Iitmains-2013 News Paper
Diunggah oleh
BNRedDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Iitmains-2013 News Paper
Diunggah oleh
BNRedHak Cipta:
Format Tersedia
Note: For the benefit of the students, specially the aspiring ones, the question of JEE2013_MAINS
are also given in this booklet. Keeping the interest of students studying in class XI, the questions
based on the topics from class XI have been marked with *, which can be attempted as a test. For
this test the time allocated in Chemistry, Mathematics and Physics are 35 minutes, 25 minutes and 20
minutes respectively.
JEE 2013
JEE 2013
MAINS
MAINS
Time: 3 Hours
Maximum Marks: 360
Do not open this Test Booklet until you are asked to do so.
Read carefully the Instructions on the Back Cover of this Test Booklet.
INSTRUCTIONS
(AS PER THE ORIGINAL JEE BOOKLET)
Important Instructions:
1. Immediately fill in the particulars on this page of the Test Booklet with
Blue/Black Ball Point Pen. Use of pencil is strictly prohibited.
2. The Answer Sheet is kept inside this Test Booklet. When you are
directed to open the Test Booklet, take out the Answer Sheet and fill in
the particulars carefully.
3. The test is of 3 hours duration.
4. The Test Booklet consists of 90 questions. The maximum marks are
360.
5. There are three parts in question paper A, B, C consisting of
Chemistry, Mathematics and Physics having 30 questions in each part
of equal weightage. Each question is allotted 4 (four) marks for correct
response.
6. Candidates will be awarded marks as stated above in instruction No. 5
for correct response of each question. (one fourth) marks will be
deducted for indicating incorrect response of each question. No
deduction from the total score will be made if no response is indicated
for an item in the answer sheet.
7. There is only one correct response for each question. Filling up more
than one response in any question will be treated as wrong response
and marks for wrong response will be deducted accordingly as per
instruction 6 above.
8. Use Blue / Black Ball Point Pen only for written particulars / marking
responses on Side1 and Side2 of the Answer Sheet. Use of pencil is
strictly prohibited.
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS20132
9. No candidate is allowed to carry and textual material, printed or
written, bits of papers, pager, mobile phone, any electronic device,
etc., except the Admit Card inside the examination hall/room.
10. Rough work is to be done on the space provided for this purpose in the
Test Booklet only. This space is given at the bottom of each page and
in 3 pages at the end of the booklet.
11. On completion of the test, the candidate must hand over the Answer
Sheet to the Invigilator on duty in the Room / Hall. However, the
candidates are allowed to take away this Test Booklet with them.
12. The CODE for this Booklet is P. Make sure that the CODE printed on
Side2 of the answer sheet is the same as the on this booklet. In case
discrepancy, the candidate should immediately report the matter to
the Invigilator for replacement of both the Test Booklet and the
Answer Sheet.
13. Do not fold or make any stray marks on the Answer Sheet.
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS20133
PART A: CHEMISTRY
1. An unknown alcohol is treated with the
Lucas reagent to determine whether the
alcohol is primary, secondary or tertiary.
Which alcohol reacts fastest and by what
mechanism:
(1) tertiary alcohol by SN1
(2) secondary alcohol by SN2
(3) tertiary alcohol by SN2
(4) secondary alcohol by SN1
1. 1
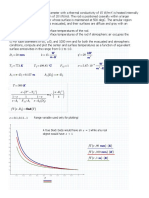
Sol. Because of more stabilized tertiary
carbocation formation.
2. The first ionisation potential of Na is 5.1 eV.
The value of electron gain enthalpy of Na
+
will be :
(1) 5.1 eV (2) 10.2 eV
(3) + 2.55 eV (4) 2.55 eV
2. 1
Sol.
( ) ( )
5.1eV
g g
I.P. Na Na e ;
+
+
Electron gain enthalpy
( )
( )
g
Na e Na g
+
+
i.e., (I.P) = 5.1 eV.
3. Stability of the species Li2,
2
Li
and
2
Li
+
increases in the order of:
(1)
2 2 2
Li Li Li
+
< < (2)
2 2 2
Li Li Li
+
< <
(3)
2 2 2
Li Li Li
+
< < (4)
2 2 2
Li Li Li
+
< <
3. 1
Sol. Bond orders of Li2,
2 2
Li &Li
+
are 1, 0.5 & 0.5
respectively (As per M.O.T). Stability bond
order, and in
2
Li
the last electron enters into
antibonding molecular orbital and hence less
stable than
2
Li
+
.
4. The molarity of a solution obtained by mixing
750 mL of 0.5(M) HCl with 250 mL of 2(M)
HCl will be:
(1) 1.00 M (2) 1.75 M
(3) 0.975 M (4) 0.875 M
4. 4
Sol.
1 1 2 2
final
final
VM V M
M
V
+
=
( ) ( ) 750 0.5 250 2
750 250
+
+
=
375 500
0.875M
1000
+
5. Which of the following is the wrong statement
?
(1) O3 molecule is bent.
(2) Ozone is violetblack in solid state
(3) Ozone is diamagnetic gas.
(4) ONCl and ONO
are not isoelectronic.
5.
Sol. All are correct statements, No answer.
6. Four successive members of the first row
transition elements are listed below with
atomic numbers. Which one of them is
expected to have the highest 3 2
0
M / M
E
+ +
value ?
(1) Mn(Z = 25) (2) Fe (Z = 26)
(3) Co (Z = 27) (4) Cr(Z = 24)
6. 3
Sol. The 3 2
0
M / M
E
+ + values for Mn(Z = 25) is
+1.57V, for Fe (Z = 26) is 0.77V, for Co (Z =
27) is 1.81V, for Cr (Z = 24) is 0.42V.
7. A solution of () 1chloro1phenylethane
in toluene racemises slowly in the presence
of a small amount of SbCl5, due to the
formation of:
(1) carbene (2) carbocation
(3) free radical (4) carbanion
7. 2
Sol. SbCl5 acts as Lewis acid and removes Cl
from the given substance and a carbocation
6 5 3
C H CHCH
+
_
,
will be formed, which leads
to racemisation because of its planarity.
8. The coagulating power of electrolytes having
ions Na
+
, Al
3+
and Ba
2+
for arsenic sulphide
sol increases in the order:
(1)
2 3
Na Ba Al
+ + +
< <
(2)
2 3
Ba Na Al
+ + +
< <
(3)
3 2
Al Na Ba
+ + +
< <
(4)
3 2
Al Ba Na
+ + +
< <
8. 1
Sol. Coagulating power is directly related to the
charge present on cation for arsenic sulphide
(vely charged sol). (HardySchulze rule).
9. How many litres of water must be added to 1
litre of an aqueous solution of HCl with a pH
of 1 to create an aqueous solution with pH of
2 ?
(1) 0.9 L (2) 2.0 L
(3) 9.0 L (4) 0.1 L
9. 3
Sol. For every 10 times dilution, the pH of the
strong acid increases by 1 unit.
Initial volume = 1 lt.
For an increase of 1 unit of pH (from 1 to
2), the final volume should be 10 lt.
water to be added = 10 lt 1 lt = 9 lt.
10. Which one of the following molecules is
expected to exhibit diamagnetic behaviour ?
(1) N2 (2) O2 (3) S2 (4) C2
10. 1, 4
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS20134
Sol. As per M.O.T,
M.O. configuration of N2 (14e
s ) is
1s
2
*
1s
2
2s
2
*
2s
2
( )
2 2 2
x y z
2p 2p 2p
M.O. configuration of C2 (12e
s ) is
1s
2
*
1s
2
2s
2
*
2s
2
( )
2 2
x y
2p 2p
There is no unpaired electrons both in N2 and
C2. hence they are diamagnetic.
11. Which of the following arrangements does
not represent the correct order of the
property stated against it ?
(1)
2 2 2 2
Ni Co Fe Mn
+ + + +
< < < ionic size
(2)
3 3 3 3
Co Fe Cr Sc :
+ + + +
< < < stability in
aqueous solution.
(3) Sc < Ti < Cr < Mn : Number of oxidation
states
(4)
2 2 2 2
V Cr Mn Fe
+ + + +
< < < : paramagnetic
behaviour
11. 4
Sol. Paramagnetic moment ( ) ( )
s
n n 2 + .
B.M.
n = number of unpaired electrons
V
2+
: [Ar] 3d
3
n = 3
Cr
+2
: [Ar] 3d
4
n = 4
Mn
+2
: [Ar] 3d
5
n = 5
Fe
+2
: [Ar] 3d
6
n = 4
Correct order = V
2+
< Cr
+2
Fe
+2
< Mn
+2
.
