Chemistry Olympiad Problems Volume 01 (1968-1988)Dokumen408 halamanInternational Chemistry Olympiad Problems Volume 01 (1968-1988)Science Olympiad Blog100% (5)
- Different Types of RefrigerantsDokumen7 halamanDifferent Types of RefrigerantsAnthony John Camacho ReburianoBelum ada peringkat
- WBM ObmDokumen27 halamanWBM ObmMygroup 5544Belum ada peringkat
- Welcome SpeechDokumen2 halamanWelcome Speechmansursk100% (3)
- Montreal Protocol protects ozone layer and climateDokumen3 halamanMontreal Protocol protects ozone layer and climatelilackill Lor100% (1)
- Ozone Layer DepletionDokumen14 halamanOzone Layer DepletionRai HrithikBelum ada peringkat
- Boiler Cycle Control: Questions and AnswersDokumen6 halamanBoiler Cycle Control: Questions and AnswersChanBoonChewBelum ada peringkat
- Water Vapor, Not Carbon Dioxide, Is Major Contributor to the Earth's Greenhouse Effect: Putting the Kibosh on Global Warming AlarmistsDari EverandWater Vapor, Not Carbon Dioxide, Is Major Contributor to the Earth's Greenhouse Effect: Putting the Kibosh on Global Warming AlarmistsBelum ada peringkat
- Industria1 C o M B U S T I o N Pollution and C o N T R o L PDFDokumen890 halamanIndustria1 C o M B U S T I o N Pollution and C o N T R o L PDFAfzaal AshrafBelum ada peringkat
- ChlorofluorocarbonsDokumen2 halamanChlorofluorocarbonsrofifah nur fadhilahBelum ada peringkat
- 03.BE REFRIGERATION AND AIR CONDIONING, (Class Room)Dokumen237 halaman03.BE REFRIGERATION AND AIR CONDIONING, (Class Room)girimech24Belum ada peringkat
- Greenhouse GasDokumen5 halamanGreenhouse Gascynthiaaa sBelum ada peringkat
- Free Radicals & Atmospheric OxidationDokumen9 halamanFree Radicals & Atmospheric OxidationUsman GhaniBelum ada peringkat
- Chlorofluorocarbon (CFC) & Hydrochlorofluorocarbon (HCFC)Dokumen18 halamanChlorofluorocarbon (CFC) & Hydrochlorofluorocarbon (HCFC)Geetha SarikaBelum ada peringkat
- FluorocarbonsDokumen3 halamanFluorocarbonsLavendar RobertsBelum ada peringkat
- HSC Chemistry Lesson Plan 26Dokumen11 halamanHSC Chemistry Lesson Plan 26Ali HaidarBelum ada peringkat
- Montreal Protocol NotesDokumen2 halamanMontreal Protocol NotesSidemen For LifeBelum ada peringkat
- Sherubtse College Faculty of Mathematics and Computer ScienceDokumen9 halamanSherubtse College Faculty of Mathematics and Computer ScienceSampa Nima YoezerBelum ada peringkat
- Atmospheric Chemistry Assignment 1: Prepared ForDokumen3 halamanAtmospheric Chemistry Assignment 1: Prepared ForNikhil AgarwalBelum ada peringkat
- Module For Climate Change Chapter 3 Greenhouse Effect and Global WarmingDokumen14 halamanModule For Climate Change Chapter 3 Greenhouse Effect and Global WarmingJohn Rey TabaraBelum ada peringkat
- CFCS, Their ReplacementsDokumen5 halamanCFCS, Their ReplacementsPaqui MiñoBelum ada peringkat
- Withdrawal of Albuterol Inhalers Containing Chlorofluorocarbon PropellantsDokumen8 halamanWithdrawal of Albuterol Inhalers Containing Chlorofluorocarbon PropellantsAli HabibBelum ada peringkat
- Brochure CFCDokumen2 halamanBrochure CFCJason Raquin RoqueBelum ada peringkat
