Tutorial 4
Diunggah oleh
Majid AishahDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Tutorial 4
Diunggah oleh
Majid AishahHak Cipta:
Format Tersedia
Faculty of Chemical Engineering CPE553 Chemical Engineering Thermodynamics
Semester March-July 2013 29 March 2013
TUTORIAL 4
1. The general form of summability relation of molar properties for a binary solution is given by
=
i
i
i
M x M
By using above equation, prove that partial molar properties
1
M and
2
M for a binary systems at
constant T and P are
= + 1
2
1
dM
M M x
dx
and
= 2
1
1
dM
M M x
dx
2. The molar volume (cm
3
mol
-1
) of a binary liquid mixture at T and P is given by
( ) = + + +
1 2 1 2 1 2
120 70 15 8 V x x x x x x
(a) Find expressions for the partial molar volumes of species 1 and 2 at T and P.
(b) Show that when these expressions are combined in accord with eq. (11.11) the given
equation for V is recovered.
(c) Show that these expressions satisfy eq. (11.14), the Gibbs/Duhem equation.
(d) Show that
= =
| | | |
= =
| |
\ . \ .
1 1
1 1 2 1
1 0
0
x x
dV dx dV dx .
(e) Plot values of
1 2
, , and V V V calculated by the given equation for V and by the equations
developed in (a) vs. x
1
. Label points
1 2 1 2
, , , and V V V V , and show their values.
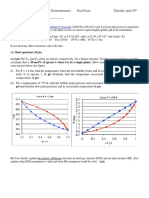
3. Figure 1 shows a plot of
1 2
, and H H H vs. x
1
(J/mol) at constant T and P for a binary liquid
mixture. Label the below points and show their values on the plot.
(a) Enthalpy of pure species 1.
(b) Enthalpy of pure species 2.
(c) Partial enthalpy of pure species 1.
(d) Partial enthalpy for pure species 2.
(e) Partial enthalpy at infinite dilution of species 1.
(f) Partial enthalpy at infinite dilution of species 2.
(g) Enthalpy of the mixture that contains 22 mole % species 2.
(h) Partial enthalpy of species 2 if the mixture contains 64 mole % species 1.
(i) Partial enthalpy of species 1 if the mixture contains 52 mole % species 2.
4. Figure 2 shows a plot of entropy (kJ/kg.K) vs. x
1
at constant T and P for a binary liquid mixture.
Explain how to obtain the below points from the plot and show the values. Then, label and show
their values on the plot.
(a) Partial entropy of pure species 1 at 60 mole % species 1.
(b) Partial entropy of pure species 2 at 60 mole % species 1.
(c) Partial entropy at infinite dilution of species 1.
(d) Partial entropy at infinite dilution of species 2.
Faculty of Chemical Engineering CPE553 Chemical Engineering Thermodynamics
Semester March-July 2013 29 March 2013
Figure 1
0
50
100
150
200
250
300
350
400
450
500
550
600
650
700
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1
x1
H
Faculty of Chemical Engineering CPE553 Chemical Engineering Thermodynamics
Semester March-July 2013 29 March 2013
Figure 2
0
20
40
60
80
100
120
140
160
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1
S
(
k
J
/
k
g
.
K
)
x1
Anda mungkin juga menyukai
- Modelling of Reaction Term of The Adr EquationDokumen9 halamanModelling of Reaction Term of The Adr Equationricardogl1982Belum ada peringkat
- Determination of Partial Molar EnthalpyDokumen21 halamanDetermination of Partial Molar EnthalpyKrizz AstorgaBelum ada peringkat
- MOLE CONCEPT SIGNIFICANT FIGURESDokumen22 halamanMOLE CONCEPT SIGNIFICANT FIGURESjohn nashBelum ada peringkat
- Answers 1997 ExamDokumen7 halamanAnswers 1997 ExamcjBelum ada peringkat
- 117 B.P.S. XI Chemistry IIT JEE Advanced Study Package 2014 15 PDFDokumen314 halaman117 B.P.S. XI Chemistry IIT JEE Advanced Study Package 2014 15 PDFVISHWAJEET100% (4)
- ReportDokumen4 halamanReportChadt Montague I'gautteBelum ada peringkat
- Chem RXN EquilDokumen12 halamanChem RXN EquilfarahanisiliasBelum ada peringkat