12. Experimentally it was found that a metal
oxide has formula
0.98
M O.
Metal M, is
present as M
2+
and M
3+
in its oxide. Fraction
of the metal which exists as M
3+
would be:
(1) 4.08% (2) 6.05%
(3) 5.08% (4) 7.01%
12. 1
Sol. Emperical formula :
0.98
M O
M98O100
For every three M
+2
ions replaced, two M
+3
ions must be introduced creating 1 cationic
vacancy.
If 2 M
+3
ions are there 1 vacancy
? 2 vacancies
no of M
+3
ions = 4
% M
+3
=
4
100 4.08
98
13. A compound with molecular mass 180 is
acylated with CH3COCl to get a compound
with molecular mass 390. The number of
amino groups present per molecule of the
former compound is:
(1) 5 (2) 4 (3) 6 (4) 2
13. 1
Sol.
2 3 3
R NH Cl CO CH R HN CO CH +
For every 1 molecule of NH2 group, the
molecular mass increases by 42 units.
In given problem, molecular mass increases
by 390 180 = 210
No. of NH2 groups =
210
5
42
14. Given
3 2
4
0 0
Cr / Cr MnO / Mn
E 0.74V; E 1.51V
+ +
2 3
2 7
0 0
Cl / Cl
Cr O / Cr
E 1.33V; E 1.36V
+
Based on the data given above, strongest
oxidizing agent will be:
(1) Cr
3+
(2) Mn
2+
(3)
4
MnO
(4) Cl
14. 3
Sol. Higher the reduction potential, greater will be
the oxidizing ability. So,
4
MnO
acts as
strongest oxidizing agent of these
2
4 2
MnO 8H 5e Mn 4H O;
+ +
+ + + 1.51 V.
15. Arrange the following compounds in order of
decreasing acidity:
OH
Cl
;
OH
CH
3
;
OH
NO
2
;
OH
OCH
3
;
(I) (II)
(III) (IV)
(1) I > II > III > IV (2) III > I > II > IV
(3) IV > III > I > II (4) II > IV > I > III
15. 2
Sol. Acidic nature of phenols is increased, if
strong electron withdrawing groups are
present on benzene ring.
CH3 weakly activating group.
OCH3 moderately activating group.
Cl Weak deactivating group
NO2 Strong deactivating group.
Acidic nature order is
OH
NO
2
>
OH
Cl
>
OH
CH
3
>
OH
OCH
3
III
I
II
IV
16. The rate of a reaction doubles when its
temperature changes from 300 K to 310 K.
Activation energy of such a reaction will be:
(R = 8.314 JK
1
mol
1
and log2 = 0.301)
(1) 48.6 kJ mol
1
(2) 58.5 kJ mol
1
(3) 60.5 kJ mol
1
(4) 53.6 kJ mol
1
16. 1
Sol. Arhenius equation is
a 2
1 1 2
E K 1 1
log
K 2.303R T T
1
1
]
a
3
E 1 1
log2
300 310
2.303 8.314 10
1
1
]
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS20135
3
a
0.3010 2.303 8.314 10 300 310
E kJ
10
= 53.598 kJ = 53.6 kJ
17. Synthesis of each molecule of glucose in
photosynthesis involves:
(1) 10 molecules of ATP
(2) 8 molecules of ATP
(3) 6 molecules of ATP
(4) 18 molecules of ATP
17. 4
Sol.
6CO
2
6 ribulose bisphosphate
( 6 x 5 = 30 carbons)
1 2 3 -phosphoglyceric acid (PGA)
( 12 x 3 = 36 carbons)
6 ADP
6 ATP
6 rubulose phosphate
( 6 x 5 = 30 carbons)
10 PGAL
( 10 x 3 = 30 carbons)
12 ATP
12 ADP
1 2 1, 3-bisphosphoglyceric acid (DPGA)
( 36 carbons)
12 NADPH 12 H
+
12 NADP
+
1 2 3-phosphoglyceraldehyde (PGAL)
( 36 carbons)
( 12 Pi & 6H
2
O)
fructose, glucose
etc.
2 PGAL
( 2 x 3 = 6 carbons)
( 6 carbons)
The Calvin Cycle
For every 3 molecules of CO2 that enters the
calvin cycle, one molecule of the three
carbon glyceraldehyde 3phosphate (G3P) is
produced. Two molecules of G3P are needed
to produce one molecule of glucose.
Therefore, the calvin cycle needs to make a
total of 6 turns to produce 2 molecules of
G3P. One turn of the calvin cycle requires 3
molecules of ATP & 2 molecules of NaDPH &
so for 6 turns.
3 x 6 = 18 ATPs
2 x 6 = 12 NADPHs
18. Which of the following complex species is not
expected to exhibit optical isomerism ?
(1)
( )
2
2
Co en Cl
+
1
]
(2) ( )
3 3
3
Co NH Cl
1
]
(3)
( ) ( )
3 2
2
Co en NH Cl
+
1
]
(4)
( )
3
3
Co en
+
1
]
18. 2
Sol. Optical isomerism in octahedral complexes is
generally observed if atleast one bidentate
ligand is present.
N
Co
N
N
Cl
N
Cl
N
Co
N
N
N
N
N
N
Co
N
Cl
NH
3
Cl
H
3
N
exhibits optical activity
, ,
compounds
19. A piston filled with 0.04 mol of an ideal gas
expands reversibly from 50.0 mL to 375 mL
at a constant temperature of 37.0C. As it
does so, it absorbs 208 J of heat. The values
of q and w for the process will be:
(R = 8.314 J/mol K) (ln 7.5 = 2.01)
(1) q = 208 J, w = 208 J
(2) q = 208 J, w = + 208 J
(3) q = + 208 J, w = + 208 J
(4) q = + 208 J, w = 208 J
19. 4
Sol. Since Temperature is constant, the process
is isothermal
dE = 0
dE = dq + dw dq = dw
dq = 208 J. dw = 208 J
20. A gaseous hydrocarbon gives upon
combustion 0.72g. of water and 3.08g. of
CO2. The empirical formula of the
hydrocarbon is:
(1) C3H4 (2) C6H5
(3) C7H8 (4) C2H4
20. 3
Sol.
2
H O
0.72
n 0.04
18
.
No of atoms of H : 0.04 x 2 = 0.08 NA
2
CO
3.08
n 0.07
44
No. of atom of C = 0.07 NA
Emperical formula = C7H8
21. The order of stability of the following
carbocations:
CH
2
CH CH
2
; CH
3
CH
2
CH
2
CH
2
III
(I) (II)
;
(1) II > III > I (2) I > II > III
(3) III > I > II (4) III > II > I
21. 3
Sol. Stability of carbocation no. of
resonance structures
no. of hyperconjugate structure
+I effect
1
I effect
Generally: Resonance effect >
hyperconjugation > Inductive effect
benzyl (III) > allyl (I) > npropyl (II) cations
stability order
22. Which of the following represents the correct
order of increasing first ionization enthalpy
for Ca, Ba, S, Se and Ar ?
(1) S < Se < Ca < Ba < Ar
(2) Ba < Ca < Se < S < Ar
(3) Ca < Ba < S < Se < Ar
(4) Ca < S < Ba < Se < Ar
22. 2
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS20136
Sol. IE of inert gases is highest in a period. Down
the group IE decrease and across the period,
IE increases.
IE of metals is usually lesser than non
metals.
Ba < Ca < Se < S < Ar IE order
23. For gaseous state, if most probable speed is
denoted by C
*
, average speed by C and
mean square speed by C, then for a large
number of molecules the ratios of these
speeds are:
(1) C : C: C 1.128 : 1.225 : 1
(2) C : C: C 1: 1.128 : 1.225
(3) C : C: C 1: 1.225 : 1.128
(4) C : C: C 1.225 : 1.128 : 1
23. 2
Sol. At a given temperature the ratio between the
most probable speed C*, average speed ( )
C
and mean square speed C of a gas is
2RT 8RT 3RT
: :
M M M
8
2 : : 3
1 : 1.128 : 1.225
24. The gas leaked from a storage tank of the
Union Carbide plant in Bhopal gas tragedy
was:
(1) Methylamine (2) Ammonia
(3) Phosgene (4) Methylisocyanate
24. 4
Sol. Fact based (CH3NCO methyl isocyanate
(MIC) gas)
25. Consider the following reaction :
2
4 2 4
xMnO yC O zH
+
+ +
2
2 2
z
xMn 2yCO H O
2
+
+ +
The values of x, y and z in the reaction are,
respectively :
(1) 2, 5 and 8 (2) 2, 5 and 16
(3) 5, 2 and 8 (4) 5, 2 and 16
25. 2
Sol. ( )
2
4 2
2 MnO 5e 8H Mn 4H O
+ +
+ + +
Reduction half reaction
( )
2
2 4 2
5 C O 2CO 2e
+
Oxidation half
reaction.
balanced equations is:
2 2
4 2 4 2 2
x z
z x y 2y
2
2MnO 5C O 16H 2Mn 10CO 8H O
+ +
+ + + +
x = 2, y = 5, z = 16.
