Spindle Cell Breast Paid Articl
Diunggah oleh
Asmara SyedHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Spindle Cell Breast Paid Articl
Diunggah oleh
Asmara SyedHak Cipta:
Format Tersedia
An Immunohistochemical Study of Metaplastic Spindle Cell Carcinoma, Phyllodes Tumor and Fibromatosis of the Breast
BARBARA DUNNE, MRCPATH, ANDREW H. S. LEE, MRCP, MRCPATH, SARAH E. PINDER, FRCPATH, JANE A. BELL, FIBMS, AND IAN O. ELLIS, FRCPATH
The diagnosis of metaplastic (sarcomatoid) carcinoma (MSC) of breast often requires immunohistochemistry with a cytokeratin (CK) panel to distinguish them from phyllodes tumors (PT), primary sarcomas, and bromatoses. CK staining may be heterogeneous in metaplastic carcinomas. The aim of the study was to investigate the theory that MSCs show evidence of myoepithelial differentiation and to evaluate immunohistochemical markers that may be helpful in distinguishing MSCs from PT and bromatosis. We reviewed histology and performed immunohistochemistry for AE1/AE3, 34E12, CK5 and CK14, Cam5.2, CK7 and CK19, epithelial membrane antigen (EMA) (B55), smooth muscle actin (SMA), S100, desmin, vimentin, CD31, CD34, and bcl-2 on parafn-embedded tissue from 18 MSCs, 26 PTs, and 8 bromatoses. We assessed staining by using a semiquantitative method. Sarcomatous areas in MSCs were positive for 34E12 in 11 cases; for SMA in 10; for CK5 in 7; for CK14 in 6; for Cam5.2, AE1/AE3, and S100 in 5; and for CK7 and CK19 in 3. No CK expression was seen in stromal areas in PT or in bromatoses. CD34 and bcl-2 were more frequently expressed in spindle cell areas in PTs (18 and 12 of 26, respectively) than in MSCs (0 and 2 of 18, respectively). MSCs show strong evidence of myoepithelial differentiation. CD34 and, to a lesser extent, bcl-2 positivity in PTs may be helpful in differentiating these two lesions from MSCs, particularly in small biopsies, because CK staining in MSCs may be heterogeneous. In our hands, 34E12 was the CK most frequently expressed in sarcomatoid areas in MSCs. HUM PATHOL 34:1009-1015. 2003 Elsevier Inc. All rights reserved. Key words: breast, metaplastic carcinoma, myoepithelial phenotype, phyllodes tumor, CD34 positivity. Abbreviations: MSC, metaplastic sarcomatoid carcinoma; PT, phyllodes tumor; CK, cytokeratin.
Breast tumors with a sarcomatous growth pattern are relatively rare and can present a diagnostic challenge to the histopathologist. Monophasic spindle cell tumors include bromatosis, spindle cell myoepithelioma, myobroblastoma, and inammatory pseudotumor, which are benign, and metaplastic (sarcomatoid) carcinoma (MSC) and the very rare primary breast sarcoma at the malignant end of the spectrum. Tumors with a biphasic pattern composed of spindle and epithelial areas are also a heterogeneous group and encompass broadenomas, PTs, and biphasic MSCs. MSCs, by denition, contain malignant sarcomatoid, usually spindle cell, areas that show, at least in part, an epithelial immunophenotype.1-4 MSCs can be biphasic and contain discrete epithelial areas in addition to sarcomatoid areas, or less commonly, they can be composed of sarcomatoid areas alone. The sarcomatoid areas, in turn, can be pure spindle cell or malignant brous histiocytoma (MFH) like or show heterologous mesenchymal differentiation, most commonly cartilaginous or osseous in type.5 The theory that MSCs are derived from myoepithelial cells rather than epithelial cells or, indeed, from a
From the Department of Pathology, City Hospital, Nottingham, United Kingdom. Accepted for publication May 1, 2003. Address correspondence and reprint requests to Barbara Dunne, MRCPath, Department of Pathology, Glasgow Royal Inrmary, Glasgow G4 OSF, United Kingdom. 2003 Elsevier Inc. All rights reserved. 0046-8177/03/3410-0007$30.00/0 doi:10.1053/S0046-8177(03)00414-3
common stem cell that is capable of dual differentiation has been debated in the literature, the earliest such proposal coming from Hamperl.6 Myoepithelial neoplasms of the breast are extremely uncommon, and their description has been limited to case reports. Adenomyoepitheliomas have been divided into 3 subtypes based on their growth pattern:7,8 spindle cell, which resembles leiomyoma but contains occasional glandular spaces, and tubular and lobular variants. They have been shown to behave as low-grade malignant neoplasms,7-10 having the potential to recur locally if not completely excised and, very rarely, to metastasize.10 The entity of malignant myoepithelioma (myoepithelial carcinoma) in its pure form is reportedly composed of spindle cells that show evidence of myoepithelial differentiation by immunohistochemistry or electron microscopy.11,12 It is recognized that there is an overlap between this entity and monophasic MSC, as both tumors may express S100, actin, and cytokeratins (CKs).1,3,13-15 PTs, in contrast, are biphasic tumors that do not show an epithelial or a myoepithelial phenotype in the stroma. They are characterized by their leaike architecture, cleftlike spaces lined by epithelium, and hypercellular stroma.16-18 Fibromatosis of breast has a similar histological appearance to the same lesion at other sites and is composed of a variably cellular proliferation of uniform, bland spindle cells arranged in fascicles with intervening hyalinized areas.19,20 It is important as a differential diagnosis for monophasic MSC, but absence of an epithelial component after careful sampling and CK negativity are useful differentiating features.
