Anthracyclines and Heart Failure: Clinical Implications of Basic Research
Diunggah oleh
Danny IndrawarmanJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Anthracyclines and Heart Failure: Clinical Implications of Basic Research
Diunggah oleh
Danny IndrawarmanHak Cipta:
Format Tersedia
The
n e w e ng l a n d j o u r na l
of
m e dic i n e
clinical implications of basic research
Anthracyclines and Heart Failure
Douglas B. Sawyer, M.D., Ph.D. Since their discovery more than 50 years ago, anthracyclines have become the mainstay for the treatment of many cancers. However, anthracyclines are associated with a risk of heart failure, with the risk proportionate to the cumulative exposure; cardiac injury appears to occur with every dose, and cardiac-biopsy specimens obtained within hours after a single dose of an anthracycline (e.g. doxorubicin or daunorubicin) show pathologic changes.1 Much effort has gone into finding ways to prevent anthracycline cardiotoxicity, yet advanced heart failure remains a consequence of anthracycline exposure. Moreover, symptomatic heart failure often occurs years after cancer treatment, making it difficult to evaluate preventive strategies. The dominant theory of how anthracyclines cause heart damage involves the generation of reactive oxygen species, which results in damage to DNA, proteins, and lipids and leads to cellular dysfunction and myocyte death. However, results from a recent study by Zhang et al.2 suggest that the first step in cardiac myocyte damage from anthracyclines is independent of reactive oxygen species and depends instead on drug interactions with a particular type of topoisomerase, an enzyme that affects the tension and topologic features of DNA. Anthracyclines disrupt tumor growth by binding to and blocking the function of topoisomerase II (TOP2). Topoisomerases break, twist, and reseal the phosphate backbone of DNA and, in so doing, permit a readjustment in the tension of the double helix during replication and transcription. Anthracyclines intercalate into DNA and form complexes with TOP2 that disrupt the activity of the enzyme and activate a DNA-damage response, leading to cell death. There are several forms of topoisomerase. Rapidly dividing tumor cells express high levels of topoisomerase II alpha (TOP2A). TOP2 beta (TOP2B) is ubiquitously expressed; cardiomyocytes express TOP2B but not TOP2A. Anthracyclines target both TOP2A and TOP2B.
1154
Anthracyclines are thought to cause cardiomyocyte damage by driving reactions that result in the formation of free radicals, which in turn can react with and disrupt the function of many cellular constituents, causing dysfunction and cell death. Numerous studies in isolated cells and in animals have shown cardioprotective effects of antioxidants, lending support to the hypothesis that, as a consequence of anthracycline exposure, reactive oxygen species wreck cardiomyocytes (Fig. 1). However, clinical trials of antioxidants for the prevention of anthracycline-induced cardiac injury have been disappointing. Nevertheless, dexrazoxane, a compound that chelates iron and prevents hydroxyl radical formation in the presence of anthracyclines, prevents cardiac injury, lending support to the hypothesis regarding reactive oxygen species. (Dexrazoxane has been approved by the Food and Drug Administration for the prevention of anthracycline cardiotoxicity.) On the other hand, some research has challenged the hypothesis regarding reactive oxygen species. Using mouse cells in culture, Lyu et al.3 have shown that anthracycline-induced DNA breaks and cell death in a cardiomyocyte cell line depend on the presence of Top2b. Building on this finding, Zhang et al. genetically engineered mice to lack Top2b specifically in their cardiomyocytes. In contrast with control mice, the mutant mice did not have acute or chronic cardiac injury after exposure to doxorubicin, a commonly used anthracycline. Nor did these mice have reductions in left ventricular ejection fraction, a key characteristic of doxorubicin cardiotoxicity. Zhang et al. observed the formation of reactive oxygen species, consequent to interactions among doxorubicin, Top2b, and DNA in the wildtype mice. The formation of reactive oxygen species appeared to be caused by the disruption of mitochondrial function, rather than a consequence of reductionoxidation cycling of doxorubicin quinones. Analysis of cardiac tissue from the doxorubicin-treated wild-type mice revealed
n engl j med 368;12 nejm.org march 21, 2013
The New England Journal of Medicine Downloaded from nejm.org on March 25, 2013. For personal use only. No other uses without permission. Copyright 2013 Massachusetts Medical Society. All rights reserved.
