Chapter Outline: Phase Transformations. Kinetics
Diunggah oleh
mpcd07Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Chapter Outline: Phase Transformations. Kinetics
Diunggah oleh
mpcd07Hak Cipta:
Format Tersedia
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Phase transformations. Kinetics. Chapter Outline: Phase Transformations in Metals
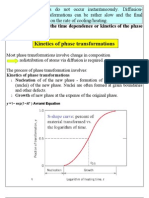
Phase transformations (change of the microstructure) can Heat Treatment (time and temperature) be divided into three categories: Microstructure Mechanical Properties ! Diffusion-dependent with no change in phase composition or number of phases present (e.g. melting, solidification of pure metal, allotropic ! transformations, Kinetics of phase transformations etc.) recrystallization, ! Multiphase Transformations ! Diffusion-dependent with changes in phase ! compositions Phase transformations in Fe-C and/or number ofalloys phases (e.g. eutectoid transformations) ! Isothermal Transformation Diagrams ! phase transformation - produces a ! Diffusionless Mechanical Behavior metastable phase by cooperative small displacements of atoms in structure (e.g. martensitic transformation ! all Tempered Martensite discussed in later in this chapter) Phase transformations do not occur instantaneously. Not tested: Diffusion-dependent phase transformations can be rather 10.6 Continuous Cooling Transformation Diagrams slow and the final structure often depend on the rate of cooling/heating.
We need to consider the time dependence or kinetics of the phase transformations.
University of Virginia, Dept. of Materials Science and Engineering 1 2
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Kinetics of phase transformations
Most phase transformations involve change in composition redistribution of atoms via diffusion is required. The process of phase transformation involves: ! Nucleation of of the new phase - formation of stable small particles (nuclei) of the new phase. Nuclei are often formed at grain boundaries and other defects. ! Growth of new phase at the expense of the original
phase.
S-shape curve: percent of material transformed vs. the logarithm of time.
University of Virginia, Dept. of Materials Science and Engineering
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Nucleation
A nucleus is only stable if further growth reduces the energy of the system. For r > rc the nucleus is stable.
University of Virginia, Dept. of Materials Science and Engineering
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Rate of phase transformations
Rate of transformation can be defined as reciprocal of time for transformation to proceed halfway to completion: r = 1 / t0.5 Rate increases with temperature according to Arrhenius equation, characteristic for thermally activated processes:
r = A exp (-QA/kT) = A exp (-Qm/ RT)
Per atom Per mole
Percent recrystallization of pure copper at different T
University of Virginia, Dept. of Materials Science and Engineering 5
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Superheating / supercooling
! Upon crossing a phase boundary on the compositiontemperature phase diagram phase transformation towards equilibrium state is induced. ! But the transition to the equilibrium structure takes time and transformation is delayed. ! During cooling, transformations occur at temperatures less than predicted by phase diagram: supercooling. ! During heating, transformations occur at temperatures greater than predicted by phase diagram: superheating. ! Degree of supercooling/superheating increases with rate of cooling/heating. ! Metastable states can be formed as a result of fast temperature change. Microstructure is strongly affected by the rate of cooling. ! Below we will consider the effect of time on phase transformations using iron-carbon alloy as an example.
University of Virginia, Dept. of Materials Science and Engineering
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
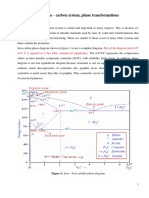
Let us consider eutectoid reaction as an example eutectoid reaction: (0.76 wt% C) (0.022 wt% C) + Fe3C
The S-shaped curves are shifted to longer times at higher T showing that the transformation is dominated by nucleation (nucleation rate increases with supercooling) and not by University of occurs faster at higher T).diffusion (whichVirginia, Dept. of
Materials Science and Engineering7
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Isothermal Transformation (or TTT) Diagrams (Temperature, Time, and % Transformation)
University of Virginia, Dept. of Materials Science and Engineering
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
TTT Diagrams
Austenite (stable)
Eutectoid temperature
ferrite
Coarse pearlite
Fe3C Fine pearlite
Austenite pearlite transformation
Denotes that a transformation is occurring
The thickness of the ferrite and cementite layers in pearlite is ~ 8:1. The absolute layer thickness depends on the temperature of the transformation.The higher the temperature, the thicker the layers.