- CH 1.1: Basic Mathematical Models Direction Fields: Differential Equations Are Equations Containing DerivativesDokumen13 halamanCH 1.1: Basic Mathematical Models Direction Fields: Differential Equations Are Equations Containing DerivativesfloopydriveBelum ada peringkat
- HHW Class XIIDokumen24 halamanHHW Class XIIMadhu kushalBelum ada peringkat
- CHEMISTRY – STUDY OF MATTERDokumen23 halamanCHEMISTRY – STUDY OF MATTERSachin KumarBelum ada peringkat
- Solution ThermoDokumen9 halamanSolution ThermofarahanisiliasBelum ada peringkat
- 10.213 Chemical Engineering Thermodynamics Spring 2002 Problem Set IDokumen2 halaman10.213 Chemical Engineering Thermodynamics Spring 2002 Problem Set IBuket IbidanBelum ada peringkat
- Lec 5 Partial Molar Property PDFDokumen7 halamanLec 5 Partial Molar Property PDFMujtabba AlkhtatBelum ada peringkat
- Rate Equations of Solid State Reactions. Euclidean and Fractal ModelsDokumen4 halamanRate Equations of Solid State Reactions. Euclidean and Fractal ModelsNadyaZulfaniBelum ada peringkat
- Applied Pipeline HydraulicsDokumen59 halamanApplied Pipeline HydraulicsOlusayoBelum ada peringkat
- Applied Pipeline HydraulicsDokumen59 halamanApplied Pipeline HydraulicsFonseca Eluards BermudezBelum ada peringkat
- Chem T1 - KineticsDokumen33 halamanChem T1 - KineticsJanani SundararajanBelum ada peringkat
- Density and Surface Tension of Binary Mixtures of Acetonitrile + 1-Alkanol at 293.15 KDokumen4 halamanDensity and Surface Tension of Binary Mixtures of Acetonitrile + 1-Alkanol at 293.15 KMariela CastilloBelum ada peringkat
- Chemengthermo Tutorial 022 K 17Dokumen4 halamanChemengthermo Tutorial 022 K 17AthinaBelum ada peringkat
- Statistical Molecular Thermodynamics HomeworkDokumen48 halamanStatistical Molecular Thermodynamics HomeworkCamila Yepes100% (1)
- Gs2019 QP CHMDokumen20 halamanGs2019 QP CHMSudip ChowdhuryBelum ada peringkat
- CBE3508 Sp21 FinalDokumen6 halamanCBE3508 Sp21 Finalsasuke uchihaBelum ada peringkat
- Dynamic stability analysis of aircraft longitudinal motionDokumen12 halamanDynamic stability analysis of aircraft longitudinal motionavianbuBelum ada peringkat
- The Thermochemistry of A Reacting Mixture of Elastic Materials With DiffusionDokumen31 halamanThe Thermochemistry of A Reacting Mixture of Elastic Materials With DiffusionSantiago Peña ClavijoBelum ada peringkat
- Partial Molal QuantitiesDokumen8 halamanPartial Molal QuantitiesLheander GernaBelum ada peringkat
- WEEK 9 - Chemical Reaction Equilibria Part 1Dokumen34 halamanWEEK 9 - Chemical Reaction Equilibria Part 1Wan Nadatul NadwaBelum ada peringkat
- CH 301 Practice Questions 2023Dokumen20 halamanCH 301 Practice Questions 2023Fortune VusheBelum ada peringkat
- ch8 ProbsDokumen4 halamanch8 ProbsEkrem GüldesteBelum ada peringkat
- Pritn HMWK 8Dokumen1 halamanPritn HMWK 8petester01Belum ada peringkat
- Chapter 13Dokumen38 halamanChapter 13Lucy BrownBelum ada peringkat
- Models - Chem.stefan TubeDokumen12 halamanModels - Chem.stefan TubeAnukul SrivastavaBelum ada peringkat
- A Thermodynamic Study of Complex Formation Between Cuii Ion and 4amino3methyl124 Triazol5thione Amtt in Binary EthanolDokumen7 halamanA Thermodynamic Study of Complex Formation Between Cuii Ion and 4amino3methyl124 Triazol5thione Amtt in Binary Ethanolsunaina agarwalBelum ada peringkat
- Vergaño-Salazar 2020 J. Phys. Conf. Ser. 1514 012004Dokumen8 halamanVergaño-Salazar 2020 J. Phys. Conf. Ser. 1514 012004ledyz cuesta herreraBelum ada peringkat
- A2 WS 4.1 (3) - Worksheet On Reaction Kinetics (Answers)Dokumen3 halamanA2 WS 4.1 (3) - Worksheet On Reaction Kinetics (Answers)Peter EdwardBelum ada peringkat
- Algebraic Representation of Thermodynamic Properties and the Classification of SolutionsDokumen4 halamanAlgebraic Representation of Thermodynamic Properties and the Classification of Solutionsgggggg82Belum ada peringkat