Clinical Implications of Basic Research
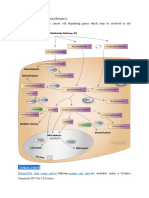
Anthracycline-Induced Heart Failure: the ROS Hypothesis
Anthracycline
O OH
TOP2B Inhibition as Mechanism for Heart Failure
O OH OH
TOP2B alters the tension of DNA during replication and transcription by breaking, twisting, and resealing DNA
TOP2B
OCH3 O
OH
O R
ROS
O2 H2O2 OH O2
e
O
Supercoiled DNA
DNA damage
Uncoiled double-stranded DNA
Quinone
Lipid peroxidation Protein carbonylation
TOP2B
Anthracyclines intercalate into DNA, forming a complex with TOP2B and thereby inhibiting its enzymatic activity.
Anthracyclines
Cellular dysfunction and cell death
DNA double-strand breaks p53 Mitochondrial dysfunction ROS Cellular dysfunction and cell death Mitochondrial biogenesis PGC1- and PGC1-
Figure 1. Mechanisms of Anthracycline-Induced Injury to Cardiac Cells. C O L O R F I G U R Eof reactive oxygen species (ROS) by the quinone moiety comThe classic model of anthracycline cardiotoxicity involves the generation 4 3/5/2013 mon to all anthracyclines. An alternative model, supported Draft by a recent study by Zhang et al.,2 posits that toxicity is caused by the disAuthor Sawyer_cibr1214975 abling of the function of topoisomerase II beta (TOP2B) by the anthracyclines. Without functional TOP2B, double-stranded DNA breaks 1 Fig # accrue, leading to events such as the activation of p53 Title tumor-suppressor mitochondrial dysfunction, and the generation of ROS Anthracyclines andprotein, Heart Failure that result in cardiac cell death. PGC1- and PGC1- denote peroxisome-proliferatoractivated receptor coactivator 1 and 1.
DE ME Artist Pub Date Phimister Laurencot Williams 3/21/2013
the activation of DNA-damageresponse pathways by means of the suppression of transcription factors known to be critical for regulation of mitochondrial biogenesis (Fig. 1). These changes were not present in cardiac tissue from the mun engl j med 368;12
Figure has been redrawn and type has been reset Please check carefully
AUTHOR PLEASE NOTE:
tant mice, a finding consistent with the observed protection against cardiotoxicity. Dexrazoxane was originally developed as a TOP2 inhibitor. In cells, dexrazoxane appears to prevent doxorubicin binding to TOP2B and thus
march 21, 2013
nejm.org
1155
The New England Journal of Medicine Downloaded from nejm.org on March 25, 2013. For personal use only. No other uses without permission. Copyright 2013 Massachusetts Medical Society. All rights reserved.
Clinical Implications of Basic Research
prevents DNA breaks and cell death. An alterna- tively binds to TOP2B could prevent its interactive mechanism for the cardioprotective effect of tion with anthracyclines and thus prevent cardiac dexrazoxane may therefore involve prevention cell death. of the binding of anthracyclines to TOP2B, rather Disclosure forms provided by the author are available with the than iron chelation and prevention of damage to full text of this article at NEJM.org. cells from reactive oxygen species. However, the From the Vanderbilt University Medical Center, Nashville. effect of dexrazoxane on TOP2A may reduce the antitumor efficacy of anthracyclines and, 1. Unverferth BJ, Magorien RD, Balcerzak SP, Leier CV, Unverferth DV. Early changes in human myocardial nuclei after doxoironically, limit its usefulness. rubicin. Cancer 1983;52:215-21. Research is needed to determine whether 2. Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the these findings have clinical relevance. In the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med event that they do, the implications of this work 2012;18:1639-42. 3. Lyu YL, Lin C-P, Azarova AM, Cai L, Wang JC, Liu LF. Role of are exciting. The development of inhibitors spe- topoisomerase II in the expression of developmentally regucific to TOP2A may lead to efficacious antitumor lated genes. Mol Cell Biol 2006;26:7929-41. therapies that have no effect on the heart. It is DOI: 10.1056/NEJMcibr1214975 also possible that a small molecule that selec- Copyright 2013 Massachusetts Medical Society.