University of Virginia, Dept. of Materials Science and Engineering
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
TTT Diagrams
! The family of S-shaped curves at different T are used to construct the TTT diagrams. ! The TTT diagrams are for the isothermal (constant T) transformations (material is cooled quickly to a given temperature before the transformation occurs, and then keep it at that temperature). ! At low temperatures, the transformation occurs sooner (it is controlled by the rate of nucleation) and grain growth (that is controlled by diffusion) is reduced. ! Slow diffusion at low temperatures leads to fine-grained microstructure with thin-layered structure of pearlite (fine pearlite). ! At higher temperatures, high diffusion rates allow for larger grain growth and formation of thick layered structure of pearlite (coarse pearlite). ! At compositions other than eutectoid, a proeutectoid phase (ferrite or cementite) coexist with pearlite. Additional curves for proeutectoid transformation must be included on TTT diagrams.
University of Virginia, Dept. of Materials Science and Engineering
10
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Formation of Bainite Microstructure (I)
If transformation temperature is low enough (540C) bainite rather than fine pearlite forms.
University of Virginia, Dept. of Materials Science and Engineering 11
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Formation of Bainite Microstructure (II)
! For T ~ 300-540C, upper bainite consists of needles of ferrite separated by long cementite particles ! For T ~ 200-300C, lower bainite consists of thin plates of ferrite containing very fine rods or blades of cementite ! In the bainite region, transformation rate is controlled by microstructure growth (diffusion) rather than nucleation. Since diffusion is slow at low temperatures, this phase has a very fine (microscopic) microstructure. ! Pearlite and bainite transformations are competitive; transformation between pearlite and bainite not possible without first reheating to form austenite
Upper bainite
Lower bainite
University of Virginia, Dept. of Materials Science and Engineering
12
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Spheroidite
Annealing of pearlitic or bainitic microstructures at elevated temperatures just below eutectoid (e.g. 24 h at 700 C) leads to the formation of new microstructure spheroidite - spheres of cementite in a ferrite matrix. Composition or relative amounts of ferrite and cementite are not changing in this transformation, only shape of the cementite inclusions is changing. Transformation proceeds by C diffusion needs high T. Driving force for the trancsformation - reduction in total ferrite - cementite boundary area
University of Virginia, Dept. of Materials Science and Engineering
13
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Martensite (I)
Martensite forms when austenite is rapidly cooled (quenched) to room T. It forms nearly instantaneously when the required low temperature is reached. The austenite-martensite does not involve diffusion no thermal activation is needed, this is called an athermal transformation. Each atom displaces a small (sub-atomic) distance to transform FCC -Fe (austenite) to martensite which has a Body Centered Tetragonal (BCT) unit cell (like BCC, but one unit cell axis is longer than the other two). Martensite is metastable - can persist indefinitely at room temperature, but will transform to equilibrium phases on annealing at an elevated temperature. Martensite can coexist with other phases and/or microstructures in Fe-C system Since martensite is metastable non-equilibrium phase, it does not appear in phase Fe-C phase diagram
University of Virginia, Dept. of Materials Science and Engineering
14
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
TTT Diagram including Martensite
A: Austenite B: Bainite
P: Pearlite M: Martensite
Austenite-to-martensite is diffusionless and very fast. The amountUniversity of Virginia,formed depends onand Engineering only.of martensite Dept. of Materials Science temperature15
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Time-temperature path microstructure
University of Virginia, Dept. of Materials Science and Engineering
16
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Mechanical Behavior of Fe-C Alloys (I)
Cementite is harder and more brittle than ferrite increasing cementite fraction therefore makes harder, less ductile material.
University of Virginia, Dept. of Materials Science and Engineering
17
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Mechanical Behavior of Fe-C Alloys (II)
The strength and hardness of the different microstructures is inversely related to the size of the microstructures (fine structures have more phase boundaries inhibiting
dislocation motion).