- Assignment Mole Concept JH Sir-2686Dokumen22 halamanAssignment Mole Concept JH Sir-2686Nitin DasBelum ada peringkat
- 6021 Fall 2004Dokumen547 halaman6021 Fall 2004combatps10% (1)
- Partial Molar Volume LabDokumen8 halamanPartial Molar Volume LabRaj MahendranBelum ada peringkat
- 2020RussiaWinterCampEngDokumen20 halaman2020RussiaWinterCampEngTəranə MəmmədovaBelum ada peringkat
- Vidyamandir Classes JEE TestDokumen16 halamanVidyamandir Classes JEE TestArshil Khan100% (1)
- Universiti Teknologi Mara Final Examination: Confidential EH/JAN 2013/CPE553Dokumen9 halamanUniversiti Teknologi Mara Final Examination: Confidential EH/JAN 2013/CPE553mhd badhrul bin baharBelum ada peringkat
- Final ExamDokumen4 halamanFinal ExamTinu Paul JepinBelum ada peringkat
- Chapter 6Dokumen48 halamanChapter 6Lucy Brown0% (1)
- Penurunan HattaDokumen18 halamanPenurunan HattaBernardinus Andrie LuirenBelum ada peringkat
- VMC TestDokumen17 halamanVMC TestTushar AgrawalBelum ada peringkat
- Executive Summary:: κ A (P P µ∗L ⇔ v= Q A κ∗∆ P μ∗LDokumen5 halamanExecutive Summary:: κ A (P P µ∗L ⇔ v= Q A κ∗∆ P μ∗LTrường Tùng LýBelum ada peringkat
- Executive Summary:: κ A (P P µ∗L ⇔ v= Q A κ∗∆ P μ∗LDokumen5 halamanExecutive Summary:: κ A (P P µ∗L ⇔ v= Q A κ∗∆ P μ∗LTrường Tùng LýBelum ada peringkat
- 5.1.2 How Far QPDokumen16 halaman5.1.2 How Far QPSir MannyBelum ada peringkat
- Phy 1321 Assignment SolutionsDokumen2 halamanPhy 1321 Assignment SolutionsNupur VijBelum ada peringkat
- University of Mauritius Faculty of EngineeringDokumen5 halamanUniversity of Mauritius Faculty of EngineeringToMemBelum ada peringkat
- Chemistry CY11003 Long Test Aut 2020-2021Dokumen14 halamanChemistry CY11003 Long Test Aut 2020-2021GokulBelum ada peringkat
- Eee L-1, T-2 (2016-2017) PDFDokumen26 halamanEee L-1, T-2 (2016-2017) PDFআশিক পালোয়ানBelum ada peringkat
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportDari EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportBelum ada peringkat
- O Level Biology Practice Questions And Answers EnzymesDari EverandO Level Biology Practice Questions And Answers EnzymesPenilaian: 5 dari 5 bintang5/5 (1)
- Selected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionDari EverandSelected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionBelum ada peringkat
- Power Geometry in Algebraic and Differential EquationsDari EverandPower Geometry in Algebraic and Differential EquationsBelum ada peringkat
- Reviews in Computational ChemistryDari EverandReviews in Computational ChemistryAbby L. ParrillBelum ada peringkat
- Production of - Phenylalanine From Glycerol by A Recombinant: Escherichia ColiDokumen8 halamanProduction of - Phenylalanine From Glycerol by A Recombinant: Escherichia ColiMajid AishahBelum ada peringkat
- SafetyDokumen1 halamanSafetyMajid AishahBelum ada peringkat
- Results Lab GenetucsDokumen1 halamanResults Lab GenetucsMajid AishahBelum ada peringkat
- 10 1 1 104 6408Dokumen127 halaman10 1 1 104 6408Majid AishahBelum ada peringkat
- How To Produce Amylase by Bacillus SubtilisDokumen1 halamanHow To Produce Amylase by Bacillus SubtilisMajid AishahBelum ada peringkat
- Experiment 1Dokumen25 halamanExperiment 1Majid AishahBelum ada peringkat
- Chapter 1 - 2012 VDokumen36 halamanChapter 1 - 2012 VMajid AishahBelum ada peringkat
- Plastic Bags ProjectDokumen8 halamanPlastic Bags ProjectMajid AishahBelum ada peringkat
- Resume AyanchattopadhyayDokumen2 halamanResume Ayanchattopadhyayapi-163237383Belum ada peringkat
- Rate of Reaction FactorsDokumen3 halamanRate of Reaction FactorsFungai mhlangaBelum ada peringkat
