Ischemic Heart Disease
Diunggah oleh
April Joy Villacorta PonceHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Ischemic Heart Disease
Diunggah oleh
April Joy Villacorta PonceHak Cipta:
Format Tersedia
What is Ischemic Heart Disease?
Ischemic heart disease is caused by an imbalance between the myocardial blood flow and the metabolic demand of the myocardium. Reduction in coronary blood flow is related to progressive atherosclerosis with increasing occlusion of coronary arteries. Blood flow can be further decreased by superimposed events such as vasospasm, thrombosis, or circulatory changes leading to hypoperfusion. (Anversa and Sonnenblick, 1990) Coronary artery perfusion depends upon the pressure differential between the ostia (aortic diastolic pressure) and coronary sinus (right atrial pressure). Coronary blood flow is reduced during systole because of Venturi effects at the coronary orifices and compression of intramuscular arteries during ventricular contraction. Factors reducing coronary blood flow include: 1. Decreased aortic diastolic pressure 2. Increased intraventricular pressure and myocardial contraction 3. Coronary artery stenosis, which can be further subdivided into the following etiologies: o Fixed coronary stenosis o Acute plaque change (rupture, hemorrhage) o Coronary artery thrombosis o Vasoconstriction 4. Aortic valve stenosis and regurgitation 5. Increased right atrial pressure 40 micron collateral vessels are present in all hearts with pressure gradients permitting flow, despite occlusion of major vessels. In general, the cross-sectional area of the coronary artery lumen must be reduced by more than 75% to significantly affect perfusion. Coronary atherosclerosis is diffuse (involving more than one major arterial branch) but is often segmental, and typically involves the proximal 2 cm of arteries (epicardial). (Anversa et al, 1995) "Thrombolytic therapy" with agents such as streptokinase or tissue plasminogen activatorS (TPA) such as atelpase is often used within the first 12 hours following onset of symptoms and with ST-segment elevation to try and lyse a recently formed thrombus. Such therapy with lysis of the thrombus can re-establish blood flow in a majority of cases. This helps to prevent significant myocardial injury, if early in the course of events, and can at least help to reduce further damage. (Kumar and Cannon, Part II, 2009) Images of coronary artery disease: 1. 2. 3. 4. Normal coronary artery, microscopic. Coronary atherosclerosis, cross sections, gross. Coronary atherosclerosis, minimal, gross. Coronary atherosclerosis, severe, gross.
5. Coronary atherosclerosis, composite, microscopic. 6. Coronary atherosclerosis, intimal plaque, microscopic. 7. Coronary atherosclerosis, complicated by calcification, microscopic. 8. Coronary atherosclerosis, occlusive, microscopic. 9. Coronary atherosclerosis, occlusive, microscopic. 10. Coronary artery, hemorrhage into plaque, gross. 11. Coronary artery, atheromatous plaque with disrupted fibrin cap, microscopic. 12. Thrombosis of coronary artery, gross. 13. Thrombosis of coronary artery, gross. 14. Thrombosis of coronary artery, microscopic. 15. Thrombosis of coronary artery, microscopic.
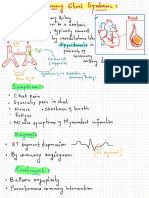
Patterns of Ischemic Heart Disease (IHD) with Acute Coronary Syndromes
Acute coronary syndromes include several patterns (Kumar and Cannon, Part I, 2009): 1. Unstable angina: there is no ST-segment change and there is not sufficient myocardial damage for for release of a biomarker such as the troponins or CKMB. There is one or more of the following: (1) rest angina, (2) new-onset severe angina, and (3) a crescendo pattern of occurrence. 2. Non-ST-segment Elevation Myocardial Infarction (NSTEMI): there is no STsegment change but there is myocardial necrosis for release of a biomarker such as the troponins or CK-MB. 3. ST-segment Elevation Myocardial Infarction (STEMI): there is ST-segment elevation and myocardial necrosis with release of a biomarker such as the troponins or CK-MB.
Myocardial Infarction (MI)
The pathogenesis can include:
Occlusive intracoronary thrombus - a thrombus overlying an plaque causes 75% of myocardial infarctions, with superficial plaque erosion present in the remaining 25%. Vasospasm - with or without coronary atherosclerosis and possible association with platelet aggregation. Emboli - from left sided mural thrombosis, vegetative endocarditis, or paradoxic emboli from the right side of heart through a patent foramen ovale.