Mechanical properties of bainite, pearlite, spheroidite Considering microstructure we can predict that ! Spheroidite is the softest ! Fine pearlite is harder and stronger than coarse pearlite ! Bainite is harder and stronger than pearlite
Mechanical properties of martensite Of the various microstructures in steel alloys ! Martensite is the hardest, strongest and the most brittle The strength of martensite is not related to microstructure. Rather, it is related to the interstitial C atoms hindering dislocation motion (solid solution hardening, Chapter 7)
and to the small number of slip systems.
University of Virginia, Dept. of Materials Science and Engineering 18
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Mechanical Behavior of Fe-C Alloys (III)
University of Virginia, Dept. of Materials Science and Engineering
19
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Tempered Martensite (I) Martensite is so brittle that it needs to be modified for practical applications. This is done by heating it to 250-650 oC for some time (tempering) which produces tempered martensite, an extremely fine-grained and well dispersed cementite grains in a ferrite matrix. ! Tempered martensite is less hard/strong as compared to regular martensite but has enhanced ductility (ferrite phase is ductile). ! Mechanical properties depend upon cementite particle size: fewer, larger particles means less boundary area and softer, more ductile material eventual limit is spheroidite. ! Particle size increases with higher tempering temperature and/or longer time (more C diffusion) - therefore softer, more ductile material.
University of Virginia, Dept. of Materials Science and Engineering
20
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Tempered Martensite (II)
Higher temperature & time: spheroidite (soft)
Electron micrograph of tempered martensite
University of Virginia, Dept. of Materials Science and Engineering 21
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Summary of austenite transformations
Austenite
Slow cooling Moderate cooling
Rapid quench
Pearlite ( + Fe3C) + a proeutectoid phase
Bainite ( + Fe3C)
Martensite (BCT phase) Reheat
Tempered martensite ( + Fe3C)
Solid lines are diffusional transformations, dashed is diffusionless martensitic transformation
University of Virginia, Dept. of Materials Science and Engineering 22
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Summary
Make sure you understand language and concepts: ! Alloy steel ! Athermal transformation ! Bainite ! Coarse pearlite ! Fine pearlite ! Isothermal transformation diagram ! Kinetics ! Martensite ! Nucleation ! Phase transformation ! Plain carbon steel ! Spheroidite ! Supercooling ! Superheating ! Tempered martensite ! Thermally activated transformation ! Transformation rate
University of Virginia, Dept. of Materials Science and Engineering
23
Introduction to Materials Science, Chapter 10, Phase Transformations in Metals
Reading for next class:
Chapter 11: Thermal Processing of Metal Alloys ! Process Annealing, Stress Relief ! Heat Treatment of Steels ! Precipitation Hardening
University of Virginia, Dept. of Materials Science and Engineering
24
Anda mungkin juga menyukai
- Materials Data for Cyclic Loading: Aluminium and Titanium AlloysDari EverandMaterials Data for Cyclic Loading: Aluminium and Titanium AlloysPenilaian: 1 dari 5 bintang1/5 (1)
- TTT and CCT Continuous Cooling TransformationDokumen6 halamanTTT and CCT Continuous Cooling TransformationurcojBelum ada peringkat