26. Which of the following exists as covalent
crystals in the solid state ?
(1) Silicon (2) Sulphur
(3) Phosphorus (4) Iodine
26. 1
Sol. In covalent solid, the bonds are strong
covalent bonds and usually form high melting
point solids. They are also called network
solids.
Ex : diamond, graphite Si, SiC
S8 and P4 are molecular solids. The individual
units are bound by weak Vanderwaals forces
of attraction. They have low MP & BP
but as per latest literature (2004 yr) the lattice
energy of I2 solid is found to be 10 times
more than that for a Vanderwaals solid &
thus its now considered as covalent crystal.
27. Compound (A), C8H9Br, gives a white
precipitate when warmed with alcoholic
AgNO3. Oxidation of (A) gives an acid (B), C-
8H6O4. (B) easily forms anhydride on heating.
Identify the compound (A).
(1)
C
2
H
5
Br
(2)
CH
2
Br
CH
3
(3)
CH
2
Br
CH
3
(4)
CH
2
Br
CH
3
27. 3
Sol. The compounds
CH
2
Br
CH
3
,
CH
2
Br
CH
3
and
CH
2
Br
CH
3
can give precipitate with aq. Alcoholic AgNO3
solution because of the stability of the
intermediate (benzylic cation)
The compound
C
2
H
5
Br
Does not give ppt with aq. Alcoholic AgNO3
because the intermediate is highly unstable
(i.e., Aryl cation)
The
CH
2
Br
CH
3
,
CH
2
Br
CH
3
and
CH
2
Br
CH
3
On oxidation gives
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS20137
COOH
COOH
,
COOH
COOH
and
COOH
COOH
respectively
But only
COOH
COOH
can form anhydride. Hence the compound
(A) is
CH
2
Br
CH
3
28. Energy of an electron is given by
2
18
2
Z
E 2.178 10 J
n
_
,
. Wavelength of
light required to excite an electron in an
hydrogen atom from level n = 1 to n = 2 will
be :
(h = 6.62 x 10
34
Js and c = 3.0 x 10
8
ms
1
)
(1) 2.816 = 10
7
m
(2) 6.500 x 10
7
m
(3) 8.500 x 10
7
m
(4) 1.214 x 10
7
m
28. 4
Sol.
18 2
2 2
1 2
hc 1 1
E 2.178 10 Z
n n
1
1
1
]
Here Z = 1 for Hatom; n1 = 1 & n2 = 2.
34 8
18 2
hc 6.625 10 3 10
2.178 10 z
2 2
1 2
1 1
n n
1
1
1
]
= 1.214 x 10
7
m.
29. An organic compound A upon reacting with
NH3 gives B. On heating, B gives C. C in
presence of KOH reacts with Br2 to give
CH3CH2NH2. A is :
(1) CH3CH2CH2COOH
(2) CH3CH(CH3)COOH
(3) CH3CH2COOH
(4) CH3COOH
29. 3
Sol.
( )
3
NH
3 2 3 2 4
A
CH CH COOH CH CH COONH
2
3 2 2
H O
CH CH CONH
2
Hoffmann Bromamide reaction
3 2 2
KOH Br
CH CH NH
+
30. In which of the following pairs of molecules/
ions, both the species are not likely to exist ?
(1)
2
2 2
H He
< (2)
2
2 2
H ,He
+
(3)
2
2 2
H He
+
< (4)
2
2 2
H ,He
+
30. 2
Sol. If bond order of a species is zero, it does not
exist.
2
2 2
H &He
+
has a B.O. of zero
do not exist.
PART B: MATHEMATICS
31. The circle passing through (1, 2) and
touching the axis of x at (3, 0) also passes
through the point:
(1) (2, 5) (2) (5, 2)
(3) (2, 5) (4) (5, 2)
31. 2
Sol. Centre (3, k)
Then (3 1)
2
+ (k + 2)
2
= k
2
4 + k
2
+ 4 + 4k = k
2
4k + 8 = 0 k = 2
Centre of circle is (3, 2)
Equation of circle: (x 3)
2
+ (y + 2)
2
= 4
Circle will passes through the point (5, 2).
(1, 2)
(3, 0)
x
32. ABCD is a trapezium such that AB and CD
are parallel and BC CD. If ADB = , BC =
p and CD = q, then AB is equal to :
(1)
2 2
p q cos
pcos qsin
+
+
(2)
2 2
2 2
p q
p cos q sin
+
+
(3)
( )
( )
2 2
2
p q sin
pcos qsin
+
+
(4)
( )
2 2
p q sin
pcos qsin
+
+
32. 4
Sol. BC = p and CD = q,
let BDC = DAB =
BD
2
= p
2
+ q
2
By sine rule in ABD,
( )
AB BD
sin sin
AB =
( ) ( )
2
BD sin BD sin
sin cos sin cos pcos qsin
+ +
( )
2 2
p q sin
pcos qsin
+
+
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS20138
D
A
C
B
p
q
33. Given: A circle 2x
2
+ 2y
2
= 5 and a parabola,
y
2
=4 5x .
Statement 1: An equation of a common
tangent to these curves is y = x + 5 .
Statement 2: If the line, y =
5
mx
m
+ (m
0) is their common tangent, then m satisfies
m
4
3m
2
+ 2 = 0.
(1) Statement 1 is true, statement 2 is true,
statement 2 is not a correct explanation for
statement 1
(2) Statement 1 is true, statement 2 is false
(3) Statement 1 is false, statement 2 is true
(4) Statement 1 is true, statement 2 is true,
statement 2 is a correct explanation for
statement 1
33. 2
Sol. Let y = mx +
5
m
is a tangent to parabola
and circle then
( )
2 2
2
5 / m 5 1 1
2 2
m 1 m
1 m
+
+
2 = m
4
+ m
2
m
4
+ m
2
2 = 0
m
4
+ 2m
2
m
2
2 = 0
(m
2
1) (m
2
+ 2) = 0
m = t1
Common tangents are y = x + 5 or
y = x 5
statement 1 is true, statement 2 is false.
34. A ray of light along x + 3y 3 gets
reflected upon reaching x-axis; the equation
of the reflected ray is:
(1) 3y x 3 (2) y = 3x 3
(3) 3y x 1 (4) y x 3 +
34. 1
Sol. y =
x
1
3
+
Slope of line along
incident Ray = tan
5
6
Slope of reflected ray =
1
3
Equation of reflected ray is; y = ( )
1
x 3
3
3y x 3
x
35. All the students of a class performed poorly
in Mathematics. The teacher decided to give
grace marks of 10 to each of the students.
Which of the following statistical measures
will not change even after the grace marks
were given ?
(1) median (2) mode
(3) variance (4) mean
35. 3
Sol. Variance =
( )
2
n
i
i 1
x
n
Variance will not change as xi and both will
be changing by 10, therefore (xi ) will not
change.
36. If x, y, z are in A.P and tan
1
x, tan
1
y and tan
1
z are also in A.P., then
(1) 2x = 3y = 6z (2) 6x = 3y = 2z (3) 6x =
4y = 3z (4) x = y = z
36. 4
Sol. tan
1
y tan
1
x = tan
1
z tan
1
y
1 1
y x z y
tan tan
1 xy 1 yz
+ +
y x z y
1 xy 1 yz
+ +
d d
1 xy 1 yz
+ +
(since x, y, z are in A.P.,
y x = z y = d)
If d = 0 x = y = z
If d 0 1 + yz = 1 + xy
y(x z) = 0 y = 0 or x = z
But x = z is not possible; otherwise d = 0
If y = 0, then x + z = 0 and tan
1
x + tan
1
z = 0
x + z = 0 x = z.