1009
HUMAN PATHOLOGY
Volume 34, No. 10 (October 2003)
TABLE 1. Histological Features of Metaplastic Carcinomas
Conventional carcinoma Case no 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Metaplastic components Invasive In situ DCIS DCIS DCIS DCIS DCIS DCIS DCIS
Spindle Spindle Spindle small squamoid foci Spindle (arising in sclerosing lesion) small cohesive foci Spindle Spindle small cohesive foci Spindle small cohesive foci Spindle small squamoid foci NST Spindle small cohesive foci Spindle small squamoid foci NST Cartilage Lobular Cartilage NST Cartilage NST Cartilage NST NST Spindle papillary Spindle cartilage osteoid NST Spindle cartilage NST Spindle cartilage NST
1:50), vimentin (Dako, 1:250), CD31 (Dako, 1:100), CD34 (Novocastra, 1:50), and bcl-2 (Dako, 1:100). An automatic immunohistochemical stainer (Dako TechMate with ChemMate Detection Kit) was used, with microwave retrieval for all antibodies except S100, EMA, SMA, and desmin. We examined the entire lesional area for each stain and assessed staining semiquantitatively, where 1 staining represented 10% positive cells; 2, 10% and 50% positive cells; 3, 50% and 90% positive cells; and 4, 90% positive cells. We considered 2 staining and above to represent positivity. Fishers exact test (SPSS for Windows, version 11) was used to test the signicance of differences in staining between MSCs and PTs. This project involved the use of routinely stored, archival, parafn-embedded tissue. All patients consented to removal of their breast tumors for treatment purposes alone. The type and extent of surgery were not modied in any way for the benet of subsequent research projects. Patient clinical notes were not consulted.
RESULTS Age and Sex of Patients All patients were female. The age range for the MSC patients was 27-82 years, and the mean, 57 years (standard deviation [SD], 16.3); for the PT patients, it was 20-92 years, and the mean, 52 years ( SD 12.9); and for the bromatoses, it was 20-64 years, and the mean, 44 years ( SD 19.4). Macroscopy and Microscopic Review Gross pathology was detailed from the pathology report. The macroscopic size for the MSCs ranged from 10 to 80 mm in maximum extent. Where circumscription was documented, 9 were described as well dened, 3 as moderately well dened, and 2 as poorly dened. Two tumors had friable/necrotic areas. The size range for the PTs was between 17 and 180 mm in maximum extent. Most of the tumors were described as welldened, lobulated, rm, cream and white lesions. In the case of the bromatoses, 10-35 mm was the size range, and the lesions were typically ill-dened and brous in nature. Table 1 summarizes the histological features of the MSCs. See also Figs 1 through 4. The bromatoses had
Abbreviations: DCIS, ductal carcinoma in situ; NST, no special type.
The aim of this study was to perform a detailed immunohistochemical study on spindle cell lesions of breast common to our practice to accomplish the following: (1) investigate the theory that MSCs show phenotypic evidence of myoepithelial differentiation and (2) show markers that may be helpful in differentiating these tumors, one from another. We used a range of CKs, including broad-spectrum CKs (AE1/AE3) and CKs known specically to be expressed in both basal (CK5, CK14, 34E12)21 and luminal (CK7,22 CK19,23 Cam5.2) epithelia. We included S100 and smooth muscle actin (SMA), which are also expressed in myoepithelial cells, and CD34 and bcl-2, which have been shown to be positive in the stroma of phyllodes tumors (PT).24-26 We completed the stromal panel with desmin, vimentin, and CD31. MATERIALS AND METHODS
We collected 18 MSCs (16 biphasic, 2 uniphasic), 26 PTs (18 benign, 5 borderline, 3 malignant), and 8 bromatoses from the les of Nottingham City Hospital. All tumors had been resected at this hospital between 1987 and 1999. We reviewed the hematoxylin and eosin (H&E) slides. The PTs were graded according to the criteria of Moffat et al.27 We performed immunohistochemistry on one representative block from each case for Cam5.2 (Becton Dickinson, Franklin Lakes, NJ, 1:2), AE1/AE3 (Dako, Glostrup, Denmark, 1:50), CK5 (The Binding Site, Birmingham, UK, 1:100), CK14 (Novocastra, Newcastle Upon Tyne, UK, 1:100), 34E12 (Dako, 1:80), CK7 (Dako, 1:50), CK19 (Dako, 1:100), epithelial membrane antigen (EMA [B55 antibody]; Nottingham University, Nottingham, UK, 1:4000), SMA (Sigma, St. Louis, MO, 1:200), S100 (Dako, 1:1000), desmin (Dako,
FIGURE 1. Biphasic metaplastic spindle cell carcinoma with squamous cell differentiation (Case 3, see Table 1; H&E, magnication 200).
1010
IMMUNOHISTOCHEMICAL DIFFERENTIATION IN BREAST (Dunne et al)
FIGURE 3. Uniphasic spindle cell carcinoma invading fat (Case 2, see Table 1; H&E, magnication 200).