- Get Free Access To This Document by Contributing To Scribd: Kenneth E. Hagin The Art of PrayerDokumen1 halamanGet Free Access To This Document by Contributing To Scribd: Kenneth E. Hagin The Art of Prayermpcd07Belum ada peringkat
- ABAP Training PlanDokumen4 halamanABAP Training PlanAhmed HamadBelum ada peringkat
- A Practical Total Synthesis of CocaineDokumen13 halamanA Practical Total Synthesis of CocaineRodrigo Fernanda100% (4)
- Chapter Outline:: Heat Treatment (And Temperature)Dokumen24 halamanChapter Outline:: Heat Treatment (And Temperature)DSGBelum ada peringkat
- TTT 11Dokumen55 halamanTTT 11MrDOTBelum ada peringkat
- Fe CDokumen34 halamanFe CZaza ArifinBelum ada peringkat
- Iron-Carbon Phase Diagram (A Review) See Callister Chapter 9Dokumen34 halamanIron-Carbon Phase Diagram (A Review) See Callister Chapter 9Zefa Erliana YullahBelum ada peringkat
- Fe-C Phase DiagramDokumen34 halamanFe-C Phase DiagramYoung-long Choi100% (1)
- TTT N AvramiDokumen40 halamanTTT N AvramiAmirul AminBelum ada peringkat
- Phase Transformation in Metals and AlloysDokumen31 halamanPhase Transformation in Metals and AlloysSyafiqah RusdiBelum ada peringkat
- EMAT 10 (2k21)Dokumen41 halamanEMAT 10 (2k21)Kumail AbbasBelum ada peringkat
- Kinetics and Heat Treatments in Metals: The IT TTT Diagram For Eutectoid SteelDokumen8 halamanKinetics and Heat Treatments in Metals: The IT TTT Diagram For Eutectoid Steelkatchani123Belum ada peringkat
- 03 - Iron - Iron CarbideDokumen35 halaman03 - Iron - Iron CarbidebotobotoakbarBelum ada peringkat
- Phase Transformation in Metals: Dr. Aneela WakeelDokumen29 halamanPhase Transformation in Metals: Dr. Aneela WakeelmazharBelum ada peringkat
- University of Kirkuk. Department of Mechanical Enginnering.: Name: Ahmed QaisDokumen2 halamanUniversity of Kirkuk. Department of Mechanical Enginnering.: Name: Ahmed QaisMostafa HamawandyBelum ada peringkat
- TTT CurvesDokumen4 halamanTTT Curvesmanas310jntuhBelum ada peringkat
- 02.Iron-Phase DiagramDokumen21 halaman02.Iron-Phase Diagrampopular102001Belum ada peringkat
- 8.heat TreatmentDokumen7 halaman8.heat Treatmentrohan_n_desai100% (2)
- Publication 4 11889 199Dokumen9 halamanPublication 4 11889 199Mulia AridhoBelum ada peringkat
- TTT Phase DiagramDokumen9 halamanTTT Phase Diagramhari krishnaBelum ada peringkat
- Computational Materials Science: R. Mahnken, A. Schneidt, S. Tschumak, H.J. MaierDokumen7 halamanComputational Materials Science: R. Mahnken, A. Schneidt, S. Tschumak, H.J. MaierJames HaidoBelum ada peringkat
- Time Temperature Transformation (TTT) Diagrams PDFDokumen108 halamanTime Temperature Transformation (TTT) Diagrams PDFSerkan Apay100% (1)
- Fe CdiagramDokumen36 halamanFe CdiagramGeorge SingerBelum ada peringkat
- Heat Treatment 1Dokumen42 halamanHeat Treatment 1Akash TiwariBelum ada peringkat
- Time-Temperature-Transformation (TTT) DiagramDokumen18 halamanTime-Temperature-Transformation (TTT) DiagramGikiTopiBelum ada peringkat
- 08 Transformasi Fasa - UASDokumen35 halaman08 Transformasi Fasa - UASDinta PratiwiBelum ada peringkat
- Material and Heat Treatment 3Dokumen5 halamanMaterial and Heat Treatment 3lastking_king17Belum ada peringkat
- TTT DiagramDokumen6 halamanTTT DiagramDeepa PujariBelum ada peringkat
- TTT DiagramDokumen17 halamanTTT DiagramGAURAV SINGHBelum ada peringkat
- 3 Fe-Fe3C Phase DiagramDokumen33 halaman3 Fe-Fe3C Phase DiagramRajat Mishra100% (1)
- TTT DiagramDokumen31 halamanTTT DiagramEducated SmugglerBelum ada peringkat