37. If f(x) dx = (x), then x
5
f(x
3
) dx is equal to:
(1) ( ) ( )
3 3 3 3
1
x x 3 x x dx C
3
+
(2) ( ) ( )
3 3 2 3
1
x x x x dx C
3
+
(3) ( ) ( )
3 3 3 3
1
x x x x dx C
3
1
+
1
]
(4) ( ) ( )
3 3 2 3
1
x x x x dx C
3
1
+
1
]
37. 2
Sol. f(x) dx = (x)
I = x
5
f(x
3
) dx = x
3
x
2
f(x
3
) dx
Let x
3
= t
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS20139
I = ( ) ( )
dt 1 1
t f t tf t dt
3 3 3
[t (t) (t)
dt] + c
=
1
3
[x
3
(x
3
) (x
3
) 3x
2
dx]
=
1
3
x
3
(x
3
) x
2
(x
3
) dx + c
38. The equation of the circle passing through
the foci of the ellipse
2 2
x y
16 9
+ = 1 and having
centre at
(0, 3) is:
(1) x
2
+ y
2
6y + 7 = 0
(2) x
2
+ y
2
6y 5 = 0
(3) x
2
+ y
2
6y + 5 = 0
(4) x
2
+ y
2
6y 7 = 0
38. 4
Sol. e =
9 7
1
16 4
foci = ( )
7
4,0 7,0
4
_
t t
,
Equation of circle having centre at (0, 3)
and passing through ( )
7,0
is
x
2
+ y
2
6y + 9 = 7 + 9
x
2
+ y
2
6y 7 = 0
39. The x-coordinate of the incentre of the
triangle that has the coordinates of mid
points of its sides as (0, 1), (1, 1) and (1, 0)
is:
(1) 2 2 (2) 1 2 +
(3) 1 2 (4) 2 2 +
39. 1
Sol. From the figure.
r r 2
r
2
+
2 2r = 2r
r =
2
2 2
2 2
+
(0, 2)
(0, 1)
(0, 0) (1, 0) (2, 0)
x + y = 2
(r, r)
Let r be the inradius of
the circle.
40. The intercepts on x-axis made by tangents to
the curve, y =
x
0
t dt
, x R, which are
parallel to the line y = 2x, are equal to:
(1) t2 (2) t3 (3) t4 (4) t1
40. 4
Sol. y =
x
0
t dt
, x R
f(x) = |x| |x| = 2 x = t2
y =
2 2
0 0
t dt t dt 2
y =
2
0
t dt 2
Points are (2, 2) and (2, 2)
Equation of tangents are y = 2x 2 and
y = 2x + 2.
41. The sum of first 20 terms of the sequence
0.7, 0.77, 0.777, ...... is
(1) ( )
20
7
99 10
9
(2) ( )
20
7
179 10
81
+
(3) ( )
20
7
99 10
9
+ (4) ( )
20
7
179 10
81
41. 2
Sol. S20 = 0.7 + 0.77 + 0.777 + ..... =
2 3
1 11 111
7 till 20 terms
10 10 10
1
+ + +
1
]
=
2 3
2 3
7 10 1 10 1 10 1
till 20 terms
9 10 10 10
1
+ + +
1
]
S20 =
20
1
1
7 1 10
20
1
9 10
1
10
1 _
_
1
, 1
1
1
, ]
( )
20 20
7 1 7
20 1 10 179 10
9 9 81
1
1 +
1 ]
]
.
42. Consider:
Statement 1: (p ~ q) (~ p q) is a
fallacy.
Statement 2: (p q) (~ q ~ p) is a
tautology.
(1) Statement 1 is true, statement 2 is true,
statement 2 is not a correct explanation for
statement 1
(2) Statement 1 is true, statement 2 is false
(3) Statement 1 is false, statement 2 is true
(4) Statement 1 is true, statement 2 is true,
statement 2 is a correct explanation for
statement 1
42. 1
Sol.
p q ~q p (~q) ~p ~p q (p ~q)
(~p q)
F
T
F
F
F
F
T
F
F
F
F
F
p q p q ~q ~p ~q ~p (p q)
(~q ~p)
T T T
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS201310
F
T
T
F
T
T
T
T
T
Statement 1 is true, statement 2 is true but
statement 2 is not correct explanation for
statement 1
43. The area (in square units) bounded by the
curves y = x , 2y x + 3 = 0, x-axis, and
lying in the first quadrant is
(1) 36 (2) 18 (3)
27
4
(4) 9
43. 4
Sol. A = ( )
9 3
3
2 2
0 0
y
2y 3 y dy y 3y 9
3
1
+ +
1
]
3
9 3
44. The expression
tanA cot A
1 cot A 1 tanA
+
can be
written as:
(1) sec A cosec A + 1 (2) tan A + cot A
(3) sec A + cosec A (4) sin A cos A + 1
44. 1
Sol.
tanA cot A
1 cot A 1 tanA
+
=
( ) ( )
2 2
sin A cos A
cosA sinA cosA sinA cosA sinA
+
=
2 2
1 sin A cos A
sinA cosA cosA sinA
' ;
=
1 sinAcosA
sinAcosA
+
= 1 + sec A cosec A
45. The real number k for which the equation, 2x
3
+ 3x + k = 0 has two distinct real roots in [0,
1]
(1) lies between 2 and 3
(2) lies between 1 and 0
(3) does not exist
(4) lies between 1 and 2
45. 3
Sol. f(x) = 2x
3
+ 3x + k
f(x) = 6x
2
+ 3 > 0 x R
f(x) will be strictly increasing function
therefore two distinct real roots are not
possible for any k.
k doesnt exist.
46.
( ) ( )
x 0
1 cos2x 3 cosx
lim
x tan4x
+
is equal to:
(1)
1
2
(2) 1 (3) 2 (4)
1
4
46. 3
Sol.
( )
( )
( )
2
2 2
x 0 x 0
x 0
x 0
sin x
lim lim 3 cosx
2sin x 3 cosx x
lim
tan4x tan4x
x 4x 2lim
4x 4x
_
+
+
,
_ _
, ,
( ) 1 3 1
4
2
2 1 2
+
47. Let Tn be the number of all possible triangles
formed by joining vertices of an nsided
regular polygon. If Tn + 1 Tn = 10, then the
value of n is:
(1) 5 (2) 10 (3) 8 (4) 7
47. 1
Sol. Number of triangles formed by Joining 3
points out of n non-collinear points =
n
C3
Given that
(n + 1)
C3
n
C3 = 10
( ) ( ) ( ) ( ) n 1 n n 1 n n 1 n 2
10
3! 3!
+
n(n 1) [n + 1 n + 2] = 60
n(n 1) = 20 n(n 1) = 5 4
n = 5
48. At present, a firm is manufacturing 2000
items. It is estimated that the rate of change
of production P w.r.t. additional number of
workers x is given by
dP
100 12 x
dx
. If the
firm employs 25 more workers, then the new
level of production of items is:
(1) 3000 (2) 3500
(3) 4500 (4) 2500
48. 2
Sol.
dP
100 12 x
dx
( )
dp 100 12 x dx
P = 100x
3/ 2
x
12 c
3/ 2
+
P = 100x 8x
3/2
+ c
At x = 0, p = 2000 c = 2000
x = 25 p = 100 25 8 (25)
3/2
+ 2000
= 2500 + 2000 1000 = 3500
49. Statement 1: The value of the integral
/ 3
/ 6
dx
1 tanx
is equal to
6
Statement 2: ( )
b b
a a
f(x) dx f a b x dx +
.
(1) Statement 1 is true, statement 2 is true,
statement 2 is not a correct explanation for
statement 1
(2) Statement 1 is true, statement 2 is false
(3) Statement 1 is false, statement 2 is true
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS201311
(4) Statement 1 is true, statement 2 is true,
statement 2 is a correct explanation for
statement 1
49. 3
Sol. I =
/ 3
/ 6
1
dx
sinx
1
cosx
I =
/ 3
/ 6
cosx
dx
cosx sinx
...... (1)
I =
/ 3
/ 6
cos x
2
dx
cos x sin x
2 2
,
_ _
+
, ,
( )
b b
a a
f(x) dx f a b x dx
1
+ 1
1
]
I =
/ 3
/ 6
sinx
dx
sinx cosx
...... (2)
(1) + (2) 2I =
/ 3
/ 6
cosx sinx
dx
cosx sinx
+
+
2 I = [ ]
/ 3
/ 3
/ 6
/ 6
dx x
2 I =
6
I =
12
Statement 1 is false, statement 2 is true.
50. If P =
1 3
1 3 3
2 4 4
1
1
1
1
]
is the adjoint of a 3 3
matrix A and |A| = 4, then is equal to:
(1) 11 (2) 5 (3) 0 (4) 4
50. 1
Sol. |Adj A| = |A|
n 1
|P| = |A|
2
.