FIGURE 2. Biphasic metaplastic spindle cell carcinoma with well-demarcated spindle and epithelial components (Case 15, see Table 1; H&E, magnication 200).
strongly positive in the sarcomatous areas in all but 1 MSC.
MSC: Epithelial/Myoepithelial Staining
typical appearances and showed broad fascicles of spindle cells with variable cellularity, sometimes intervening hyalinized areas and inltrative edges. Results of Immunohistochemical Staining
MSC: Spindle and Heterologous Staining
The differential staining pattern of CKs, EMA, S100, and SMA in spindle cell and heterologous areas of the MSCs is summarized in Table 2. Overall, a greater number of cases stained positively for the myoepithelial markers 34E12 (11 cases), SMA (10 cases), CK5 (7 cases), and CK14 (6 cases) (see Fig 5) than for the broad-spectrum CK AE1/AE3 (5 cases) and the luminal CKs cam5.2 (5 cases), CK7 (3 cases), and CK19 (3 cases). Five cases stained positively for S100. In the majority of cases, the spindle areas stained positively for both myoepithelial and luminal CKs, and in some cases there appeared to be positive staining in the same cells for the 2 different subtypes of keratin. The staining was patchy in most cases. The most diffuse staining was seen with AE1/AE3, for which all positive cases showed positive staining in 50% of cells. In the 7 cases in which chondrosarcoma-like areas were present, SMA was positive in 2 cases and S100 in 5, with diffuse 4 staining in 2 of these. Of the CKs, 34E12 was positive in cartilaginous areas in 5 cases (see Fig 6) and was positive for CK5, CK14, AE1/AE3, and cam5.2 in 1 case each; staining for CK7 and CK19 was negative. There was no positivity when using any of the antibodies in the osteoid present in 1 case. EMA was negative in all cases. A comparison of mesenchymal staining in MSCs, PTs, and bromatoses is summarized in Table 3. None of the MSCs showed CD34 expression in the spindle cell component. There was positive staining for bcl-2 in 2 MSCs; 1 of these was in a chondroid area, and 1 was in a spindle cell area. CD31 was positive in pleomorphic spindle cells in 1 MSC. There was desmin positivity in 3 MSCs, 2 in cartilage and 1 in spindle cells. Vimentin was
Epithelial and myoepithelial marker expression in nonsarcomatous areas of MSCs is summarized in Table 4. The epithelial areas in the MSCs showed mixed expression for both basal and luminal CKs and EMA. Fourteen cases expressed 34E12; 13 expressed AE1/ AE3, Cam5.2, and CK7; 12 expressed EMA; 11 expressed CK5 and CK19; 10 expressed CK14; 7 expressed S100; and 4 showed SMA positivity. There was positive staining for bcl-2 in the epithelial component of 7 MSCs; two of these showed 4 staining, but there was no positivity for CD34, CD31, desmin, and vimentin.
PTs: Stromal Staining
See Table 3 and Table 5. There was CD34 expression in the stroma of 18 of 26 PTs. Positive staining was seen in 1 (2 staining) of 3 malignant, in 3 (4 staining in 1, 2 staining in 2) of 5 borderline, and in 14 benign tumors (4 in 3, 3 in 2, and 2 in 9; see Fig
FIGURE 4. Biphasic metaplastic carcinoma showing heterologous cartilaginous differentiation (Case 17, see Table 1; H&E, magnication 200).
1011
HUMAN PATHOLOGY
Volume 34, No. 10 (October 2003)
TABLE 2. Epithelial and Myoepithelial Marker Expression in Spindle and Heterologous Elements in Metaplastic Spindle Cell Carcinomas
Case number SMA S100 34E12 CK5 CK14 AE1/AE3 Cam5.2 CK7 CK19 EMA
1 S2 S0 S1 S1 S1 S1 S0 S1 S0 S0 2 S3 S1 S3 S4 S3 S3 S1 S0 S0 S0 3 S0 S0 S3 S2 S2 S4 S4 S2 S2 S0 4 S3 S0 S0 S1 S0 S0 S1 S1 S0 S0 5 S1 S0 S2 S4 S4 S3 S3 S0 S0 S0 6 S4 S0 S2 S4 S3 S3 S1 S2 S0 S0 7 S2 S0 S2 S4 S4 S0 S2 S0 S0 S0 8 S0 S0 S2 S3 S1 S0 S4 S2 S2 S0 9 S2 S1 S0 S0 S0 S0 S0 S0 S0 S0 10 S0 S0 S1 S0 S0 S0 S0 S1 S4 S0 11 C3 C4 C4 C0 C0 C0 C0 C0 C0 C0 12 C0 C4 C4 C2 C2 C3 C3 C1 C1 C0 13 C0 C2 C2 C0 C1 C1 C1 C0 C0 C0 14 C2 C1 C1 C0 C1 C1 C0 C0 C0 C0 15 S3 S1 S1 S0 S0 S0 S0 S1 S0 S0 16 S3, C1, B0 S2, C2, B0 S2, C2, B0 S0, C0, B0 S0, C0, B0 S0, C0, B0 S0, C0, B0 S0, C0, B0 S0, C0, B0 S0, C0, B0 17 S1, C0 S1, C0 S1, C0 S0, C0 S0, C0 S0, C0 S0, C0 S0, C0 S0, C0 S0, C0 18 S1, C0 S2, C2 S2, C2 S0, C0 S0, C0 S0, C0 S0, C0 S0, C0 S0, C0 S0, C0 Total positive 2 10 5 11 7 6 5 5 3 3 0 1 staining 10% positive cells, 2 10% and 50% positive cells, 3 50% and 90% positive cells, 4 90% positive cells. Abbreviations: S, spindle cell areas; C, cartilaginous areas; B, osteoid areas.