- Harmful Chemicals RevisedDokumen2 halamanHarmful Chemicals RevisedMarian LuegoBelum ada peringkat
- Ozone Depletion: EX - NO: DateDokumen2 halamanOzone Depletion: EX - NO: DateHailin ArumigaBelum ada peringkat
- Advantages of Ammonia as an Environmentally-Friendly RefrigerantDokumen4 halamanAdvantages of Ammonia as an Environmentally-Friendly RefrigerantJhanZaibBelum ada peringkat
- Cfcschlorofluorocarbons Ea14Dokumen2 halamanCfcschlorofluorocarbons Ea14api-313086327Belum ada peringkat
- SLG Chem3 LG 2.15 Application of HalogenoalkanesDokumen4 halamanSLG Chem3 LG 2.15 Application of HalogenoalkanesLorraine CalacsanBelum ada peringkat
- EPA Questions and Answers On Ozone DepletionDokumen4 halamanEPA Questions and Answers On Ozone DepletionAndrew GrantBelum ada peringkat
- MRACDokumen205 halamanMRACritesh kumarBelum ada peringkat
- Chemistry IcseDokumen10 halamanChemistry Icses.reeva.jainBelum ada peringkat
- Week 12 Lecture Notes 2Dokumen7 halamanWeek 12 Lecture Notes 2FarhBelum ada peringkat
- Ozone Layer Showing 'Signs of Recovery', UN Says: by Roger HarrabinDokumen6 halamanOzone Layer Showing 'Signs of Recovery', UN Says: by Roger HarrabinShekinah MizpahBelum ada peringkat
- F322 Atmospheric ChemistryDokumen6 halamanF322 Atmospheric ChemistryDoc_CrocBelum ada peringkat
- RHVAC Components and Environmental IssuesDokumen2 halamanRHVAC Components and Environmental IssuesRIDHO BAGUS NURROHMANBelum ada peringkat
- CFCs and Halons Deplete Ozone LayerDokumen2 halamanCFCs and Halons Deplete Ozone LayerMarian LuegoBelum ada peringkat
- Research Paper About Ozone DepletionDokumen7 halamanResearch Paper About Ozone Depletiondxvxhvhkf100% (1)
- Addressing The Need For Halon Replacement: Sheinson@code6185.nrl - Navy.milDokumen13 halamanAddressing The Need For Halon Replacement: Sheinson@code6185.nrl - Navy.milHSE nestBelum ada peringkat
- GHG and Its ImpactDokumen5 halamanGHG and Its ImpactAditya RajBelum ada peringkat
- AIR POLLUTION: PAN, CFCs & MTBEDokumen35 halamanAIR POLLUTION: PAN, CFCs & MTBEAleena NaeemBelum ada peringkat
- Uses of HalogenoalkanesDokumen5 halamanUses of HalogenoalkanesLorenz SmallBelum ada peringkat
- Ozone Depletion Potential of Refrigerants and Steps to Counter ItDokumen4 halamanOzone Depletion Potential of Refrigerants and Steps to Counter ItFazeel Sajid100% (1)
- OTBA 2015 For Class 11 For Geography (English Version)Dokumen23 halamanOTBA 2015 For Class 11 For Geography (English Version)Mota Chashma100% (1)
- Destruction of Ozone LayerDokumen15 halamanDestruction of Ozone LayerIanne Mae E. LongosBelum ada peringkat
- Montreal and Kyoto ProtocolDokumen47 halamanMontreal and Kyoto ProtocolJabel Pates100% (1)
- Review of Related Literature of Ozone Layer DepletionDokumen7 halamanReview of Related Literature of Ozone Layer Depletiongw15ws8jBelum ada peringkat
- Spring 2011 Fire Detection & Suppression MagazineDokumen10 halamanSpring 2011 Fire Detection & Suppression Magazineminiongsky100% (1)
- Global WarmingDokumen7 halamanGlobal Warmingcarlo78Belum ada peringkat