1156
n engl j med 368;12 nejm.org march 21, 2013
The New England Journal of Medicine Downloaded from nejm.org on March 25, 2013. For personal use only. No other uses without permission. Copyright 2013 Massachusetts Medical Society. All rights reserved.
Anda mungkin juga menyukai
- Prostaglandins, Platelets, Lipids: New Developments in AtherosclerosisDari EverandProstaglandins, Platelets, Lipids: New Developments in AtherosclerosisBelum ada peringkat
- NIH Public Access: Dipyrone Inhibits Neuronal Cell Death and Diminishes Hypoxic/ Ischemic Brain InjuryDokumen26 halamanNIH Public Access: Dipyrone Inhibits Neuronal Cell Death and Diminishes Hypoxic/ Ischemic Brain InjuryYulian 53Belum ada peringkat
- Renu 2017Dokumen72 halamanRenu 2017ramangBelum ada peringkat
- 97 312 PDFDokumen5 halaman97 312 PDFdupuytrenBelum ada peringkat
- Ijerph 07 04111Dokumen16 halamanIjerph 07 04111Sukma ArdillaBelum ada peringkat
- 667 FullDokumen10 halaman667 FullMustafa KadhemBelum ada peringkat
- Higashi 2014Dokumen13 halamanHigashi 2014Lavínia FernandaBelum ada peringkat
- Ni Hms 681370Dokumen18 halamanNi Hms 681370Camilo SotomayorBelum ada peringkat
- Review Article Potential Role of Antioxidants As Adjunctive Therapy in Chagas DiseaseDokumen13 halamanReview Article Potential Role of Antioxidants As Adjunctive Therapy in Chagas DiseaseColo VoltaBelum ada peringkat
- Glutathione, Oxidative Stress and Neurodegeneration: MinireviewDokumen8 halamanGlutathione, Oxidative Stress and Neurodegeneration: Minireviewnurul hanifahBelum ada peringkat
- Gene CVD OxidatDokumen15 halamanGene CVD OxidatvshiniBelum ada peringkat
- Dhami Et Al-2019-Journal of Neurochemistry PDFDokumen18 halamanDhami Et Al-2019-Journal of Neurochemistry PDFJawad A. KhanBelum ada peringkat
- Crocin Attenuates Oxidative Stress and Myocardial Infarction Injury in RatsDokumen7 halamanCrocin Attenuates Oxidative Stress and Myocardial Infarction Injury in RatsAdrian BratuBelum ada peringkat
- Jurnal PDFDokumen9 halamanJurnal PDFFerdy Arif FadhilahBelum ada peringkat
- Pathophysiology, Bioelectricidad, Review Stroke TINS99Dokumen7 halamanPathophysiology, Bioelectricidad, Review Stroke TINS99Carla Saramy MachillandaBelum ada peringkat
- Apoptosis in The Failing Human HeartDokumen11 halamanApoptosis in The Failing Human HeartBetza CxBelum ada peringkat
- Cisplatin As An Anti Cancer DrugDokumen10 halamanCisplatin As An Anti Cancer DrugMahima KamraBelum ada peringkat
- Elucidation of The Role of Notch Pathway in Doxorubicin-Induced CardiotoxicityDokumen13 halamanElucidation of The Role of Notch Pathway in Doxorubicin-Induced Cardiotoxicitymeraj21Belum ada peringkat
- Aspirin and Clopidogrel Treatment Impair Nitric Oxide Biosynthesis by PlateletsDokumen7 halamanAspirin and Clopidogrel Treatment Impair Nitric Oxide Biosynthesis by PlateletsBryam Smith MolinaBelum ada peringkat
- E-Empagliflozin Attenuates Doxorubicin-Induced CardiotoxicityDokumen8 halamanE-Empagliflozin Attenuates Doxorubicin-Induced CardiotoxicityЈован БаљакBelum ada peringkat
- Efectos Del Ejercicio en La Funcion EndotelialDokumen21 halamanEfectos Del Ejercicio en La Funcion EndotelialOscarDavidGordilloGonzalezBelum ada peringkat
- Ischemic StrokeDokumen8 halamanIschemic StrokeRinavi Adrin RirinBelum ada peringkat