7). Bcl-2 was positive in the stroma of 12 PTs, 2 of 3 malignant, 3 of 5 borderline, and 7 of 18 benign tumors (see Fig 8). There was 3 staining in the malignant tumor and in 2 of the benign lesions, but the remainder of the staining was in 50% of cells. Seventeen PTs showed stromal positivity for SMA, 2 of 3 malignant, 3 of 5 borderline, and 11 of 18 benign lesions. Three PTs (1 malignant, 1 borderline, and 1 benign) showed stromal positivity for S100. CD31 staining was negative. There was desmin positivity in 3 tumors (1 malignant, 1 borderline, and 1 benign). Vimentin was diffusely positive in the stromal component of all lesions and CK, and EMA staining was negative.
PTs: Epithelial Staining
All cases showed 4 positivity for AE1/AE3, Cam5.2, CK7, and EMA, where in the case of each antibody, the positive cells lined the luminal surfaces of the clefts or glandular spaces. 34E12 stained positively in all but one case, and S100, positively in all cases, with a mixed basal and luminal pattern of positivity. CK5, CK14, and SMA showed strong positivity in the epithelial areas of all cases, but this time, the pattern of staining was predominantly basal, where the positive cells were those lying adjacent to the basement membrane and there were often overlying negative luminal cells. There was positive staining in the epithelial areas for bcl-2 in 24 of 26 PTs; this was predom-
FIGURE 5. Uniphasic spindle cell carcinoma showing strong expression for CK14 by immunohistochemistry (Case 2, see Table 1; H&E, magnication 100).
FIGURE 6. Immunohistochemical staining for 34E12 in heterologous cartilage in a metaplastic carcinoma (Case 17, see Table 1; H&E, magnication 400).
1012
IMMUNOHISTOCHEMICAL DIFFERENTIATION IN BREAST (Dunne et al)
TABLE 3. Comparison of Staining in Spindle Cell Areas of Metaplastic Carcinomas, Phyllodes Tumors, and Fibromatoses
Metaplastic carcinoma (n 18) 0 2 10 10 1 3 17 Phyllodes tumour (n 26) 18 12 17 3 0 3 26 P value (Fishers exact test) 0.0001 0.02 0.54 0.002 0.41 0.66 0.41 Fibromatosis (n 8) 0 0 5 0 0 1 7
Staining CD34 Bcl-2 SMA S100 CD31 Desmin Vimentin
inantly luminal in distribution. There was no positivity in the epithelial areas of PTs for CD34, CD31, desmin, or vimentin.
Fibromatosis
See Table 3. Five of 8 bromatoses showed positivity for SMA, 1 showed 4 positivity, and 4 showed 2 positivity. Vimentin was diffusely positive in 7 of 8 cases. There was negative staining in all cases for S100, bcl-2, CD31, all CKs, and EMA. Staining for CD34 was also interpreted as negative, although some of the cases had positive staining at the interface between the lesion and normal breast tissue. One case showed 2 positivity for desmin. DISCUSSION We reviewed all of the spindle cell tumors of breast that were recorded in our practice over a 12-year period. We carried out a detailed immunohistochemical
analysis of the tumors by using antibodies against a range of CKs and mesenchymal markers. Our aim was to try to better understand the histogenesis of these tumors and to identify potential antibodies that might aid the histopathologist in his or her diagnosis. The mesenchymal elements of the MSCs showed heterogeneous immunohistochemical staining for both epithelial and myoepithelial markers, and there was coexpression of both luminal and basal CKs in some of the cases. Overall, the pattern of immunoreactivity favored a myoepithelial phenotype, with 34E12 and CKs 5 and 14 staining positively in 11 (61%), 7 (39%), and 6 (33%) of cases, respectively, and SMA and S100 staining positively in 10 (55%) and 5 (28%) of cases. AE1/ AE3 (5 cases, 28%) and luminal CKs Cam5.2 (5 cases, 28%), CK7 (3 cases, 16%), and CK19 (3 cases, 16%) were also positive in sarcomalike areas in some of the cases. Thus, in our hands, 34E12 is positive in mesenchymal areas in the greatest number of MSCs. This nding concurs with other reports in which this antibody has been the most sensitive of all the CKs in metaplastic carcinomas.5,28 Overall, however, the staining for CKs was variable in distribution, which highlights the need to use a panel of CK antibodies in the workup of a potential MSC. Epithelial areas in the biphasic MSCs showed strong diffuse staining for all of the CKs, both basal and luminal types and for S100. SMA was positive in epithelial areas in 4 (25%) cases. Other investigators have also reported evidence of a myoepithelial phenotype in MSCs by using immunohistochemical methods, either alone1,3 or in combination with ultrastructural examination.2,29,30 Contrasting reports state that the presence of similar tumors in other organs in which myoepithelial cells are absent makes this an unlikely cell of origin.13 Although one
TABLE 4. Epithelial and Myoepithelial Marker Expression in Epithelial Areas in Metaplastic Spindle Cell Carcinomas
Case number 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Total positive 2 SMA 0 0 4 0 4 0 0 0 4 0 2 0 0 0 0 0 4 S100 0 4 3 2 0 2 1 0 0 2 1 2 0 2 1 1 7 34E12 2 4 3 4 2 4 4 2 3 4 1 0 2 2 4 4 14 CK5 4 2 4 4 4 4 4 1 4 3 0 0 0 1 2 2 11 CK14 3 4 4 4 4 4 4 1 4 4 1 1 0 1 1 4 10 AE1/AE3 4 4 4 4 4 4 4 4 0 4 1 1 4 4 4 4 13 Cam5.2 4 4 4 4 4 2 4 4 1 4 1 0 4 4 4 4 13 CK7 2 4 4 3 4 2 4 4 2 4 0 1 0 4 4 4 13 CK19 2 4 3 0 1 3 4 4 0 2 0 0 4 4 4 4 11 EMA 0 4 4 2 4 3 4 4 2 4 0 0 1 4 4 4 12
NOTE. 1 staining 10% positive cells, 2 10% and 50% positive cells, 3 50% and 90% positive cells, 4 90% positive cells.