1(12 12) (4 6) + 3(4 6) = 4
2
2 6 = 16 2 = 22 = 11
51. The number of values of k, for which the
system of equations:
(k + 1)x + 8y = 4k
kx + (k + 3)y = 3k 1
has no solution, is:
(1) 1 (2) 2 (3) 3 (4) infinite
51. 1
Sol. For no solution:
k 1 8 4k
k k 3 3k 1
+
+
k
2
+ 4k + 3 = 8k k
2
4k + 3 = 0
(k 1)(k 3) = 0
k = 1, 3
If k = 1, then system has infinite solutions
k = 3
Number of values of k such that system does
not have any solution is 1.
52. If y = sec (tan
1
x), then
dy
dx
at x = 1 is equal
to:
(1)
1
2
(2) 1 (3) 2 (4)
1
2
52. 4
Sol. y = sec (tan
1
x)
( ) ( )
1 1
2
dy 1
sec tan x tan tan x
dx 1 x
+
( )
1
x 1
dy 1 1 1
sec tan 1 1 2
dx 1 1 2
2
_ _
+
, ,
53. If the lines
x 2 y 3 z 4 x 1 y 4 z 5
and
1 1 k k 2 1
are coplanar, then k can have:
(1) exactly one value
(2) exactly two values
(3) exactly three values
(4) any value
53. 2
Sol.
2 1 3 4 4 5
1 1 k
k 2 1
= 0
1 1 1
1 1 k
k 2 1
= 0
(1 + 2k) + 1(1 + k
2
) (2 k) = 0
1 + 2k + 1 + k
2
2 + k = 0 k
2
+ 3k
= 0 k = 0, 3
k have two values
54. Let A and B be two sets containing 2
elements and 4 elements respectively. The
number of subsets of A B having 3 or more
elements is:
(1) 220 (2) 219 (3) 211 (4) 256
54. 2
Sol. Number of elements in
A B = n(A) n(B) = 2 4 = 8.
Number of subsets of A B having 3 or more
elements.
=
8
C3 +
8
C4 +
8
C5 +
8
C6 +
8
C7 +
8
C8 = 2
8
8
C0
8
C1
8
C2
= 256 1 8 28 = 256 37 = 219
55. If the vectors
AB 3i 4k and AC 5i 2j 4k + +
uuur uuur
are the
sides of a triangle ABC, then the length of the
median through A is:
(1) 72 (2) 33 (3) 45
(4) 18
55. 2
Sol. Length of median through A
AB AC 3i 4k 5i 2j 4k 8i 2j 8k
2 2 2
+ + + + +
uuur uuur
4i j 4k 16 1 16 33 + + +
.
56. A multiple choice examination has 5
questions. Each question has three
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS201312
alternative answers of which exactly one is
correct. The probability that a student will get
4 or more correct answers just by guessing
is:
(1)
5
13
3
(2)
5
11
3
(3)
5
10
3
(4)
5
17
3
56. 2
Sol. P(E) =
4 5
5 5
4 5
5 5 5
1 2 1 5 2 1 11
C C
3 3 3 3 3 3
_ _ _
+ +
, , ,
57. If z is a complex number of unit modulus and
argument , then
1 z
arg
1 z
+ _
+
,
equals:
(1)
2
(2) (3) (4)
57. 2
Sol. Let z = e
i
, then
1 z
arg
1 z
+ _
+
,
=
1 cos i sin
arg
1 cos i sin
+ + _
+
,
=
2cos cos i sin
2 2 2
arg
2cos cos i sin
2 2 2
_ _
+
,
_
, ,
=
i / 2
i / 2
e
arg
e
=
58. If the equation x
2
+ 2x + 3 = 0 and ax
2
+ bx +
c = 0, a, b, c R, have a common root, then
a : b : c is:
(1) 3 : 2 : 1 (2) 1 : 3 : 2
(3) 3 : 1 : 2 (4) 1 : 2 : 3
58. 4
Sol. For the equation x
2
+ 2x + 3 = 0 D < 0
both roots will be common.
a : b : c = 1 : 2 : 3.
59. Distance between two parallel planes 2x + y
+ 2z = 8 and 4x + 2y + 4z + 5 = 0 is:
(1)
5
2
(2)
7
2
(3)
9
2
(4)
3
2
59. 2
Sol. Distance between parallel plane
=
( )
2 2 2
5
8
21 7
2
6 2
2 1 2
+ +
.
60. The term independent of x in expansion of
10
2/ 3 1/ 3 1/ 2
x 1 x 1
x x 1 x x
+ _
+
,
is:
(1) 120 (2) 210 (3) 310 (4) 4
60. 2
Sol.
10
2/ 3 1/ 3 1/ 2
x 1 x 1
x x 1 x x
+ _
+
,
=
( ) ( )
( )
( ) ( )
( )
10
1/ 3 2/ 3 1/ 3 1/ 2 1/ 2
2/ 3 1/ 3 1/ 2 1/ 2
x 1 x x 1 x 1 x 1
x x 1 x x 1
_
+ + +
+
,
=
( ) ( ) ( ) ( )
10
1/ 3 1/ 2 1/ 3 1/ 2
x 1 1 x x x
+ +
Tr + 1 =
10
Cr (x
1/3
)
10 r
(x
1/2
)
r
, then
If Tr + 1 is independent of x
10 r r
0
3 2
r = 4
Value of term =
10
C4 (1)
4
= 210
PART C: PHYSICS
61. In an LCR circuit as shown below both
switches are open initially. Now switch S1 is
closed S2 kept open. (q is charge on the
capacitor and = RC is capacitive time
constant). Which of the following statement is
correct ?
(1) at t = ,
CV
q
2
(2) at t = 2,
2
q CV(1 e )
(3) at t
2
,
1
q CV(1 e )
(4) work done by the battery is half of the
energy dissipated in the resistor
L
V
C
S
1
S
2
R
61. 2
Sol. Charge q on capacitor after time t will be,
( )
t
RC
q CV 1 e
_
,
at t = , ( )
1
q CV 1 e
1
]
at t = 2 , q = CV (1e
2
)
at
1
2
t , q CV 1 e
2
_
,
work done by the battery is equal to twice the
energy dissipated in the resistor.
62. A diode detector is used to detect an
amplitude modulated wave of 60%
modulation by using a condenser of capacity
250 pico farad in parallel with a load
resistance 100 kilo ohm. Find the maximum
modulated frequency which could be
detected by it:
(1) 10. 62 kHz (2) 5.31 MHz
(3) 5.31 kHz (4) 10.62 MHz
62. 1
Sol. For resonating condition,
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS201313
1
R
C
1
f
2 RC
Maximum modulated frequency,
max
1 100
f
2 RC 60
= 10.62 kHz
63. The supply voltage to a room is 120 V. The
resistance of the lead wires is 6 . A 60 W
bulb is already switched on. What is the
decrease of voltage across the bulb, when a
240 W heater is switched on in parallel to the
bulb?
(1) 2.9 volt (2) 13.3 volt
(3) 10.04 volt (4) zero volt
63. 3
Sol. Resistance of 60 W bulb for 120 V is
1
120 120
R
60
= 240
Similarly, resistance of 290 W bulb for 120 V
is,
2
120 120
R
240
= 60
Initial current in the circuit is,
1
120
i
6 240
+
=
120
A
246
, Voltage across bulb,
1
120
V 120 6 V
246
_
,
after heater is switched on,
2
i
(through the
battery)
120 120
240 60
54
6
240 60
+
+
A
, Voltage across bulb,
2
120
V 120 6 V
54
_
,
, decrease in voltage = V1 V2.