- FTPDokumen7 halamanFTPAlema PelesićBelum ada peringkat
- Wang 2021Dokumen18 halamanWang 2021h234074Belum ada peringkat
- Possible Mechanisms of Disease Development in Tuberous SclerosisDokumen7 halamanPossible Mechanisms of Disease Development in Tuberous Sclerosisplastioid4079Belum ada peringkat
- Beta-Block The Septic Heart 2010Dokumen5 halamanBeta-Block The Septic Heart 2010Ivann FloresBelum ada peringkat
- 0792 Group 8 Roca, Alexandra Margarette Rentiquiano, Desiree Ruiz, Chrinssie Vivien Ruiz, Kimberly Santos, CandiceDokumen5 halaman0792 Group 8 Roca, Alexandra Margarette Rentiquiano, Desiree Ruiz, Chrinssie Vivien Ruiz, Kimberly Santos, CandiceKim RuizBelum ada peringkat
- Dosis DoxoDokumen12 halamanDosis DoxodeyaBelum ada peringkat
- Naoxinqing, An Anti-Stroke Herbal Medicine, Reduces Hydrogen Peroxide-Induced Injury in Ng108-15 CellsDokumen4 halamanNaoxinqing, An Anti-Stroke Herbal Medicine, Reduces Hydrogen Peroxide-Induced Injury in Ng108-15 CellsZahra NisaBelum ada peringkat
- Oxidative Stress and Stroke A Review of Upstream - 2019Dokumen12 halamanOxidative Stress and Stroke A Review of Upstream - 2019Patus QuakusBelum ada peringkat
- Disruption of The 5-Lipoxygenase Pathway Attenuates Atherogenesis Consequent To COX-2 Deletion in MiceDokumen6 halamanDisruption of The 5-Lipoxygenase Pathway Attenuates Atherogenesis Consequent To COX-2 Deletion in MicePaul KelnerBelum ada peringkat
- Fendo 12 683151Dokumen13 halamanFendo 12 683151Leilane GlienkeBelum ada peringkat
- ArtigoDokumen12 halamanArtigoGabrielBelum ada peringkat
- Alzheimer's Disease Pathogenesis: Role of AgingDokumen7 halamanAlzheimer's Disease Pathogenesis: Role of AgingHaseeba KhanBelum ada peringkat
- Critical Role of Oxidant and Antioxidant in Cancer 2168 9547.1000e110Dokumen2 halamanCritical Role of Oxidant and Antioxidant in Cancer 2168 9547.1000e110Paulo César López BarrientosBelum ada peringkat
- Alkaloids Induce Programmed Cell Death in Bloodstream Forms of Trypanosomes (Trypanosoma B. Brucei)Dokumen12 halamanAlkaloids Induce Programmed Cell Death in Bloodstream Forms of Trypanosomes (Trypanosoma B. Brucei)Kayo PaivaBelum ada peringkat
- Actualidades en NeuroprotecciónDokumen23 halamanActualidades en Neuroprotección.............. .................. ............Belum ada peringkat
- Pan2018 STRESS OXIDATIVO E NEUROTOXICIDADEDokumen35 halamanPan2018 STRESS OXIDATIVO E NEUROTOXICIDADEFran CiBelum ada peringkat
- Gamma Radiation Induces p53 Mediated Cell Cycle Arrest in Bone Marrow CellsDokumen20 halamanGamma Radiation Induces p53 Mediated Cell Cycle Arrest in Bone Marrow CellsVanina TucciBelum ada peringkat
- Molecules 23 00814 v2Dokumen26 halamanMolecules 23 00814 v2M.S GRAPHICSBelum ada peringkat
- Pharmacological Inhibition of HDAC6 Attenuates Endothelial Barrier Dysfunction Induced by ThrombinDokumen9 halamanPharmacological Inhibition of HDAC6 Attenuates Endothelial Barrier Dysfunction Induced by ThrombinA2ZBelum ada peringkat
- 9 - Co-Author - Sci Publication PDFDokumen15 halaman9 - Co-Author - Sci Publication PDFAnanth BabuBelum ada peringkat