1013
HUMAN PATHOLOGY
Volume 34, No. 10 (October 2003)
TABLE 5. Staining in Stromal Areas of Phyllodes Tumors in Relation to Grade of Tumor
Staining CD34 Bcl-2 SMA S100 Vimentin Desmin CD31 Benign (n 18) 14 7 11 1 18 1 0 Borderline (n 5) 3 3 3 1 5 1 0 Malignant (n 3) 1 2 2 1 3 1 0
recent report has shown positive staining in MSCs for the novel putative myoepithelial markers maspin, pcadherin, and p63,31 immunohistochemical evidence of myoepithelial differentiation previously has been limited to showing actin, S100, and nonspecic CK in sarcomatoid areas. In this series, as well as actin and S100, we have shown frequent expression of CKs 5 and 14, which are specically expressed in myoepithelial cells in both sarcomatoid and epithelial elements of biphasic MSCs, lending further and stronger evidence in favor of myoepithelial differentiation. It is most likely that the sarcomatous areas of these tumors develop along a particular pathway of myoepithelial differentiation rather than that they are truly derived histogenetically from myoepithelium. This theory is in keeping with our nding of strong diffuse staining for both basal and luminal CKs in epithelial areas of MSCs, coupled with differential expression of more basal-specic proteins in mesenchymal areas in many of the tumors. Kaufman et al32 have suggested that the myoepithelial phenotype may represent a transition between the transformation from epithelial to sarcomatous lines of differentiation. Another study, using microsatellite analysis, showed a single clonal origin for carcinomatous and sarcomatoid elements in a case of biphasic MSC by showing similar loss of heterozygosity patterns in both areas.33
FIGURE 8. Positive immunohistochemical staining for bcl-2 in a benign phyllodes tumor (H&E, magnication 200).
FIGURE 7. Strong positive immunohistochemical staining for CD34 in a benign phyllodes tumor (H&E, magnication 100).
Because of the rarity of MSCs and thus the paucity of large series with long-term follow-up and because of the lack of uniformity of grading between series, it has been difcult to fully elucidate the prognosis of these lesions. Some reports have suggested an unfavorable outcome for the group as a whole,34 whereas others have shown evidence of a variable prognosis depending on the histological pattern.2,30,35 Clearly, much more needs to be learned about the biology of these tumors if we are to predict survival and response to treatment. The second important nding in this study was the signicantly greater frequency of expression of CD34 (P 0.0001) and bcl-2 (P 0.02) in mesenchymal areas of PTs compared with in MSCs. Previous reports have similarly described expression of CD3424,25 and bcl-224,26 in the stroma of broadenomas and PTs. Combining the results of the current series with those of a previous series24 and with those from other recent cases in our department, we have seen CD34 expression in 58 of 66 PTs. Of these, 38 of 40 benign tumors were positive, 8 of 9 borderline tumors showed positivity, and 12 of 15 malignant PTs were positive. S100 was found to be expressed in a signicantly higher number of MSCs than PTs (P 0.002), and SMA was expressed in a roughly similar proportion of PTs and MSCs (P 0.54). The differential diagnosis between biphasic MSC and PT is clear-cut in some cases. On H&E staining, a leaike architecture and bland epithelium lining cleftlike spaces points to the latter diagnosis, whereas malignant epithelial elements and heterologous differentiation, if present, are more likely to be present in the former. In the preoperative setting, in which only a small quantity of tissue in a core biopsy may be available for assessment, positive stromal CK staining (or its absence) can often be the deciding factor in differentiating these two lesions. Because axillary surgery is more often undertaken in MSC than in PT, accurate preoperative diagnosis is important. As we have shown, CK staining is rarely diffuse in MSC, and heterologous
1014
IMMUNOHISTOCHEMICAL DIFFERENTIATION IN BREAST (Dunne et al)