- FALLSEM2019-20 MEE1005 ETH VL2019201001078 Reference Material I 29-Aug-2019 Fe-Fe3C Phase DiagramDokumen33 halamanFALLSEM2019-20 MEE1005 ETH VL2019201001078 Reference Material I 29-Aug-2019 Fe-Fe3C Phase DiagramFazal KhanBelum ada peringkat
- Phase Transformation in Metals1Dokumen3 halamanPhase Transformation in Metals1sridharan.engineer01Belum ada peringkat
- Review MetalDokumen38 halamanReview MetalnisannisaBelum ada peringkat
- TTTDokumen7 halamanTTTMuhammad Hery Al-GhiffaryBelum ada peringkat
- TTT and CCT DiagramDokumen24 halamanTTT and CCT DiagramArun Raj A C100% (2)
- Microstructure Properties: II Martensitic TransformationsDokumen38 halamanMicrostructure Properties: II Martensitic TransformationsRecep VatanseverBelum ada peringkat
- Platinum Alloys For Shape Memory ApplicationsDokumen15 halamanPlatinum Alloys For Shape Memory ApplicationsKursalBelum ada peringkat
- Kinetics - Heat TreatmentDokumen18 halamanKinetics - Heat TreatmentUnknownBelum ada peringkat
- Heat Treatment of 1045 Steel PDFDokumen17 halamanHeat Treatment of 1045 Steel PDFH_DEBIANEBelum ada peringkat
- ME 216 - Engineering Materials II: Heat Treatment (Part I)Dokumen15 halamanME 216 - Engineering Materials II: Heat Treatment (Part I)ozanBelum ada peringkat
- Time Temperature Transformation With ReferncesDokumen12 halamanTime Temperature Transformation With ReferncesEllie BrooklynBelum ada peringkat
- Phase Transformations: Ferrite - BCCDokumen65 halamanPhase Transformations: Ferrite - BCCAhsan JunaidBelum ada peringkat
- Iron Carbon Phase DiagramDokumen4 halamanIron Carbon Phase DiagramMizanur RahmanBelum ada peringkat
- The Role of Manganese and Copper in The Eutectoid Transformation of Spheroidal Graphite Cast IronDokumen11 halamanThe Role of Manganese and Copper in The Eutectoid Transformation of Spheroidal Graphite Cast IronChanthar SoeBelum ada peringkat
- Deformation-Induced Transformation of Retained Austenite in Transformation Induced Plasticity-Aided Steels: A Thermodynamic ModelDokumen10 halamanDeformation-Induced Transformation of Retained Austenite in Transformation Induced Plasticity-Aided Steels: A Thermodynamic ModelAhmed El-SaiedBelum ada peringkat
- Microstructure-Properties: II Martensitic TransformationsDokumen38 halamanMicrostructure-Properties: II Martensitic TransformationsnotsofarBelum ada peringkat
- Materials Science & Engineering A: SciencedirectDokumen11 halamanMaterials Science & Engineering A: SciencedirectDaniel TibataBelum ada peringkat
- Iron Carbon DiagramDokumen43 halamanIron Carbon Diagramhrpatel_165Belum ada peringkat
- Ch-8 Compatibility ModeDokumen58 halamanCh-8 Compatibility Modedreamgurl9011Belum ada peringkat
- Sample Questions For Phase DiagramDokumen5 halamanSample Questions For Phase DiagramMohaiminul Islam TalhaBelum ada peringkat
- DivaCalista 1806187902 CCT&TTTdiagramsDokumen7 halamanDivaCalista 1806187902 CCT&TTTdiagramsAlishia LoshBelum ada peringkat
- Trans FasesDokumen6 halamanTrans FasesIveth Carmona GonzalezBelum ada peringkat
- Trans FasesDokumen6 halamanTrans FasesIveth Carmona GonzalezBelum ada peringkat
- Time-Temperature-Transformation Diagram Within The Bainitic Temperature Range in A Medium Carbon SteelDokumen10 halamanTime-Temperature-Transformation Diagram Within The Bainitic Temperature Range in A Medium Carbon SteelAnonymous Fty6OOHlBelum ada peringkat
- Dataset We UsedDokumen11 halamanDataset We UsedDev Raj SoniBelum ada peringkat
- An Overview On Bainite Formation in SteelsDokumen10 halamanAn Overview On Bainite Formation in SteelssajadBelum ada peringkat