- Apes - Powerpoint Notes - Chapter 25 Ozone Depletion: Epidemic of Skin Cancers in The United StatesDokumen5 halamanApes - Powerpoint Notes - Chapter 25 Ozone Depletion: Epidemic of Skin Cancers in The United StatesFedel AliBelum ada peringkat
- Science Focus 9 Unit 3 Topic 6Dokumen26 halamanScience Focus 9 Unit 3 Topic 6mills.home.eduBelum ada peringkat
- Performance and Safety of LPG RefrigerantsDokumen18 halamanPerformance and Safety of LPG RefrigerantsarumbinarBelum ada peringkat
- Ozone Depletion Power PointDokumen49 halamanOzone Depletion Power PointthebrajeshkumarBelum ada peringkat
- Ccac - To Reduce SLCPDokumen11 halamanCcac - To Reduce SLCPAlejandra MuñozBelum ada peringkat
- Hot issue: HFC use is climbing as there is consumer demand for electrical products that require insulation foam and refrigerantsDokumen5 halamanHot issue: HFC use is climbing as there is consumer demand for electrical products that require insulation foam and refrigerantsKokila MageswaranBelum ada peringkat
- An Overview of The Impacts of Chlorofluorocarbon On The Port Harcourt Environment in NigeriaDokumen13 halamanAn Overview of The Impacts of Chlorofluorocarbon On The Port Harcourt Environment in NigeriaKIU PUBLICATION AND EXTENSIONBelum ada peringkat
- Vrefrigerants: A. Ideal Properties of RefrigerantsDokumen3 halamanVrefrigerants: A. Ideal Properties of RefrigerantsLonie ReyesBelum ada peringkat
- Project Report on Ozone Layer Depletion & SolutionsDokumen14 halamanProject Report on Ozone Layer Depletion & Solutionsvikeshjha18Belum ada peringkat
- Nuclear Waste and It's DisposalDokumen6 halamanNuclear Waste and It's DisposalKaustubh ZawarBelum ada peringkat
- Reducing Air Pollution EmissionsDokumen16 halamanReducing Air Pollution EmissionsMarie FranchescaBelum ada peringkat
- Sample Research Paper Ozone DepletionDokumen5 halamanSample Research Paper Ozone Depletionaflbojhoa100% (1)
- International Education Centre CFCs, Fire Retardants and the Ozone LayerDokumen7 halamanInternational Education Centre CFCs, Fire Retardants and the Ozone LayerJunaidahMubarakAliBelum ada peringkat
- Global Atm ChangesDokumen7 halamanGlobal Atm ChangesmanipsgBelum ada peringkat
- Environmental Assessment of Air Pollution in Khartoum North Thermal Power Generation 1. Air PollutionDokumen8 halamanEnvironmental Assessment of Air Pollution in Khartoum North Thermal Power Generation 1. Air PollutionLemia ELtyeb ELfadelBelum ada peringkat
- Ozone Depletion and Global Warming: The Threat of UV RadiationDokumen15 halamanOzone Depletion and Global Warming: The Threat of UV RadiationSarah ShahBelum ada peringkat
- Greenhouse Gases, Ozone Layer, and Recycling ExplainedDokumen9 halamanGreenhouse Gases, Ozone Layer, and Recycling ExplainedLakshan Selvarajah100% (1)
- IJCRT2306620Dokumen5 halamanIJCRT2306620mansikakaniBelum ada peringkat
- RohanDokumen21 halamanRohanmansikakaniBelum ada peringkat
- Department of Civil Engineering: TEACHING PLAN, 2018-2019Dokumen4 halamanDepartment of Civil Engineering: TEACHING PLAN, 2018-2019mansikakaniBelum ada peringkat