*64. A uniform cylinder of length L and mass M
having crosssectional area A is suspended,
with its length vertical, from a fixed point by a
massless spring, such that it is half
submerged in a liquid of density at
equilibrium position. The extension x0 of the
spring when it is in equilibrium is:
(1)
Mg LA
1
k M
_
,
(2)
Mg LA
1
k 2M
_
,
(3)
Mg LA
1
k M
_
+
,
(4)
Mg
k
(Here k is spring constant)
64. 2
Sol. In equilibrium,
0
V
kx g Mg
2
+
,
0
V g
Mg
2
x
k
Mg AL
1
k 2M
1
1
]
kx
0
Mg
65. Two charges, each equal to q, are kept at x =
a and x = a on the x - axis. A particle of
mass m and charge
0
q
q
2
is placed at the
origin. If charge q0 is given a small
displacement (y << a) along the yaxis, the
net force acting on the particle is proportional
to:
(1) y (2)
1
y
(3)
1
y
(4) y
65. 4
Sol. Force on q0 due to one of the q charge is
0
2 2
kqq
F
(a y )
+
, net resultance force on q0 will be = 2F cos
0
2 2 2 2 1/ 2
2 kqq y
(a y ) (a y )
+ +
0
3
2kqq
y (as y a)
a
_
<<
,
, Fnet y
F
F
y
q
0
y
x
q q
a +a
66. A beam of unpolarised light of intensity I0 is
passed through a Polaroid A and then
through another Polaroid B which is oriented
so that its principal plane makes an angle of
45 relative to that of A. The intensity of the
emergent light is:
(1)
0
I
2
(2)
0
I
4
(3)
0
I
8
(4) I0
66. 2
Sol. After passing through the Polaroid A,
unpolarized light of intensity I0 will reduced to
polarized light of intensity
0
I
2
.
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS201314
Now this polarized light of intensity
0
I
2
when
passed through another polarizer, intensity of
emergent light will be,
=
2 0 0
I
cos (45 )
2
_
,
=
0
I
4
67. The anode voltage of a photocell is kept
fixed. The wavelength of the light falling on
the cathode is gradually changed. The plate
current I of the photocell varies as follows:
I
O
I
O
(1) (2)
I
O
I
O
(3) (4)
67. 3
Sol. For low wavelength current will be maximum
and as wavelength of incident light
approaches threshold value of wavelength,
the current will approach to zero.
68. Two coherent point sources S1 and S2 are
separated by a small distance d as shown.
The fringes obtained on the screen will be:
S
1
S
2
d
Screen
D
(1) straight lines (2) semi circles
(3) concentric circles (4)points
68. 3
Sol. Locus of
1 2
(S P S P)
on the screen will be
concentric circle.
(where P is a point on the screen)
69. A metallic rod of length l is tied to a string of
length 2 l and made to rotate with angular
speed on a horizontal table with one end of
the string fixed. If there is a vertical magnetic
field 'B' in the region, the e.m.f. induced
across the ends of the rod:
(1)
2
3B
2
l
(2)
2
4B
2
l
(3)
2
5B
2
l
(4)
2
2B
2
l
2l
69. 3
Sol. emf induced across one element of the
conducting rod of length dx at a distance x
from the fixed end of the string will be, d =
(B) (dx) (x)
emf induced across the ends of the rod is,
3L
2L
B x dx
=
2
5
B L
2
70. In a hydrogen like atom electron makes
transition from an energy level with quantum
number n to another with quantum number (n
1). If n >> 1, the frequency of radiation
emitted is proportional to:
(1)
2
1
n
(2)
3 / 2
1
n
(3)
3
1
n
(4)
1
n
70. 3
Sol. As energy E of nth level is
2
1
n
2 2
1 1
E
(n 1) n
1
1
]
2 2
2 2
n (n 1)
n (n 1)
1
]
2 2
2n 1
n (n 1)
1
]
3
1
(as n 1)
n
>>
*71. Assume that a drop of liquid evaporates by
decrease in its surface energy, so that its
temperature remains unchanged. What
should be the minimum radius of the drop for
this to be possible? The surface tension is T,
density of liquid is and L is its latent heat of
vaporization:
(1)
T
L
(2)
T
L
(3)
2T
L
(4)
L
T
71. 3
Sol. Decrease in surface energy of a drop of
radius r, when a layer of thickness dr
evaporates is,
dU (8 rdr)T
Hence,
T(8 r dr) (dm)L
2
T(8 r dr) (4 r dr) L
2T
r
L
72. The graph between angle of deviation () and
angle of incidence (i) for a triangular prism is
represented by :
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS201315
O
i
O
i
(1) (2)
O
i
O
i
(3) (4)
72. 2
Sol. Angle of deviation () first decreases and
then increases non linearly for a triangular
prism when angel of incidence is increased.
Angle of deviation is minimum when angle of
incidence is equal to angle of emergence.
73. Let
0
denote the dimensional formula of the
permittivity of vacuum. If M = mass, L=
length, T = time and A = electric current, then
:
(1)
1 3 4 2
0
[M L T A ]
(2)
1 2 1 2
0
[M L T A ]
(3)
1 2 1
0
[M L T A]
(4)
1 3 2
0
[M L T A]
73. 1
Sol.
1 2
2
0
q q 1
F
4 r
2 1 2
0
2
q q
[F] [MLT ]
4 r F
1 3 4 2
0
2 2
[AT][AT]
[ ] [M L T A ]
[L] [MLT ]
*74. The above p-v diagram represents the
thermodynamic cycle of an engine, operating
with an ideal monoatomic gas. The amount
of heat, extracted from the source in a single
cycle is :
P
A D
B C
2P
0
P
0
V
0
2V
0
V
(1)
0 0
13
p v
2
_
,
(2)
0 0
11
p v
2
_
,
(3) 4p0v0 (4) p0v0
74. 1
Sol. AB is isochoric
v
3
Q U nc T nR T
2
B B A A
3 3
P V P V
2 2
0 0
3
P V
2
BC is isobaric
P C C B B
5 5 5
Q nC T nR T P V P V
2 2 2
0 0
5
(2P V )
2
Q in CD and DA are negative since
temperature is decreasing
Heat taken from source
=
AB BC 0 0
13
Q Q P V
2
+
P
A D
B C
2P
0
P
0
V
0
2V
0
V
75. A sonometer wire of length 1.5 m is made of
steel. The tension in it produces an elastic
strain of 1%. What is the fundamental
frequency of steel if density and elasticity of
steel are 7.7 x 10
3
kg/m
3
and 2.2 x 10
11
N/m
2
respectively?
(1) 178.2 Hz (2) 200.5 Hz
(3) 770 Hz (4) 188.5 Hz
75. 1
Sol. Fundamental frequency =
1 T
2 l
= (density) (crossectional area).
= A
T YA
_
,
l
l
1
100
l
l
YA
1
f
2 A
_
,
l
l
l
= 178.2Hz.
76. This question has Statement I and Statement
II. Of the four choices given after the
Statements, choose the one that best
describes the two Statements.
Statement - I : Higher the range, greater is
the resistance of ammeter.
Statement - II: To increase the range of ammeter,
additional shunt needs to be used across it.
(1) Statement-I is true, Statement-II is true,
Statement-II is not the correct explanation of
Statement-1.
(2) Statement - I is true, Statement - II is
false.
(3) Statement - I is false, Statement II is
true.
(4) Statement-I is true, Statement - II is true,
Statement -II is the correct explanation of
Statement - 1.
76. 3
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS201316
Sol.
g
g
R S
i i
S
+ 1
1
]
Smaller the shunt used, higher is the current
in ammeter for same galvanometer current.
*77. What is the minimum energy required to
launch a satellite of mass m from the surface
of a planet of mass M and radius R in a
circular orbit at an altitude of 2R ?
(1)
2GmM
3R
(2)
GmM
2R
(3)
GmM
3R
(4)
5GmM
6R
77. 4
Sol. Total energy of satellite at surface of planet =
GMm
R
Potential energy of satellite in orbit at altitude
of
GMm
2R
3R
Orbital velocity =
GM
3R
Kinetic energy at altitude of
2
1 GM
2R m
2 3R
_
,
GMm
6R
Total energy in orbit of altitude
GMm
2R
6R
Hence minimum energy required to launch in
orbit =
GMm GMm
R 6R
=
5GmM
6R
*78. A projectile is given an initial velocity of
(i 2j)m/ s + , where
i
is along the ground and
j is along the vertical. If g = 10 m/s
2
, the
equation of its trajectory is:
(1) y = 2x 5x
2
(2) 4y = 2x 5x
2
(3) 4y = 2x 25x
2
(4) y = x 5x
2
78. 1
Sol. Initial velocity
x y
u i 2j u 1, u 2 +
x y
a g j a 0, a 10
2
x x
1
x x u t a t
2
+
= t
2 2
y y
1
y y u t a t 2t 5t
2
+
Substituting x = t in y = 2t 5t
2
We get y = 2x 5x
2
79. Two capacitors C1 and C2 are charged to 120
V and 200 V respectively. It is found that by
connecting them together the potential on
each one can be made zero. Then :
(1) 3C1 = 5C2 (2) 3C1 + 5C2= 0
(3) 9C1 = 9C2 (4) 5C1 = 3C2
79. 1
Sol. Let q1 and q2 be charges on capacitors C1
and C2 respectively.