- FeC and TTT DiagramsDokumen12 halamanFeC and TTT DiagramsMohamed El-WakilBelum ada peringkat
- Thermomechanical Processing of High-Strength Low-Alloy SteelsDari EverandThermomechanical Processing of High-Strength Low-Alloy SteelsBelum ada peringkat
- Sundram FastenersDokumen4 halamanSundram Fastenersmpcd07Belum ada peringkat
- Technical Comparision - BoilerDokumen2 halamanTechnical Comparision - Boilermpcd07Belum ada peringkat
- Boa PromisesbibleDokumen26 halamanBoa Promisesbiblempcd07Belum ada peringkat
- Objective Chemistry For ..Dokumen3 halamanObjective Chemistry For ..mpcd070% (1)
- Upload A Document For Free Download Access.: Select Files From Your Computer or Choose Other Ways To Upload BelowDokumen5 halamanUpload A Document For Free Download Access.: Select Files From Your Computer or Choose Other Ways To Upload Belowmpcd07Belum ada peringkat
- Get Free Access To This Document by Contributing To Scribd: NTN Handbook of Roller BearingDokumen2 halamanGet Free Access To This Document by Contributing To Scribd: NTN Handbook of Roller Bearingmpcd07Belum ada peringkat
- ST Lawrence Ultra-MetDokumen6 halamanST Lawrence Ultra-Metmpcd07Belum ada peringkat
- Get Free Access To This Document by Contributing To Scribd: MuthuswamyDokumen2 halamanGet Free Access To This Document by Contributing To Scribd: Muthuswamympcd07Belum ada peringkat
- Get Free Access To This Document by Contributing To Scribd: Six Sigma, Kaizen & 5SDokumen2 halamanGet Free Access To This Document by Contributing To Scribd: Six Sigma, Kaizen & 5Smpcd07Belum ada peringkat
- Objective Chemistry For ..Dokumen3 halamanObjective Chemistry For ..mpcd070% (1)
- 1 150 - Questions Paper I 2012 TetDokumen5 halaman1 150 - Questions Paper I 2012 Tetmpcd07Belum ada peringkat
- Get Free Access To This Document by Contributing To Scribd: QSP-211-02 3C 5S Improvment SheetDokumen2 halamanGet Free Access To This Document by Contributing To Scribd: QSP-211-02 3C 5S Improvment Sheetmpcd07Belum ada peringkat
- Get Free Access To This Document by Contributing To Scribd: Bible StoriesDokumen2 halamanGet Free Access To This Document by Contributing To Scribd: Bible Storiesmpcd07Belum ada peringkat
- This Document by Contributing To Scribd: 5S ModulDokumen2 halamanThis Document by Contributing To Scribd: 5S Modulmpcd07Belum ada peringkat
- This Document by Contributing To Scribd: Upload NowDokumen2 halamanThis Document by Contributing To Scribd: Upload Nowmpcd07Belum ada peringkat
- This Document by Contributing To Scribd: Spoken English To TamilDokumen1 halamanThis Document by Contributing To Scribd: Spoken English To Tamilmpcd07Belum ada peringkat
- Get Free Access To This Document by Contributing To Scribd: (2) Weld Deposit CalculationDokumen1 halamanGet Free Access To This Document by Contributing To Scribd: (2) Weld Deposit Calculationmpcd07Belum ada peringkat
- 1 PDFDokumen1 halaman1 PDFmpcd07Belum ada peringkat
- 1 PDFDokumen1 halaman1 PDFmpcd07Belum ada peringkat
- This Document by Contributing To Scribd: Bible StoriesDokumen2 halamanThis Document by Contributing To Scribd: Bible Storiesmpcd07Belum ada peringkat
- Chief Educational Officer: RecentDokumen2 halamanChief Educational Officer: Recentmpcd07Belum ada peringkat
- Related Keywords: 1,689,989 People Like This. To See What Your Friends LikeDokumen11 halamanRelated Keywords: 1,689,989 People Like This. To See What Your Friends Likempcd07Belum ada peringkat
- 1 PDFDokumen6 halaman1 PDFmpcd07Belum ada peringkat