staining can lead to false negatives. We propose that the addition of CD34, bcl-2, and S100 antibodies to a CK panel would be of benet in the workup of spindle cell lesions of breast, CD34 and bcl-2 positivity would favor PT, and S100 positivity would favor MSC. The bromatoses showed positivity for vimentin in 7 cases, for SMA in 5, and for desmin in 1 case. Staining with S100 and bcl-2 was negative. Unsurprisingly, no immunoreactivity was seen with CKs or EMA, a feature that differentiates these lesions from bland uniphasic MSCs. We saw no true lesional positivity for CD34 in bromatoses, although some of the cases showed positivity at the edge of the lesion in the stromal interface between the lesion and adjacent normal breast. In summary, in our hands, MSCs of breast showed strong immunohistochemical evidence of myoepithelial differentiation, often expressing basal-specic CKs. They also showed that they can coexpress specic luminal CKs, suggesting an origin from a stem cell that is capable of dual differentiation. An immunohistochemical panel composed of S100, SMA, and at least 4 CKs, including basal-specic 34E12 and CK14 or CK5, is valuable in the assessment of a suspected MSC. CD34 and, to a lesser extent, bcl-2 positivity in the stroma of PTs may be useful in differentiating them from MSC of breast, particularly in the preoperative setting. Our ndings are useful to diagnostic histopathologists in their attempt to accurately categorize these lesions. REFERENCES
1. Meis JM, Nelson Gordonez NG, Gallagher HS: Sarcomatoid carcinoma of the breast: An immunohistochemical study of six cases. Virchows Arch A 410:415-421, 1987 2. Wargotz ES, Deos OH, Norris HJ: Metaplastic carcinomas of the breast. II. Spindle cell carcinoma. Hum Pathol 20:732-740, 1989 3. Eusebi V, Cattani MG, Ceccarelli C, et al: Sarcomatoid carcinomas of the breast: An immunohistochemical study of 14 cases. Prog Surg Pathol 10:83-89, 1989 4. Ellis IO, Bell J, Ronan JE, et al: Immunocytochemical investigation of intermediate lament proteins and epithelial membrane antigen in spindle cell tumours of the breast. J Pathol 154:157-165, 1988 5. Rosen PP: Rosens Breast Pathology, 2nd ed. Philadelphia, PA, Lippincott Williams &Wilkins, 2001 6. Hamperl H: The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol 53; 161-220, 1970 7. Tavassoli FA: Myoepithelial lesions of the breast: Myoepitheliosis, adenomyoepithelioma and myoepithelial carcinoma. Am J Surg Pathol 15:554-568, 1991 8. Rosen PP: Adenomyoepithelioma of the breast. Hum Pathol 18:1232-1237, 1987 9. Kaier H, Nielsen B, Paulsen S, et al: Adenomyoepithelial adenosis and low-grade malignant adenomyoepithelioma of the breast. Virchows Arch A Pathol Anat 405:55-67, 1984 10. Loose JH, Patchefsky AS, Hoolandes IJ, et al: Adenomyoepithelioma of the breast. A spectrum of biologic behaviour. Am J Surg Pathol 16:868-876, 1992 11. Schurch W, Potvin C, Seemayer TA: Malignant myoepitheli-
oma (myoepithelial carcinoma) of the breast: An ultrastructural and immunohistochemical study. Ultrastruct Pathol 8:1-11, 1985 12. Thorner PS, Kahn HJ, Baumal R, et al: Malignant myoepithelioma of the breast. An immunohistochemical study by light and electron microscopy. Cancer 57:745-750, 1986 13. Foschini MP, Dina RE, Eusebi V: Sarcomatoid neoplasms of the breast: Proposed denitions for biphasic and monophasic sarcomatoid mammary carcinomas. Semin Diagn Pathol 10:128-136, 1993 14. Lakhani SR, OHare MJ, Monaghan P, et al: Malignant myoepithelioma (myoepithelial carcinoma) of the breast: A detailed cytokeratin study. J Clin Pathol 48:164-167, 1995 15. Foschino MP, Eusebi V: Carcinomas of the breast showing myoepithelial cell differentiation. A review of the literature. Virchows Arch 432:303-310, 1998 16. Treves N: A study of cystosarcom phyllodes. Ann N Y Acad Sci 114:922-936, 1964 17. Pietruszka M, Barnes L: Cystosarcoma phyllodes. A clinicopathological analysis of 42 cases. Cancer 41:-1983, 1978 1974 18. Ward RM, Evans HL: Cystosarcoma phyllodes. A clinicopathologic study of 26 cases. Cancer 58:2282-2289, 1986 19. Wargotz ES, Norris HJ, Austin RM, et al: Fibromatosis of the breast: A clinical and pathologic study of 28 cases. Am J Surg Pathol 11:38-47, 1987 20. Bogomoletz WV, Boulenger E, Simatos A: Inltrating bromatosis of the breast. J Clin Pathol 34:30-34, 1981 21. Purkis PE, Steel JB, Mackenzie IC, et al: Antibody markers of basal cells in complex epithelia. J Cell Sci 97:39-50s, 1990 22. Van Niekerk CC, Jap PHK, Ramaekers FCS, et al: Immunohistochemical demonstration of keratin 7 in routinely xed parafn embedded human tissues. J Pathol 165:145-152, 1991 