The potential can be made zero by
connecting them across each other with
opposite terminals connected.
Final charges can be zero if
q1 q2 = 0
q1 = 120 C1 and q2 = 200 C2
120C1 = 200C2 3C1 = 5C2
q
1
q
1
q
2
q
2
C
2
C
1
*80. A hoop of radius r and mass m rotating with
an angular velocity 0 is placed on a rough
horizontal surface. The initial velocity of the
centre of the hoop is zero. What will be the
velocity of the centre of the hoop when it
ceases to slip?
(1)
0
r
3
(2)
0
r
2
(3) r 0 (4)
0
r
4
80. 2
Sol. Let final velocity and angular velocity of hoop
be v and when it starts pure rolling.
Such that
v r
Angular momentum about a point P on the
ground initially
2
i 0
L mr
f P
0
Angular momentum about P finally when
hoop starts pure rolling
2 2
f
L mr mvr 2mr +
Angular momentum about P is conserved.
Hence
2 2
i f 0
L L mr 2mr
0
2
Hence velocity of center of mass finally,
0
r
v
2
v
*81. An ideal gas enclosed in a vertical cylindrical
container supports a freely moving piston of
mass M. The piston and the cylinder have
equal cross sectional area A. When the
piston is in equilibrium, the volume of the gas
is V0 and its pressure is P0 The piston is
slightly displaced from the equilibrium
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS201317
position and released. Assuming that the
system is completely isolated from its
surrounding, the piston executes a simple
harmonic motion with frequency :
(1)
0 0
2
V MP 1
2 A
(2)
2
0
0
A P 1
2 MV
(3)
0
0
MV 1
2 A P
(4)
0
0
A P 1
2 V M
81. 2
Sol. Initially at equilibrium
a 0
P A Mg P A +
Let piston be displaced by x
Volume = V0 Ax
Since gas undergoes adiabatic change
0 0
P V PV
=
0
P(V Ax)
0
x
0
0 0
0 0
V Ax
P P P 1
V Ax V
1 1
1 1
] ]
a a
P A Mg PA M +
a 0 a
0
Ax
P A Mg P A 1 M
V
1
+
1
]
Since Ax << V0
2
0
a 0 a
0
P A x
P A Mg P A M
v
+
2
0
0
P A
a x
MV
The piston undergoes SHM with
2
2 0
0
P A
MV
frequency =
2
0
0
P A 1
2 2 MV
82. A charge Q is uniformly distributed over a
long rod AB of length L as shown in the
figure. The electric potential at the point O
lying at a distance L from the end A is:
O
B A
L L
(1)
0
3Q
4 L
(2)
0
Q
4 Lln2
(3)
0
Qln2
4 L
(4)
0
Q
8 L
82. 3
Sol. Consider on element of length dx at distance
x from 0.
Potential due to element at 0
=
0
1 dq
4 x
charge q
dq dx dx
length L
_
,
O
dx B A
L L
x
Net potential
0
1 dq
4 x
2L
0
L
1 q dx
4 L x
0
1 q
. .ln2
4 L
83. A circular loop of radius 0.3 cm lies parallel to
a much bigger circular loop of radius 20 cm.
The centre of the small loop is on the axis of
the bigger loop. The distance between their
centres is 15 cm. If a current of 2.0 A flows
through the smaller loop, then the flux linked
with bigger loop is
(1) 6 x 10
11
weber
(2) 3.3 x 10
11
weber
(3) 6.6 x 10
9
weber
(4) 9.1 x 10
11
weber
83. 4
Sol.
2
0 1
2 2 3 / 2
i R
B
2(R x )
+
2
2 0 1
2
2 2 3 / 2
i R
r
2(R x )
+
2 2
0
2 i
2 2 3 / 2
r R
i
2(R x )
+
x
R
i
1
i
2
P
r
2 2
0
2 2 3/ 2
r R
M
2(R x )
+
1 2
Mi
2 2
0 2
2 2 3 / 2
r R i
2(R x )
+
11
9.1 10
Wb.
*84. If a piece of metal is heated to temperature
and then allowed to cool in a room which is
at temperature 0, the graph between the
temperature T of the metal and time t will be
closest to :
T
t O
0
T
t O
0
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS201318
(1) (2)
T
t O
0
T
t O
(3) (4)
84. 1
Sol. Body was by newtons law of cooling
0
d
k( )
dt
i
t
0
0
d
k dt
0
i 0
log kt
kt
0 i 0
e ( )
kt
0 i 0
( )e
+
So, exponentially decreasing to
0
.
85. The I V characteristic of an LED is
I
V O
B
G
Y
R
I
V O
(1) (2)
I
O
B
G
Y
R
V
I
V O
(R) (Y) (G) (B)
R
e
d
Y
e
l
l
o
w
G
r
e
e
n
B
l
u
e
(3) (4)
85. 4
Sol. LED is pn junction diode.
A diode operates in forward bias
hc
E
so smaller wavelength for higher
energy.
So, when voltage is less, then smaller energy
is acquired by electron in conduction band
and hence while jumping to occupy a hole it
releases lesser energy and hence higher
wavelength (i.e. Red) and so on.
*86. This question has Statement I and Statement
II. Of the four choices given after the
Statements, choose the one that best
describes the two Statements.
Statement - I: A point particle of mass m
moving with speed v collides with stationary
point particle of mass M. If the maximum
energy loss possible is given as
2
1 m
f mv then f
2 M m
_ _
+
, ,
.
Statement - II : Maximum energy loss occurs
when the particles get stuck together as a
result of the collision.
(1) Statement - I is true, Statement - II is true,
Statement - II is a correct explanation of
Statement -I.
(2) Statement - I is true, Statement - II is
false.
(3) Statement - I is false, Statement II is
true.
(4) Statement - I is true, Statement-II is true,
Statement-II is not a correct explanation of
Statement-I
86. 3
Sol. MV = MV1 + MV2
Maximum energy lost when V1 = V2 = V0
(e = 0)
mV = (m + M)v0
0
mv
v
m M
_
+
,
2 2
0
1 1
E mv (m M)V
2 2
+
2 2
2
2
1 1 m v
mv (m M)
2 2 (m M)
+
+
2
1 m
mv 1
2 m M
1
1
+
]
2
1 M
E mv
2 m M
_
+
,
2
1
E f mv
2
_
,
M
f
m M
+
*87. The amplitude of a damped oscillator
decreases to 0.9 times its original magnitude
in 5s. In another 10s it will decrease to
times its original magnitude, where equals:
(1) 0.81 (2) 0.729 (3) 0.6 (4) 0.7
87. 2
Sol.
bt
1 2
y e [C cos t C sin t]
+
bt
y Ce cos( t )
+
A = Ce
bt
0.9 C = C e
b x 5
log (0.9) = 5b
1 10
log 15
5 9
A' Ce
_
,
3
10
log
9
Ce
_
= 0.729 C
= 0.729
88. Diameter of a plano convex lens is 6 cm
and thickness at the centre is 3 mm. If speed
of light in material of lens is 2 x 10
8
m/ s, the
focal length of the lens is :
(1) 20 cm (2) 30 cm
(3) 10 cm (4) 15 cm
88. 2
Sol. C = 2 x 10
8
m/s
8
8
3 10
1.5
2 10
(R 0.3)
2
+ 3
2
= R
2
R
2
+ 0.09 0.6R + 9 = R
2
0.6R = 9.09, R = 15.15 cm
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
JEE MAINS201319
1 1 1
(1.5 1)
f 15.15
_
,
1 1
0.5
f 15.15
f 30 cm
3cm
R0.3
R
0.3
89. The magnetic field in a travelling
electromagnetic wave has a peak value of 20
nT. The peak value of electric field. strength
is :
(1) 6 V/m (2) 9 V/m
(3) 12 V/m (4) 3 V/m
89. 1
Sol.
0
0
E
C
B
E0 = B0C = 20 x 10
9
x 3 x 10
8
E0 = 6 V/m
90. Two short bar magnets of length 1 cm each
have magnetic moments 1.20 Am
2
and 1.00
Am
2
respectively. They are placed on a
horizontal table parallel to each other with
their N poles pointing towards the South.
They have a common magnetic equator and
are separated by a distance of 20.0 cm. The
value of the resultant horizontal magnetic
induction at the mid - point O of the line
joining their centres is close to (Horizontal
component of earth's magnetic induction is
3.6 x 10
5
Wb/m
2
)
(1) 2.56 x 10
4
Wb/m
2
(2) 3.50 x 10
4
Wb/m
2
(3) 5.80 x 10
4
Wb/m
2
(4) 3.6 x 10
5
Wb/m
2
90. 1
Sol.