- Earthquake Resistant Buildings Dynamic Analyses Numerical Computations Codified Methods Case Studies and ExamplesDokumen12 halamanEarthquake Resistant Buildings Dynamic Analyses Numerical Computations Codified Methods Case Studies and ExamplesSindura SwarnakariBelum ada peringkat
- Dowel Bar-Tie Bar-IRC-58-2015Dokumen3 halamanDowel Bar-Tie Bar-IRC-58-2015SONU SINGHBelum ada peringkat
- Robot DynamicsDokumen52 halamanRobot Dynamicssilviocus88Belum ada peringkat
- 3-7 Fluids in Rigid-Body Motion: - We ObtainedDokumen6 halaman3-7 Fluids in Rigid-Body Motion: - We ObtainedAsmaa Ali El-AwadyBelum ada peringkat
- Experiment No 8: Aim: To Determine The Frequency & Wavelength in A Rectangular Waveguide Working inDokumen5 halamanExperiment No 8: Aim: To Determine The Frequency & Wavelength in A Rectangular Waveguide Working inSanab KumarBelum ada peringkat
- Margarine 12Dokumen74 halamanMargarine 12the_gunners2004Belum ada peringkat
- Design, Analysis and Fabrication of Split Braking SystemDokumen6 halamanDesign, Analysis and Fabrication of Split Braking Systemhabib nawazBelum ada peringkat
- Non Linear Analysis of RC Column PDFDokumen61 halamanNon Linear Analysis of RC Column PDFLabinotMMorinaBelum ada peringkat
- Physics of Electromagnetic Calorimeters Based On Crystal ScintillatorsDokumen49 halamanPhysics of Electromagnetic Calorimeters Based On Crystal ScintillatorsVigneshRamakrishnanBelum ada peringkat
- Internal Flows (Laminar Flow) : Lecture - 04Dokumen25 halamanInternal Flows (Laminar Flow) : Lecture - 04غيث منعمBelum ada peringkat
- FEA 2 McqsDokumen26 halamanFEA 2 Mcqsrak RoyBelum ada peringkat
- torque 정의Dokumen40 halamantorque 정의valmaxjeonBelum ada peringkat
- Quantum NumbersDokumen2 halamanQuantum NumbersWong Weng SiongBelum ada peringkat
- Physics Investigatory Project: Electromagnetic InductionDokumen16 halamanPhysics Investigatory Project: Electromagnetic InductionRajesh ChoudharyBelum ada peringkat
- Chapter 1 Units and VectorsDokumen33 halamanChapter 1 Units and VectorslozzzzzBelum ada peringkat
- Unit 2 HydrostaticsDokumen17 halamanUnit 2 HydrostaticsRin MoonBelum ada peringkat
- Vesic 1975 Bearing Capacity of Shallow FoundationsDokumen27 halamanVesic 1975 Bearing Capacity of Shallow Foundationst.w.c.100% (2)
- Ground Fault Protection and Coordination in Industrial and Commercial Power SystemsDokumen46 halamanGround Fault Protection and Coordination in Industrial and Commercial Power Systemsgerrzen64100% (1)
- Dynamic Parameter Identification of The Universal Robots UR5 PDFDokumen7 halamanDynamic Parameter Identification of The Universal Robots UR5 PDFAyman DamounBelum ada peringkat
- Forane 22 Saturation Pressure Temperature DataDokumen1 halamanForane 22 Saturation Pressure Temperature Datavineeth100% (1)
- Cbiescsu 06Dokumen4 halamanCbiescsu 06neomatrix70Belum ada peringkat
- Earth Pressure & Retaining Walls-5Dokumen28 halamanEarth Pressure & Retaining Walls-5Suraj PandeyBelum ada peringkat
- Wiles1994 - in Situ Stress Determination Using The Under-Excavation Technique - I. TheoryDokumen8 halamanWiles1994 - in Situ Stress Determination Using The Under-Excavation Technique - I. TheoryRisantoBelum ada peringkat
- JEE Advanced 2020 Analysis JEE Advanced 2021 Analysis: WWW - Motion.ac - inDokumen16 halamanJEE Advanced 2020 Analysis JEE Advanced 2021 Analysis: WWW - Motion.ac - inSarthak OmarBelum ada peringkat
- Lever Problems 1bDokumen2 halamanLever Problems 1bmaylynXiXBelum ada peringkat
- Electricity Class Notes Latest 21.10. 2019Dokumen3 halamanElectricity Class Notes Latest 21.10. 2019bittuchintuBelum ada peringkat
- Applied Thermodynamics Tutorial Reciprocating CompressorsDokumen2 halamanApplied Thermodynamics Tutorial Reciprocating CompressorsChris ZiyuenBelum ada peringkat