- Thioredoxin-2 Inhibits Mitochondrial Reactive Oxygen Species Generation and Apoptosis Stress Kinase-1 Activity To Maintain Cardiac FunctionDokumen8 halamanThioredoxin-2 Inhibits Mitochondrial Reactive Oxygen Species Generation and Apoptosis Stress Kinase-1 Activity To Maintain Cardiac FunctionSo Ra AhnBelum ada peringkat
- Zarco Ordóñez KarlaDokumen2 halamanZarco Ordóñez KarlaKarla ZarcoBelum ada peringkat
- Abstracts Free Radical Biology and Medicine 165 (2021) 26 - 59Dokumen1 halamanAbstracts Free Radical Biology and Medicine 165 (2021) 26 - 59Irina ASBelum ada peringkat
- Molecular Pathophysiology of StrokeDokumen10 halamanMolecular Pathophysiology of StrokeKahubbi Fatimah Wa'aliyBelum ada peringkat
- Yokochi 2004Dokumen6 halamanYokochi 2004hasanbatuhan küçükBelum ada peringkat
- Circulation 2004 Zhu 2109 15Dokumen8 halamanCirculation 2004 Zhu 2109 15gekayuuBelum ada peringkat
- Antioxidant in ACDDokumen8 halamanAntioxidant in ACDMuhammad Al GifariBelum ada peringkat
- Ifosfamide PathwayDokumen6 halamanIfosfamide PathwayDiana AhmadBelum ada peringkat
- Clinical Implications of The Genetic Architecture of Dilated CardiomyopathyDokumen11 halamanClinical Implications of The Genetic Architecture of Dilated CardiomyopathyIsabella-Diana ChelbanBelum ada peringkat
- Contribution of TRPC Channels in Neuronal Excitotoxicity Associated With Neurodegenerative Disease and Ischemic StrokeDokumen17 halamanContribution of TRPC Channels in Neuronal Excitotoxicity Associated With Neurodegenerative Disease and Ischemic Strokediego.battiatoBelum ada peringkat
- CNS 8 353Dokumen8 halamanCNS 8 353Adinda MelaniBelum ada peringkat
- Toxicity DocDokumen2 halamanToxicity DocRegulatory AffairsBelum ada peringkat
- DNA ROS p53 2021Dokumen14 halamanDNA ROS p53 2021Maria Elena MaldonadoBelum ada peringkat
- Microglia Clean Up Toxic Lipids in Multiple Sclerosis: News & ViewsDokumen2 halamanMicroglia Clean Up Toxic Lipids in Multiple Sclerosis: News & ViewsericBelum ada peringkat
- TFAMDokumen12 halamanTFAMKurtisBelum ada peringkat
- Apoptosis and Therapy: Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, U.S.ADokumen11 halamanApoptosis and Therapy: Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, U.S.AAntonio RolonBelum ada peringkat
- Non-Coding RNA Mediates Endoplasmic Reticulum Stress-Induced Apoptosis in Heart DiseaseDokumen11 halamanNon-Coding RNA Mediates Endoplasmic Reticulum Stress-Induced Apoptosis in Heart DiseaseWaldo IzyBelum ada peringkat
- Sex Linked WorksheetDokumen4 halamanSex Linked WorksheetKathelyn Ruiz-Sumando100% (1)
- WSC 22-23 Conf 9 Illustrated Results - FinalDokumen21 halamanWSC 22-23 Conf 9 Illustrated Results - FinalGustavo MurgaBelum ada peringkat
- Chain of InfectionDokumen2 halamanChain of Infectionapi-251976647Belum ada peringkat
- All English Editorial 14.08.2022Dokumen25 halamanAll English Editorial 14.08.2022anila rathodaBelum ada peringkat
- Profiles of Drug Substances Vol 05Dokumen556 halamanProfiles of Drug Substances Vol 05Binhnguyen Nguyen100% (3)
- Women and ArthritisDokumen15 halamanWomen and Arthritisratnav_ratanBelum ada peringkat
- Acupuncture Against Depression Acupuncture Against Depression Acupuncture Against Depression Acupuncture Against DepressionDokumen20 halamanAcupuncture Against Depression Acupuncture Against Depression Acupuncture Against Depression Acupuncture Against DepressionFrancois du RizBelum ada peringkat