In 2000, the European Society of Cardiology and the American College of Cardiology Consensus group redefined myocardial infarction, with the definition being based on myocyte necrosis as determined by troponins in the clinical setting of ischaemia. (White and Chew, 2008) The molecular events during MI relate to the initial ischemic event, reperfusion, and subsequent inflammatory response. Up to 6 hours following the initial ischemic event,
most cell loss occur via apoptosis. After that, necrosis predominates. Ischemic endothelial cells express adhesion molecules that attract neutrophils that subsequently migrate into damaged myocardium. The gross morphologic appearance of a myocardial infarction can vary. Patterns include:
Transmural infarct - involving the entire thickness of the left ventricular wall from endocardium to epicardium, usually the anterior free wall and posterior free wall and septum with extension into the RV wall in 15-30%. Isolated infarcts of RV and right atrium are extremely rare. Subendocardial infarct - multifocal areas of necrosis confined to the inner 1/3-1/2 of the left ventricular wall. These do not show the same evolution of changes seen in a transmural MI.
Gross morphologic changes evolve over time as follows: Time from Onset 18 - 24 Hours 24 - 72 Hours 3 - 7 Days 10 - 21 Days 7 weeks Gross Morphologic Finding Pallor of myocardium Pallor with some hyperemia Hyperemic border with central yellowing Maximally yellow and soft with vascular margins White fibrosis
Microscopic morphologic changes evolve over time as follows: Time from Onset 1 - 3 Hours 2 - 3 Hours 4 - 12 Hours 18 - 24 Hours
Microscopic Morphologic Finding Wavy myocardial fibers Staining defect with tetrazolium or basic fuchsin dye Coagulation necrosis with loss of cross striations, contraction bands, edema, hemorrhage, and early neutrophilic infiltrate Continuing coagulation necrosis, pyknosis of nuclei, and marginal contraction bands
24 - 72 Hours Total loss of nuclei and striations along with heavy neutrophilic infiltrate 3 - 7 Days Macrophage and mononuclear infiltration begin, fibrovascular response begins
10 - 21 Days 7 Weeks
Fibrovascular response with prominent granulation tissue Fibrosis
The above gross and microscopic changes over time can vary. In general, a larger infarct will evolve through these changes more slowly than a small infarct. Clinical complications of myocardial infarction will depend upon the size and location of the infarction, as well as pre-existing myocardial damage. Complications can include:
Arrhythmias and conduction defects, with possible "sudden death" Extension of infarction, or re-infarction Congestive heart failure (pulmonary edema) Cardiogenic shock Pericarditis Mural thrombosis, with possible embolization Myocardial wall rupture, with possible tamponade Papillary muscle rupture, with possible valvular insufficiency Ventricular aneurysm formation
Sudden death occurs within an hour of onset of symptoms. Such an occurrence often complicates ischemic heart disease. Such patients tend to have severe coronary atherosclerosis (>75% lumenal narrowing). Often, a complication such as coronary thrombosis or plaque hemorrhage or rupture has occurred. The mechanism of death is usually an arrhythmia.
Ischemic Cardiomyopathy
In this condition, there may be previous myocardial infarction, but the disease results from severe coronary atherosclerosis involving all major branches. The result is an inadequate vascular supply which leads to myocyte loss. The myocyte loss coupled with fibrosis in the form of interstitial collagen deposition results in decreased compliance, which along with the accompanying cardiac dilation, results in overload of remaining myocytes. This keeps the process going, with compensation by continuing myocyte hypertrophy. There may even be compensation through hyperplasia as well as hypertrophy, which can explain the enormous size (2 to 3 times normal size) of the resulting heart. Eventually, the heart can no longer compensate, and cardiac failure ensues with arrhythmias and/or ischemic events. There is slow, progressive heart failure with or without a history of a previous MI or anginal pain. Ischmic cardiomyopathy is responsible for as much as 40% of the mortality in IHD. (Anversa et al, 1995) Images of myocardial injury: 1. Normal myocardium, microscopic. 2. Early acute myocardial infarction (<12 hours) with loss of cross striations, microscopic.