- Work and Energy Chapter SummaryDokumen10 halamanWork and Energy Chapter SummaryMohit Kumar100% (2)
- Unit IIDokumen17 halamanUnit IImansikakaniBelum ada peringkat
- Unit 3 FinalDokumen24 halamanUnit 3 FinalmansikakaniBelum ada peringkat
- Unit 4 Part - IIDokumen9 halamanUnit 4 Part - IImansikakaniBelum ada peringkat
- Gaurav Burhade's Mechanics Problems and SolutionsDokumen14 halamanGaurav Burhade's Mechanics Problems and SolutionsmansikakaniBelum ada peringkat
- Unit 1 FinalDokumen15 halamanUnit 1 FinalmansikakaniBelum ada peringkat
- SNJB's Late Sau. Kantabai Bhavarlalji Jain College of Engineering, Chandwad Department of Computer Engineering Gap AnalysisDokumen1 halamanSNJB's Late Sau. Kantabai Bhavarlalji Jain College of Engineering, Chandwad Department of Computer Engineering Gap AnalysismansikakaniBelum ada peringkat
- 12-Gap AnalysisDokumen1 halaman12-Gap AnalysismansikakaniBelum ada peringkat
- Singrauli Lab Report October 16 FinalDokumen47 halamanSingrauli Lab Report October 16 FinalmansikakaniBelum ada peringkat
- Direct Emissions From Landfilling Municipal Solid WasteDokumen28 halamanDirect Emissions From Landfilling Municipal Solid WastemansikakaniBelum ada peringkat
- Indian Tube LightDokumen40 halamanIndian Tube LightAgrim KhatryBelum ada peringkat
- LyricsDokumen35 halamanLyricsmansikakaniBelum ada peringkat
- Ironsteel PPAHDokumen5 halamanIronsteel PPAHShefiu KareemBelum ada peringkat
- Pages577 585 PDFDokumen9 halamanPages577 585 PDFDinsun IhunnaBelum ada peringkat
- Water Requirements of The Iron and Steel IndustryDokumen58 halamanWater Requirements of The Iron and Steel IndustrymansikakaniBelum ada peringkat
- P 537Dokumen16 halamanP 537chawla20208819Belum ada peringkat
- Plastic Rules 1999Dokumen7 halamanPlastic Rules 1999Deepak RavichandranBelum ada peringkat
- 14-Asthma and Air PollutionDokumen25 halaman14-Asthma and Air PollutionmansikakaniBelum ada peringkat
- 03 Chapter 1Dokumen112 halaman03 Chapter 1mansikakaniBelum ada peringkat
- WebBased Access and Visualization of Hazardous Air PollutantsDokumen14 halamanWebBased Access and Visualization of Hazardous Air PollutantsmansikakaniBelum ada peringkat
- Plastic Manufacturing Emissions SourcesDokumen6 halamanPlastic Manufacturing Emissions SourcesmansikakaniBelum ada peringkat
- Rainfall Runoff ModellingDokumen23 halamanRainfall Runoff ModellingmansikakaniBelum ada peringkat
- 06-Environmental Pollution From Automobile Vehiclejune2012Dokumen10 halaman06-Environmental Pollution From Automobile Vehiclejune2012mansikakaniBelum ada peringkat
- Plastic Manufacturing Emissions SourcesDokumen6 halamanPlastic Manufacturing Emissions SourcesmansikakaniBelum ada peringkat
- Ifferent Types of Leakages - What Kind Is Yours?Dokumen3 halamanIfferent Types of Leakages - What Kind Is Yours?mansikakaniBelum ada peringkat
- TUTORIAL SHEET 2019 On Hydrostatic PressureDokumen5 halamanTUTORIAL SHEET 2019 On Hydrostatic PressureAndrew ChikuselaBelum ada peringkat
- Syllabus 8Dokumen1 halamanSyllabus 8harrypaswan87Belum ada peringkat