23. Bartek J, Durban EM, Hallowes RC, et al: A subclass of luminal epithelial cells in the human mammary gland, dened by antibodies to cytokeratins. J Cell Sci 75:17-33, 1985 24. Moore T, Lee AHS: Expression of CD34 and bcl-2 in phyllodes tumours, broadenomas and spindle cell lesions of the breast. Histopathology 38:62-67, 2001 25. Silverman JS, Tamsen A: Mammary broadenoma and some phyllodes tumour stroma are composed of CD34 broblasts and factor XIIIa dendrophages. Histopathology 29:411-419, 1996 26. Keunen-Boumeester V, Henzen-Logmans SC, Timmermans MM, et al: Altered expression of p53 and its regulated proteins in phyllodes tumours of the breast. J Pathol 189:169-175, 1999 27. Moffat CJC, Pinder SE, Dixon AR, et al: Phyllodes tumours of the breast: A clinicopathologic review of thirty-two cases. Histopathology 27:205-218, 1995 28. Chieng C, Cranor M, Lesser ME, et al: Metaplastic carcinoma of the breast with osteocartilagenous heterologous elements. Am J Surg Pathol 22:188-194, 1998 29. Wargotz ES, Norris HJ: Metaplastic carcinomas of the breast. I. Matrix-producing carcinoma. Hum Pathol 20:628-635s, 1989 30. Wargotz ES, Norris HJ: Metaplastic carcinoma of the breast. III. Carcinosarcoma. Cancer 64:1490-1499, 1989 31. Reis-Filho JS, Milanezi F, Paredes J, et al: Novel and classic myoepithelial/stem cell markers in metaplastic carcinomas of the breast. Appl Immunohistochem Mol Morphol 11:1-8, 2003 32. Kaufman MW, Marti JR, Gallager HS, et al. Carcinoma of the breast with pseudosarcomatous metaplasia. Cancer 53:-1917, 1984 1908 33. Kung FYL, Tse GMK, Lo KW, et al: Metachronous bilateral mammary metaplastic and inltrating duct carcinomas: A molecular study for clonality. Hum Pathol 33:677-679, 2002 34. Oberman HA: Metaplastic carcinoma of the breast. A clinicopathologic study of 29 patients. Am J Surg Pathol 11:918-929, 1987 35. Sneige N, Yaziji H, Mandavilli SR, et al: Low-grade (bromatosis-like) spindle cell carcinoma of the breast. Am J Surg Pathol 25:1009-1016, 2001
1015
Anda mungkin juga menyukai
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- Histogenesis Pleomorphic AdenomaDokumen6 halamanHistogenesis Pleomorphic AdenomaAsmara SyedBelum ada peringkat
- Surgical Pathology Unknown Case Conference 3/26/07Dokumen22 halamanSurgical Pathology Unknown Case Conference 3/26/07Asmara SyedBelum ada peringkat
- ThymomasDokumen6 halamanThymomasAsmara SyedBelum ada peringkat
- Spindle Cell Lesions BreastDokumen6 halamanSpindle Cell Lesions BreastAsmara SyedBelum ada peringkat
- Keratoacanthoma DDXDokumen1 halamanKeratoacanthoma DDXAsmara SyedBelum ada peringkat
- Sclerosing PerineuromaDokumen7 halamanSclerosing PerineuromaAsmara SyedBelum ada peringkat
- Immunohistochemical MarkersDokumen12 halamanImmunohistochemical MarkersAsmara SyedBelum ada peringkat
- Myxofibrosarcoma GradingDokumen2 halamanMyxofibrosarcoma GradingAsmara Syed0% (1)
- Difficult Patterns in HistopathologyDokumen8 halamanDifficult Patterns in HistopathologyAsmara SyedBelum ada peringkat
- The Heart 16th March 13Dokumen105 halamanThe Heart 16th March 13Asmara SyedBelum ada peringkat
- Esophagus GrossDokumen7 halamanEsophagus GrossAsmara SyedBelum ada peringkat
- 2991 10414 1 PBDokumen5 halaman2991 10414 1 PBAsmara SyedBelum ada peringkat
- AtherosclerosisDokumen52 halamanAtherosclerosisAsmara SyedBelum ada peringkat
- RPReview 3Dokumen22 halamanRPReview 3Asmara SyedBelum ada peringkat
- Acute Inflammation ExudateDokumen140 halamanAcute Inflammation ExudateAsmara SyedBelum ada peringkat
- EdemaDokumen60 halamanEdemaAsmara SyedBelum ada peringkat
- Kidney Structure and FunctionDokumen68 halamanKidney Structure and FunctionAsmara Syed100% (1)
- IhdDokumen73 halamanIhdAsmara SyedBelum ada peringkat
- Respiratory Tract PathologyDokumen154 halamanRespiratory Tract PathologyAsmara SyedBelum ada peringkat
- Esophagus WorkshopDokumen48 halamanEsophagus WorkshopAsmara SyedBelum ada peringkat
- Cutaneous MalignanciesDokumen88 halamanCutaneous MalignanciesAsmara SyedBelum ada peringkat
- Ectopic MeningiomaDokumen4 halamanEctopic MeningiomaAsmara SyedBelum ada peringkat
- T - Cell LymphomasDokumen40 halamanT - Cell LymphomasAsmara SyedBelum ada peringkat
- Allred ScoreDokumen1 halamanAllred ScoreAsmara SyedBelum ada peringkat
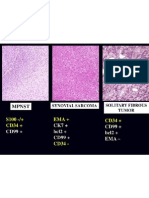