- Hunger, Eating, and HealthDokumen6 halamanHunger, Eating, and HealthMary Ann EspinosaBelum ada peringkat
- Kelainan Morf EritrositDokumen12 halamanKelainan Morf EritrositTrio Singgih SaputroBelum ada peringkat
- Evaluación Física y Clínica de Sementales Bovinos en Dos Municipios de La Costa Chica de Guerrero, MéxicoDokumen7 halamanEvaluación Física y Clínica de Sementales Bovinos en Dos Municipios de La Costa Chica de Guerrero, MéxicoChacharitas IxtapalucachalcoBelum ada peringkat
- Solution Manual For Laboratory Manual For Anatomy Physiology Featuring Martini Art Main Version Plus Masteringap With Etext Package 5 e Michael G WoodDokumen4 halamanSolution Manual For Laboratory Manual For Anatomy Physiology Featuring Martini Art Main Version Plus Masteringap With Etext Package 5 e Michael G WoodKarenAcevedotkoi100% (40)
- AlbuminDokumen27 halamanAlbuminSanjay VeerasammyBelum ada peringkat
- SketchyPath ChecklistDokumen1 halamanSketchyPath ChecklistFajar Raza100% (1)
- Revelance of Shat Kriyakala in The Diagnosis and Management of Disease - .Docx by Shubham RaoDokumen6 halamanRevelance of Shat Kriyakala in The Diagnosis and Management of Disease - .Docx by Shubham RaoSHUBHAM RAOBelum ada peringkat
- Vector DiseaseDokumen2 halamanVector DiseasenallurihpBelum ada peringkat
- HemophiliaDokumen19 halamanHemophiliaHatem EletrebyBelum ada peringkat
- Virulence and PathogenesisDokumen52 halamanVirulence and PathogenesisAlmoatazbellah AbdallahBelum ada peringkat
- Biology 9648/01: Temasek Junior College Preliminary Examinations 2015 Higher 2Dokumen18 halamanBiology 9648/01: Temasek Junior College Preliminary Examinations 2015 Higher 2Sun WeilingBelum ada peringkat
- CCO Metastatic NSCLC SlidesDokumen67 halamanCCO Metastatic NSCLC Slidesfedervacho1Belum ada peringkat
- Analysis of Microarray Gene Expression Data - M. Lee (KluwerDokumen398 halamanAnalysis of Microarray Gene Expression Data - M. Lee (KluwerciuckyBelum ada peringkat
- Adolescence: Reporter: Jo Ann M. VelascoDokumen26 halamanAdolescence: Reporter: Jo Ann M. Velascojungkook'sBelum ada peringkat
- Biochem Booklet Web NEWDokumen11 halamanBiochem Booklet Web NEWshanea bucknorBelum ada peringkat
- Pediatric Dentistry Dental Caries: Dr. Mukhaled Louay Alfalluji B.D.S. M.SCDokumen64 halamanPediatric Dentistry Dental Caries: Dr. Mukhaled Louay Alfalluji B.D.S. M.SCDr-Kinan Khalil ShraifyBelum ada peringkat
- Cia 2 347Dokumen15 halamanCia 2 347AnisBelum ada peringkat
- Endoderm AtfDokumen3 halamanEndoderm AtfMiss Tan100% (1)
- DNA ProfilingDokumen4 halamanDNA ProfilingVictorija DeldioBelum ada peringkat
- Care of Terminally Ill PatientDokumen30 halamanCare of Terminally Ill PatientSREEVANI THORVANTH67% (6)
- Chapter 6 VariationDokumen12 halamanChapter 6 VariationBro TimeBelum ada peringkat
- Forest Nursery Pests-WebDokumen212 halamanForest Nursery Pests-WebSrivani GayathriBelum ada peringkat
- Blood TransfusionDokumen5 halamanBlood TransfusionMoustafa Hazzaa100% (1)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsDari EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsBelum ada peringkat