- NMR for sequence and structural isomerism analysisDokumen19 halamanNMR for sequence and structural isomerism analysisSyed Hashim Shah HashmiBelum ada peringkat
- DP Chem Unit 3 PerodicityDokumen5 halamanDP Chem Unit 3 PerodicityPatrick AbidraBelum ada peringkat
- Set 1Dokumen18 halamanSet 1RON MARK EDWARD ANDALUZBelum ada peringkat
- USA5892ps 10193Dokumen5 halamanUSA5892ps 10193Mubashar HassanBelum ada peringkat
- Heat 1Dokumen36 halamanHeat 1ZainabBelum ada peringkat
- The Effects of Rotary Kiln Operating Conditions and Design On Burden Heating Rates As Determined by A Mathematical Model of Rotary Kiln Heat Transfer PDFDokumen9 halamanThe Effects of Rotary Kiln Operating Conditions and Design On Burden Heating Rates As Determined by A Mathematical Model of Rotary Kiln Heat Transfer PDFSHRAVAN KUMARBelum ada peringkat
- CH1502LDokumen87 halamanCH1502LDivyakumar PatelBelum ada peringkat
- Bpharmacy 1 Sem Pharmaceutics 1 Set P 2018Dokumen3 halamanBpharmacy 1 Sem Pharmaceutics 1 Set P 2018Mandlik PritiBelum ada peringkat
- Anios RHW - Sell Sheet PDFDokumen2 halamanAnios RHW - Sell Sheet PDFgadhang dewanggaBelum ada peringkat
- Heat Transfer PDFDokumen3 halamanHeat Transfer PDFTahmeed AzizBelum ada peringkat
- Annealing Process Explained in 40 CharactersDokumen8 halamanAnnealing Process Explained in 40 CharactersRohit SharmaBelum ada peringkat
- 11.VIA Group ElementsDokumen3 halaman11.VIA Group ElementsANIL KumarBelum ada peringkat
- O Level Physics Pressure NotesDokumen28 halamanO Level Physics Pressure NotesMarvel ComicsBelum ada peringkat
- Drug Targeting May Improve Drug Delivery Through Prodrug StrategiesDokumen61 halamanDrug Targeting May Improve Drug Delivery Through Prodrug StrategiesBima AnestyaBelum ada peringkat
- Xampler HFDokumen8 halamanXampler HFAnil ReddyBelum ada peringkat
- PHY 201 - Thermodynamics and Kinetic Theory of Gases: Unit Name of The Unit SyllabusDokumen1 halamanPHY 201 - Thermodynamics and Kinetic Theory of Gases: Unit Name of The Unit SyllabusBhoomi ShettyBelum ada peringkat
- Lab Report MayonnaiseDokumen5 halamanLab Report Mayonnaiseapi-295870217Belum ada peringkat
- Bore-log analysis and interpretationDokumen6 halamanBore-log analysis and interpretationKaaviyan ThirunyanamBelum ada peringkat
- Claus Plants Prove FlexibleDokumen3 halamanClaus Plants Prove Flexiblebakhtiari_afBelum ada peringkat
- High-Solids Polyester Resins For Appliance and General Metal CoatingsDokumen6 halamanHigh-Solids Polyester Resins For Appliance and General Metal CoatingsSyed Ubaid AliBelum ada peringkat
- Chemistry For Today General Organic and Biochemistry 8Th Edition Seager Solutions Manual Full Chapter PDFDokumen36 halamanChemistry For Today General Organic and Biochemistry 8Th Edition Seager Solutions Manual Full Chapter PDFelise.green301100% (11)
- Realization Phase of Model in Terms of EcodesignDokumen7 halamanRealization Phase of Model in Terms of EcodesignZvonimir MajićBelum ada peringkat
- HEC Past PaperDokumen21 halamanHEC Past PaperTalha Aslam100% (1)
- Adblue Diesel Exhaust Fluid-PI SheetDokumen2 halamanAdblue Diesel Exhaust Fluid-PI SheetAntonio AriasBelum ada peringkat