- MPNST Vs Synovial SarcomaDokumen1 halamanMPNST Vs Synovial SarcomaAsmara SyedBelum ada peringkat
- Her2 StainingDokumen11 halamanHer2 StainingAsmara SyedBelum ada peringkat
- Copy Stomach WorkshopDokumen86 halamanCopy Stomach WorkshopAsmara SyedBelum ada peringkat
- Beautiful DesignDokumen1 halamanBeautiful DesignAsmara SyedBelum ada peringkat
- Cutaneous Soft Tissue TumorsDokumen44 halamanCutaneous Soft Tissue TumorsAsmara SyedBelum ada peringkat
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Different Pfemp1-Expressing Plasmodium Endothelial Transcriptional Responses During Co-CultureDokumen27 halamanDifferent Pfemp1-Expressing Plasmodium Endothelial Transcriptional Responses During Co-CultureBerita TerkiniBelum ada peringkat
- L6-Steczina, Sonette BIOEN345 Lecture 20200415Dokumen22 halamanL6-Steczina, Sonette BIOEN345 Lecture 20200415asdfghjklBelum ada peringkat
- Gizmo Cell Structure FormDokumen3 halamanGizmo Cell Structure FormDeyanat AliBelum ada peringkat
- Cell Structure ActivityDokumen3 halamanCell Structure ActivityCogie PeraltaBelum ada peringkat
- Liver Fibrosis Causes and Methods of Assessment ADokumen11 halamanLiver Fibrosis Causes and Methods of Assessment AmichaelBelum ada peringkat
- Biocomp Exam 2009Dokumen12 halamanBiocomp Exam 2009martynapet100% (1)
- Growth Factor For Bone RegenerationDokumen17 halamanGrowth Factor For Bone RegenerationOluwasegun ModupeBelum ada peringkat
- Molecular and Biological Compatibility With Host Alpha-Synuclein Influences Fibril PathogenicityDokumen16 halamanMolecular and Biological Compatibility With Host Alpha-Synuclein Influences Fibril PathogenicityKantiaeBelum ada peringkat
- School of Food Science and Environmental Health Lab Report Gram StainDokumen8 halamanSchool of Food Science and Environmental Health Lab Report Gram StainOliaBelum ada peringkat
- DR P Gupta ImtechDokumen4 halamanDR P Gupta ImtechaaimpossibleBelum ada peringkat
- Metabolic Response in InjuryDokumen27 halamanMetabolic Response in InjuryAhmed Kh. Abu Warda100% (1)
- Group 5 - Experiment No.9 - Preparation of Bacterial Smear and Gram StainingDokumen10 halamanGroup 5 - Experiment No.9 - Preparation of Bacterial Smear and Gram StainingPMG BrightBelum ada peringkat
- Lecture 4 Technical Aspects of Cell Biology 2Dokumen21 halamanLecture 4 Technical Aspects of Cell Biology 2Jana Moataz Mohamed G02Belum ada peringkat
- Xi Botany em MLM-1Dokumen48 halamanXi Botany em MLM-1prabhu100% (1)
- @MedicalBooksStoreS 2017 Acute IschemicDokumen273 halaman@MedicalBooksStoreS 2017 Acute IschemicAditya Perdana Dharma Wiguna100% (1)
- The Role of Cytokines in Orthodontic Tooth MovemenDokumen17 halamanThe Role of Cytokines in Orthodontic Tooth MovemenvivigaitanBelum ada peringkat
- Science 7 - Summative Test - Q2 - PrintDokumen2 halamanScience 7 - Summative Test - Q2 - PrintLenette AlagonBelum ada peringkat
- Junctional EpitheliumDokumen88 halamanJunctional Epitheliumshashi kant chaudhary100% (3)
- Discussion Bio460Dokumen2 halamanDiscussion Bio460MUHAMMAD SYAHMI BIN AZMIBelum ada peringkat
- Quarter 1 - GenBio 1-Week 1 (23-24)Dokumen41 halamanQuarter 1 - GenBio 1-Week 1 (23-24)Adrienne Skyle SibalaBelum ada peringkat
- DIagnosis of Neonatal Sepsis - Past, Present and FutureDokumen14 halamanDIagnosis of Neonatal Sepsis - Past, Present and FuturedrdrBelum ada peringkat
- 04 - 2reproduction in ProtozoaDokumen5 halaman04 - 2reproduction in ProtozoaDipankar RoyBelum ada peringkat
- Building an Epigenetics ModelDokumen2 halamanBuilding an Epigenetics ModelAshley ArnoldBelum ada peringkat
- Polyphony: Superposition Independent Methods For Ensemble-Based Drug DiscoveryDokumen19 halamanPolyphony: Superposition Independent Methods For Ensemble-Based Drug DiscoveryIarinaBelum ada peringkat
- Test Bank For Human Anatomy 9th Edition by MartiniDokumen31 halamanTest Bank For Human Anatomy 9th Edition by MartiniAnthony Cohen100% (38)
- Bio411 C1Dokumen1 halamanBio411 C1Aqiena BalqisBelum ada peringkat
- Metabolism of Xenobiotics: A Two-Phase ProcessDokumen4 halamanMetabolism of Xenobiotics: A Two-Phase ProcessChingHuaBelum ada peringkat
- Genesis PRP (En) - 160129Dokumen35 halamanGenesis PRP (En) - 160129Andrea BiancaBelum ada peringkat
- Immunological BioinformaticsDokumen332 halamanImmunological BioinformaticsDiana GabrielaBelum ada peringkat
- Endocrine 1 PageDokumen3 halamanEndocrine 1 PagetimbitBelum ada peringkat