- C.KESAVAN - Diploma EEE: Phone No Mail IdDokumen3 halamanC.KESAVAN - Diploma EEE: Phone No Mail IdKesavan ChinaswmiBelum ada peringkat
- Coding Assignment 18-WPS OfficeDokumen9 halamanCoding Assignment 18-WPS Officetamj tamjBelum ada peringkat
- HV Filter Carts 1Dokumen2 halamanHV Filter Carts 1paulpopBelum ada peringkat
- A B C D: Choose Only One Answer For Each QuestionDokumen10 halamanA B C D: Choose Only One Answer For Each QuestionAchitt AchitBelum ada peringkat
- Lec1 PDFDokumen12 halamanLec1 PDFtogarsBelum ada peringkat
- CISCO Router Software - Configuration PDFDokumen408 halamanCISCO Router Software - Configuration PDFasalihovicBelum ada peringkat
- UMTS Optimization GuidelineDokumen84 halamanUMTS Optimization GuidelineEvelio Sotolongo100% (3)
- Assigment Comouter Science BSCDokumen3 halamanAssigment Comouter Science BSCutkarsh9978100% (1)
- Final Thesis Owura Kofi AmoabengDokumen84 halamanFinal Thesis Owura Kofi AmoabengKunal AgarwalBelum ada peringkat
- Differential Pr. Gauges Bellow Type 1Dokumen2 halamanDifferential Pr. Gauges Bellow Type 1Vara PrasadBelum ada peringkat
- Solution of Linear System Theory and Design 3ed For Chi Tsong ChenDokumen106 halamanSolution of Linear System Theory and Design 3ed For Chi Tsong ChensepehrBelum ada peringkat
- Chapter - Four Soil Permeability and SeepageDokumen19 halamanChapter - Four Soil Permeability and SeepageBefkadu KurtaileBelum ada peringkat
- 1 s2.0 0304386X9190055Q MainDokumen32 halaman1 s2.0 0304386X9190055Q MainJordan Ulloa Bello100% (1)
- Physical Quantities and Unit: 9th GradeDokumen28 halamanPhysical Quantities and Unit: 9th GradeAlexanderBelum ada peringkat
- W.R. Klemm (Auth.) - Atoms of Mind - The - Ghost in The Machine - Materializes-Springer Netherlands (2011)Dokumen371 halamanW.R. Klemm (Auth.) - Atoms of Mind - The - Ghost in The Machine - Materializes-Springer Netherlands (2011)El equipo de Genesis ProjectBelum ada peringkat
- Avh-X8550bt Operating Manual Eng-Esp-PorDokumen7 halamanAvh-X8550bt Operating Manual Eng-Esp-PorRannie IsonBelum ada peringkat
- UNIT 14 - On-Screen DigitizingDokumen6 halamanUNIT 14 - On-Screen DigitizingResti KharismaBelum ada peringkat
- Assignment 1Dokumen2 halamanAssignment 1Alif Bukhari Imran NaimBelum ada peringkat
- Practical - Magnetic - Design (Fill Factor) PDFDokumen20 halamanPractical - Magnetic - Design (Fill Factor) PDFAhtasham ChaudhryBelum ada peringkat
- 5-Canal Irrigation SystemDokumen23 halaman5-Canal Irrigation Systemwajid malikBelum ada peringkat
- Applications of Modern RF PhotonicsDokumen213 halamanApplications of Modern RF PhotonicsrmcmillanBelum ada peringkat
- Presentation5 EV ArchitectureDokumen26 halamanPresentation5 EV ArchitectureJAYKUMAR MUKESHBHAI THAKORBelum ada peringkat
- DLP Mathematics 6 q1 w6Dokumen5 halamanDLP Mathematics 6 q1 w6Kathlyn PerezBelum ada peringkat
- Four Bolt Unstiffened End PlateDokumen7 halamanFour Bolt Unstiffened End PlateRnD2013Belum ada peringkat
- 8 Coil PWM Drivers PDFDokumen4 halaman8 Coil PWM Drivers PDFDuzng Hoang TriBelum ada peringkat
- PLX7100A Digital Mobile C-Arm X-Ray Machine: 1. Technical SpecificationDokumen3 halamanPLX7100A Digital Mobile C-Arm X-Ray Machine: 1. Technical SpecificationAbdalhakeem AlturkyBelum ada peringkat
- Pearson Product-Moment Correlation Coefficient Table of Critical ValuesDokumen2 halamanPearson Product-Moment Correlation Coefficient Table of Critical ValuesOdy AjjaBelum ada peringkat
- App NandDokumen30 halamanApp NandRajesh MedampudiBelum ada peringkat