3. Early acute myocardial infarction (<1 day) with contraction band necrosis, microscopic. 4. Acute myocardial infarction (1 - 2 days) with early neutrophilic infiltrate, microscopic. 5. Acute myocardial infarction (1 - 2 days), hyperemic border, microscopic. 6. Acute myocardial infarction (3 - 4 days), extensive neutrophilic infiltrate, microscopic. 7. Acute myocardial infarction, gross. 8. Acute myocardial infarction, gross. 9. Acute myocardial infarction with rupture, gross. 10. Acute myocardial infarction with rupture and tamponade, gross. 11. Intermediate (healing) myocardial infarction (2 - 3 weeks), microscopic. 12. Remote myocardial infarction (weeks to years), microscopic. 13. Remote myocardial infarction (weeks to years), microscopic. 14. Remote myocardial infarction (weeks to years), gross. 15. Left ventricular aneurysm, gross. 16. Left ventricular aneurysm containing mural thrombus, gross. 17. Ischemic cardiomyopathy, microscopic.
Laboratory Diagnosis of Myocardial Infarction
A number of laboratory biomarkers for myocardial injury are available. None is completely sensitive and specific for myocardial infarction, particularly in the hours following onset of symptoms. Timing is important, as are correlation with patient symptoms, electrocardiograms, and angiographic studies. The following biomarkers have been described in association with acute myocardial infarction: Creatine Kinase - Total: The total CK is a simple and inexpensive test that is readily available using many laboratory instruments. However, an elevation in total CK is not specific for myocardial injury, because most CK is located in skeletal muscle, and elevations are possible from a variety of non-cardiac conditions. (Chattington et al, 1994) Creatine Kinase - MB Fraction: Creatine kinase can be further subdivided into three isoenzymes: MM, MB, and BB. The MM fraction is present in both cardiac and skeletal muscle, but the MB fraction is much more specific for cardiac muscle: about 15 to 40% of CK in cardiac muscle is MB, while less than 2% in skeletal muscle is MB. The BB fraction (found in brain, bowel, and bladder) is not routinely measured. The creatine kinase-MB fraction (CK-MB) is part of total CK and more specific for cardiac muscle that other striated muscle. It tends to increase within 3 to 4 hours of myocardial necrosis, then peak in a day and return to normal within 36 hours. It is less sensitive than troponins. (Saenger and Jaffe, 2007) (Kumar and Cannon, Part I, 2009)
The CK-MB is also useful for diagnosis of reinfarction or extensive of an MI because it begins to fall after a day, so subsequent elevations are indicative of another event. (Chattington et al, 1994) Troponins: Troponin I and T are structural components of cardiac muscle. They are released into the bloodstream with myocardial injury. They are highly specific for myocardial injury-more so than CK-MB--and help to exclude elevations of CK with skeletal muscle trauma. Troponins will begin to increase following MI within 3 to 12 hours, about the same time frame as CK-MB. However, the rate of rise for early infarction may not be as dramatic as for CK-MB. Troponins will remain elevated longer than CK--up to 5 to 10 days for troponin I and up to 2 weeks for troponin T. This makes troponins a superior marker for diagnosing myocardial infarction in the recent past--better than lactate dehydrogenase (LDH). However, this continued elevation has the disadvantage of making it more difficult to diagnose reinfarction or extension of infarction in a patient who has already suffered an initial MI. Troponin T lacks some specificity because elevations can appear with skeletal myopathies and with renal failure. (Kost et al, 1998) (Kumar and Cannon, Part I, 2009) Myoglobin: Myoglobin is a protein found in skeletal and cardiac muscle which binds oxygen. It is a very sensitive indicator of muscle injury. However, it is not specific for cardiac muscle, and can be elevated with any form of injury to skeletal muscle. The rise in myoglobin can help to determine the size of an infarction. A negative myoglobin can help to rule out myocardial infarction. It is elevated even before CK-MB. (Kumar and Cannon, Part I, 2009) BNP: B-type natriuretic peptide (BNP) is released from ventricular myocardium. BNP release can be stimulated by systolic and diastolic left ventricular dysfunction, acute coronary syndromes, stable coronary heart disease, valvular heart disease, acute and chronic right ventricular failure, and left and right ventricular hypertrophy secondary to arterial or pulmonary hypertension. BNP is a marker for heart failure. (Saenger and Jaffe, 2007) CRP: C-reactive protein (CRP) is an acute phase protein elevated when inflammation is present. Since inflammation is part of atheroma formation, then CRP may reflect the extent of atheromatous plaque formation and predict risk for acute coronary events. However, CRP lacks specificity for vascular events. (Saenger and Jaffe, 2007)
Anda mungkin juga menyukai
- Training Manual For Medical RepresentativesDokumen74 halamanTraining Manual For Medical RepresentativesAman Ghayas50% (4)
- OCR AS Biology Unit 1 Revision NotesDokumen22 halamanOCR AS Biology Unit 1 Revision NotesLydiaaxoxBelum ada peringkat
- Ischemic Heart DiseaseDokumen67 halamanIschemic Heart Diseasealfaz lakhani80% (5)
- Anatomy Myocardial InfarctionDokumen5 halamanAnatomy Myocardial InfarctionLyka Milo AvilaBelum ada peringkat
- Cardiac SystemDokumen7 halamanCardiac Systemsccctutor100% (3)
- Cardiogenic Shock HandoutsDokumen2 halamanCardiogenic Shock HandoutsAileenBelum ada peringkat
- Acute Myocardial InfarctionDokumen14 halamanAcute Myocardial InfarctionJardee DatsimaBelum ada peringkat
- Myocardial InfarctionDokumen20 halamanMyocardial Infarction* mokhtar !!50% (2)
- Lecture 2 Ischemic Heart DiseasesDokumen19 halamanLecture 2 Ischemic Heart DiseasesOsama MalikBelum ada peringkat
- CardiomyopathyDokumen98 halamanCardiomyopathyZellanien hdBelum ada peringkat
- STROKE: Handbook with activities, exercises and mental challengesDari EverandSTROKE: Handbook with activities, exercises and mental challengesBelum ada peringkat
- Pathology+101 Complete)Dokumen147 halamanPathology+101 Complete)Goh Kah Yong100% (2)
- Sim LabDokumen4 halamanSim Laballycat2390% (10)
- Sun Simiao - Recipes Worth A Thousand GoldDokumen56 halamanSun Simiao - Recipes Worth A Thousand Goldnqngestion100% (3)
- Ischemic Heart DiseaseDokumen5 halamanIschemic Heart DiseaseBert DivinagraciaBelum ada peringkat
- Harrison Self-Assessment and Board Review (1) - 403-490Dokumen88 halamanHarrison Self-Assessment and Board Review (1) - 403-490Cristobal Andres Fernandez Coentrao100% (2)
- ACN Unit3Dokumen35 halamanACN Unit3Kamran AltafBelum ada peringkat
- Myocardial Infarction AssignmentDokumen23 halamanMyocardial Infarction AssignmentPums100% (6)
- SAB HLTAAP001 Recognise Healthy Body SystemsDokumen67 halamanSAB HLTAAP001 Recognise Healthy Body SystemsBbas T.95% (20)
- Pathology of Cardiovascular SystemDokumen75 halamanPathology of Cardiovascular Systemefri100% (2)
- Atheroma: 2 History of ResearchDokumen7 halamanAtheroma: 2 History of ResearchZiedTrikiBelum ada peringkat
- Cardio 1stSemSY2014-15 Course SyllabusDokumen6 halamanCardio 1stSemSY2014-15 Course SyllabusBeryl Ben MergalBelum ada peringkat
- Posterior and Anterior MediastinumDokumen6 halamanPosterior and Anterior MediastinumYheng GaosaiiBelum ada peringkat
- IntroductionDokumen13 halamanIntroductionSiyara AntonyBelum ada peringkat