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDDari EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDPenilaian: 5 dari 5 bintang5/5 (3)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionDari EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionPenilaian: 4 dari 5 bintang4/5 (404)
- The Age of Magical Overthinking: Notes on Modern IrrationalityDari EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityPenilaian: 4 dari 5 bintang4/5 (32)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDari EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BePenilaian: 2 dari 5 bintang2/5 (1)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaDari EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Dari EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Penilaian: 3 dari 5 bintang3/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedDari EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedPenilaian: 4.5 dari 5 bintang4.5/5 (82)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsDari EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsPenilaian: 4 dari 5 bintang4/5 (4)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisDari EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisPenilaian: 4.5 dari 5 bintang4.5/5 (42)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsDari EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsPenilaian: 5 dari 5 bintang5/5 (1)
- The Obesity Code: Unlocking the Secrets of Weight LossDari EverandThe Obesity Code: Unlocking the Secrets of Weight LossPenilaian: 4 dari 5 bintang4/5 (6)
- Manipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesDari EverandManipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesPenilaian: 4.5 dari 5 bintang4.5/5 (1412)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeDari EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifePenilaian: 4.5 dari 5 bintang4.5/5 (254)
- The Comfort of Crows: A Backyard YearDari EverandThe Comfort of Crows: A Backyard YearPenilaian: 4.5 dari 5 bintang4.5/5 (23)
- The Marshmallow Test: Mastering Self-ControlDari EverandThe Marshmallow Test: Mastering Self-ControlPenilaian: 4.5 dari 5 bintang4.5/5 (60)
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisDari EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisPenilaian: 5 dari 5 bintang5/5 (8)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Dari EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Penilaian: 4.5 dari 5 bintang4.5/5 (110)
- Why We Die: The New Science of Aging and the Quest for ImmortalityDari EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityPenilaian: 4 dari 5 bintang4/5 (5)
- To Explain the World: The Discovery of Modern ScienceDari EverandTo Explain the World: The Discovery of Modern SciencePenilaian: 3.5 dari 5 bintang3.5/5 (51)
- Summary: Thinking, Fast and Slow: by Daniel Kahneman: Key Takeaways, Summary & Analysis IncludedDari EverandSummary: Thinking, Fast and Slow: by Daniel Kahneman: Key Takeaways, Summary & Analysis IncludedPenilaian: 4 dari 5 bintang4/5 (61)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisDari EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisPenilaian: 3.5 dari 5 bintang3.5/5 (2)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryDari EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryPenilaian: 4 dari 5 bintang4/5 (46)