1 2
0 0
1 2
3 3
M M
B (N), B (N)
4 4 d d
ur ur
ur ur
0 0 1 1
1 2
3 3 3
M M
B [M M ]
4 d d 4 d
1
+ +
1
]
ur ur ur
7
4 2
3
10
B [2.2] 2.2 10 Wb/ m (N)
(0.1)
ur
S
N
S
N
1 cm 1 cm
d d
20cm
S
N
W E
O
net H B B B +
ur ur ur
= 2.2 x 10
4
+ 3.6 x 10
5
= 2.56 x 10
4
Wb/m
2
(N).
* * *
FIITJEE (Hyderabad Classes) Limited. 5-9-14/B, Saifabad, (Opp. Secretariat) Hyderabad. 500 063. Phone: 040-66777000 03 Fax: 040-66777004
Anda mungkin juga menyukai
- At The Innovation SprintDokumen2 halamanAt The Innovation SprintBNRedBelum ada peringkat
- Chemistry Is Central ScienceDokumen3 halamanChemistry Is Central ScienceBNRedBelum ada peringkat
- Doing Science Is The Greatest Invention of Mankind Among All The Other WorksDokumen1 halamanDoing Science Is The Greatest Invention of Mankind Among All The Other WorksBNRedBelum ada peringkat
- FiDokumen1 halamanFiBNRedBelum ada peringkat
- Exponential Function WebsitesDokumen1 halamanExponential Function WebsitesBNRedBelum ada peringkat
- PEG Gels M Zimm PlotsDokumen1 halamanPEG Gels M Zimm PlotsBNRedBelum ada peringkat
- Hot Water Freezes Faster Than The Cold WaterDokumen1 halamanHot Water Freezes Faster Than The Cold WaterBNRedBelum ada peringkat
- FiDokumen1 halamanFiBNRedBelum ada peringkat
- FiDokumen1 halamanFiBNRedBelum ada peringkat
- Exponential Function WebsitesDokumen1 halamanExponential Function WebsitesBNRedBelum ada peringkat
- Plan of Next WeekDokumen4 halamanPlan of Next WeekBNRedBelum ada peringkat
- Exponential Function WebsitesDokumen1 halamanExponential Function WebsitesBNRedBelum ada peringkat
- One Should Not Get Disillusioned by PHDDokumen1 halamanOne Should Not Get Disillusioned by PHDBNRedBelum ada peringkat
- Just Think If We Could Make Plumbene, We May Be Able To Make Millimeter Thin Sheets of 100% Radioactive Resistant CoatingsDokumen1 halamanJust Think If We Could Make Plumbene, We May Be Able To Make Millimeter Thin Sheets of 100% Radioactive Resistant CoatingsBNRedBelum ada peringkat
- Just Think If We Could Make Plumbene, We May Be Able To Make Millimeter Thin Sheets of 100% Radioactive Resistant CoatingsDokumen1 halamanJust Think If We Could Make Plumbene, We May Be Able To Make Millimeter Thin Sheets of 100% Radioactive Resistant CoatingsBNRedBelum ada peringkat
- Dear ScientistsDokumen1 halamanDear ScientistsBNRedBelum ada peringkat
- Just Think If We Could Make Plumbene, We May Be Able To Make Millimeter Thin Sheets of 100% Radioactive Resistant CoatingsDokumen1 halamanJust Think If We Could Make Plumbene, We May Be Able To Make Millimeter Thin Sheets of 100% Radioactive Resistant CoatingsBNRedBelum ada peringkat
- One Should Not Get Disillusioned by PHDDokumen1 halamanOne Should Not Get Disillusioned by PHDBNRedBelum ada peringkat
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Thermal Physics - Rod in CylinderDokumen5 halamanThermal Physics - Rod in CylinderLUIS HUMBERTO MARTINEZ PALMETHBelum ada peringkat
- Che433 - 483 (3) Past Year PaperDokumen5 halamanChe433 - 483 (3) Past Year Paperthe5sos100% (1)
- LPX M930aDokumen39 halamanLPX M930aJhonatan CastroBelum ada peringkat
- Lab Experiment: Viscosity and Density: September 18, 2018Dokumen15 halamanLab Experiment: Viscosity and Density: September 18, 2018Luqman HakimBelum ada peringkat
- Basic Electrical Engineering: BY R. Sivaprasad, Lecturer in Eee, Govt. Polytechnic, SatyaveduDokumen77 halamanBasic Electrical Engineering: BY R. Sivaprasad, Lecturer in Eee, Govt. Polytechnic, Satyavedurathina4careerBelum ada peringkat
- AQA Physics Students Book AnswersDokumen3 halamanAQA Physics Students Book AnswersT-Forty ArrowBelum ada peringkat
- Fluid Mechanics Fundamentals and Applications 4Th Edition Cengel Solutions Manual Full Chapter PDFDokumen67 halamanFluid Mechanics Fundamentals and Applications 4Th Edition Cengel Solutions Manual Full Chapter PDFfionaalexandrahukc100% (10)
- Projectile MotionDokumen15 halamanProjectile MotionAlina PetrușBelum ada peringkat
- Form 4 Physics Formula ListDokumen7 halamanForm 4 Physics Formula ListNoraini Ibrahim33% (3)
- Introduction EM Waves WorksheetDokumen3 halamanIntroduction EM Waves WorksheetUmer AbdullahBelum ada peringkat
- Chapter 3 - Differential CalculusDokumen25 halamanChapter 3 - Differential CalculusEdison SioBelum ada peringkat
- Kimet JusufiDokumen7 halamanKimet JusufiFatlum RushitiBelum ada peringkat
- Chemical Solution Density & ViscosityDokumen18 halamanChemical Solution Density & ViscosityLee JianBelum ada peringkat
- Unit & DimensionsDokumen10 halamanUnit & DimensionstosifBelum ada peringkat
- Cambridge International AS & A Level: Physics 9702/13Dokumen20 halamanCambridge International AS & A Level: Physics 9702/13NigelBelum ada peringkat
- Technorama Hausprospekt enDokumen42 halamanTechnorama Hausprospekt enSriheri DeshpandeBelum ada peringkat
- Burland, J. B. (1973) - Shaft Friction of Piles in Clay - A Simple Fundamental Approach. Ground Engineering, 6 (3), 30-42Dokumen6 halamanBurland, J. B. (1973) - Shaft Friction of Piles in Clay - A Simple Fundamental Approach. Ground Engineering, 6 (3), 30-42Jennifer Miller100% (1)
- Flow Through Packed and Fluidized BedsDokumen6 halamanFlow Through Packed and Fluidized BedsJamie SamuelBelum ada peringkat
- Rheology of The Earth's Mantle: A Historical Review: Gondwana Research July 2010Dokumen30 halamanRheology of The Earth's Mantle: A Historical Review: Gondwana Research July 2010MarcosGouveaBelum ada peringkat
- YZ250F Matlab DocumentationDokumen12 halamanYZ250F Matlab DocumentationvivekpattniBelum ada peringkat
- 1802 04173 PDFDokumen87 halaman1802 04173 PDFÇhura CristianBelum ada peringkat
- Shaftings: Cebu Institute of Technology - UniversityDokumen29 halamanShaftings: Cebu Institute of Technology - UniversityRenniel DingcongBelum ada peringkat
- Triaxial TestDokumen67 halamanTriaxial TestJeffBelum ada peringkat
- P30 Unit Three Diploma ReviewDokumen30 halamanP30 Unit Three Diploma ReviewhelloBelum ada peringkat
- Magnetic Flow MeterDokumen3 halamanMagnetic Flow Meterniazakhtar100% (1)
- Refrigeration Unit: Muhammad Tarmizi Bin Abdul Murad (2019883632)Dokumen18 halamanRefrigeration Unit: Muhammad Tarmizi Bin Abdul Murad (2019883632)Lehbron JemsBelum ada peringkat
- Improved Reactive Power Capability With Grid Connected Doubly Fed Induction GeneratorDokumen14 halamanImproved Reactive Power Capability With Grid Connected Doubly Fed Induction GeneratorUday Wankar100% (2)
- Lateral Earth PressureDokumen122 halamanLateral Earth PressureEric DelunaBelum ada peringkat
- ORS SystemDokumen4 halamanORS SystemlaxmanBelum ada peringkat
- Chipman TransmissionLines TextDokumen244 halamanChipman TransmissionLines TextMau Gar Ec83% (6)