- Pathology of Acute Myocardial InfarctionDokumen13 halamanPathology of Acute Myocardial InfarctionNaily Nuzulur RohmahBelum ada peringkat
- Acute Coronary Syndrome: Aminah DalimuntheDokumen48 halamanAcute Coronary Syndrome: Aminah Dalimunthesintesis obatBelum ada peringkat
- 6 - Ischemic Heart DiseaseDokumen38 halaman6 - Ischemic Heart DiseaseHamzehBelum ada peringkat
- CVS 4 PDFDokumen24 halamanCVS 4 PDFafaq alismailiBelum ada peringkat
- Ischaemic Heart Disease (IHD) Is Defined As Acute or Chronic Form of Cardiac Disability Arising FromDokumen5 halamanIschaemic Heart Disease (IHD) Is Defined As Acute or Chronic Form of Cardiac Disability Arising FromIsak ShatikaBelum ada peringkat
- Acute Coronary SyndromesDokumen5 halamanAcute Coronary SyndromesHutomo Budi Hasnian SyahBelum ada peringkat
- Acute Coronary Syndrome ACSDokumen9 halamanAcute Coronary Syndrome ACSChen BrionesBelum ada peringkat
- نسخة HeartDokumen29 halamanنسخة HeartKyunaBelum ada peringkat
- Acute Myocardia L Infarction: Subtit LEDokumen33 halamanAcute Myocardia L Infarction: Subtit LEGabiiGabriiela100% (1)
- 3 IschemicHeartDiseasesDokumen17 halaman3 IschemicHeartDiseasesHarsha MaheshwariBelum ada peringkat
- Pathology Case Study - EvangelistaDokumen17 halamanPathology Case Study - EvangelistaAubrey Unique EvangelistaBelum ada peringkat
- Disorders of Cardiac FunctionDokumen60 halamanDisorders of Cardiac FunctionSaif AliBelum ada peringkat
- StemiDokumen7 halamanStemiAsri ParamythaBelum ada peringkat
- Acute Coronary SyndromeDokumen45 halamanAcute Coronary SyndromeParsa EbrahimpourBelum ada peringkat
- Myocardial InfarctionDokumen6 halamanMyocardial InfarctionMaicie Rose Bautista VallesterosBelum ada peringkat
- Pathology & Microbiology Chapter C.V.S Disorders: Lecture By: Dr. Bari MengalDokumen14 halamanPathology & Microbiology Chapter C.V.S Disorders: Lecture By: Dr. Bari Mengalaza bellaBelum ada peringkat
- How To Diagnose STEMI?Dokumen6 halamanHow To Diagnose STEMI?Adhie BadriBelum ada peringkat
- Tinywow - L.4 Ischemic Cardiomyopathy - 28139370Dokumen19 halamanTinywow - L.4 Ischemic Cardiomyopathy - 28139370filymascoloBelum ada peringkat
- Clinicas CardioDokumen5 halamanClinicas CardioNatali Guzman CortezBelum ada peringkat
- Myocardial Infarction: Seminar OnDokumen17 halamanMyocardial Infarction: Seminar OnSuhas IngaleBelum ada peringkat
- Myocardial InfarctionDokumen19 halamanMyocardial InfarctionkiflomBelum ada peringkat
- Cardiovascular Systems - 2 (Blood and Lymphatic Vessel)Dokumen37 halamanCardiovascular Systems - 2 (Blood and Lymphatic Vessel)RezaBelum ada peringkat
- Infarction PDFDokumen24 halamanInfarction PDFAmandeep Kaur ChabbraBelum ada peringkat
- PATHO-II Unit V Cardiovascular DisordersDokumen52 halamanPATHO-II Unit V Cardiovascular DisordersMuhammad ShayanBelum ada peringkat
- Myocardial InfarctionDokumen3 halamanMyocardial InfarctionMarah NillascaBelum ada peringkat
- Acute Coronary Syndrome: A Leading Cause of Mortality: Essence of This ArticleDokumen5 halamanAcute Coronary Syndrome: A Leading Cause of Mortality: Essence of This ArticletangledprincessBelum ada peringkat
- Acute StrokeDokumen13 halamanAcute StrokeJoel CanenciaBelum ada peringkat
- Pathophysiology Worksheet MIDokumen2 halamanPathophysiology Worksheet MIpjbedelBelum ada peringkat
- Coronay Artery Disease: Dr. Bibek Poudel Department of Medicine City Medical College HospitalDokumen22 halamanCoronay Artery Disease: Dr. Bibek Poudel Department of Medicine City Medical College HospitalitsmailbbkBelum ada peringkat
- Atherosclerosis and Myocardial InfractionDokumen26 halamanAtherosclerosis and Myocardial InfractionElina GBelum ada peringkat
- Angina Pectoris Myocardial Infarction: Bahuguna, Nimisha Imd-Batch 3Dokumen19 halamanAngina Pectoris Myocardial Infarction: Bahuguna, Nimisha Imd-Batch 3Nimisha BahugunaBelum ada peringkat
- 1.old Posterior-WPS OfficeDokumen6 halaman1.old Posterior-WPS Officeashokyd1411Belum ada peringkat
- Class 17Dokumen46 halamanClass 17Nishani SatiyaseelanBelum ada peringkat
- Ischaemic Heart Disease: EtiopathogenesisDokumen12 halamanIschaemic Heart Disease: Etiopathogenesisritika shakkarwalBelum ada peringkat
- Ischemic StrokeDokumen17 halamanIschemic StrokejamalBelum ada peringkat
- DR Dhiman BanikCariogenic Shock Final 2022 DDDokumen59 halamanDR Dhiman BanikCariogenic Shock Final 2022 DDCloudySkyBelum ada peringkat
- Acute Coronary SyndromeDokumen12 halamanAcute Coronary SyndromeAnamaria SBelum ada peringkat
- Pathology EssayDokumen19 halamanPathology EssayARUNSKBelum ada peringkat
- Shock, Arrest, Syncope PhysiopathologyDokumen54 halamanShock, Arrest, Syncope PhysiopathologyParsa EbrahimpourBelum ada peringkat
- Cardiovascular Disease: Coronary Heart Disease Refers To The Failure of The Coronary Circulation To Supply AdequateDokumen4 halamanCardiovascular Disease: Coronary Heart Disease Refers To The Failure of The Coronary Circulation To Supply AdequateAnant Kumar SrivastavaBelum ada peringkat
- Acute Mesenteric Arterial OcclusionDokumen28 halamanAcute Mesenteric Arterial OcclusionJaime Jose Ortiz Andrade100% (2)
- Pathophys VHD-Final (Budi S. Pikir)Dokumen155 halamanPathophys VHD-Final (Budi S. Pikir)Muhammad DaviqBelum ada peringkat
- Acute Myocardial InfarctionDokumen36 halamanAcute Myocardial InfarctionTa HiraBelum ada peringkat
- Ischemic Heart Disease: Dr. Pearl Myers Path Fall 2011 With Acknowledgement To DR SADokumen67 halamanIschemic Heart Disease: Dr. Pearl Myers Path Fall 2011 With Acknowledgement To DR SADylan KremerBelum ada peringkat
- Circulatory System FKM'12Dokumen55 halamanCirculatory System FKM'12muhammadsudrajadBelum ada peringkat
- Vital SIgns - First Responder - August 2013 - v2.3Dokumen18 halamanVital SIgns - First Responder - August 2013 - v2.3Rayan JaafeerBelum ada peringkat
- What Is CardiomyopathyDokumen8 halamanWhat Is CardiomyopathysakuraleeshaoranBelum ada peringkat
- Term 1 Questions - Life ProcessesDokumen30 halamanTerm 1 Questions - Life ProcessesAmbitious StudentBelum ada peringkat
- PacemakerDokumen13 halamanPacemakeralainzkie100% (2)
- 03 IjnhDokumen4 halaman03 Ijnhalev palantökenBelum ada peringkat
- Ecg - AclsDokumen338 halamanEcg - AclsPete Cobra CobraitiBelum ada peringkat
- Lab08 Frog HeartDokumen4 halamanLab08 Frog HeartAbie CaponponBelum ada peringkat
- Chapter 10 - Web Quiz 1Dokumen4 halamanChapter 10 - Web Quiz 1Nicky PhakathiBelum ada peringkat
- 8c99f2 PDFDokumen52 halaman8c99f2 PDFKrisParadajsBelum ada peringkat
- Diagnostic Pacing Maneuvers For Supraventricular Tachycardia - Part 2Dokumen13 halamanDiagnostic Pacing Maneuvers For Supraventricular Tachycardia - Part 2aafagihBelum ada peringkat
- Focused Ultrasonography For Septic Shock ResuscitationDokumen7 halamanFocused Ultrasonography For Septic Shock ResuscitationntnquynhproBelum ada peringkat
- 9 Heart MuscleDokumen31 halaman9 Heart Muscleshoeb4uBelum ada peringkat
- Immunology Tonsil 2Dokumen31 halamanImmunology Tonsil 2ariienndrrahanniie100% (1)
- Ginkgo Biloba - Mother Tincture - ABC Homeopathy ForumDokumen2 halamanGinkgo Biloba - Mother Tincture - ABC Homeopathy Forumcet.ranchi7024Belum ada peringkat
- 1 8 KardiologiaDokumen78 halaman1 8 KardiologiaRati ZivzivadzeBelum ada peringkat
- NCM 118 L 3rd ExamDokumen3 halamanNCM 118 L 3rd Examj UBelum ada peringkat
- Coronary Steal SyndromeDokumen3 halamanCoronary Steal SyndromeEdward XiamBelum ada peringkat
- CardiologyDokumen29 halamanCardiologyVimal NishadBelum ada peringkat
- Integrated Control of The Cardiovascular SystemDokumen5 halamanIntegrated Control of The Cardiovascular SystemPatricia SanchezBelum ada peringkat