James Lee Enzyme Kinetics Solution
Diunggah oleh
VPrasarnth RaajDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
James Lee Enzyme Kinetics Solution
Diunggah oleh
VPrasarnth RaajHak Cipta:
Format Tersedia
BK10110302 V.
PRASARNTH RAAJ VEERA RAO BIOPROCESS
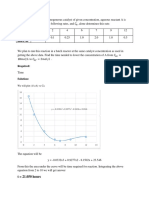
Solution 2.1
Total volume = 44 + 5 + 1= 50ml
From the graph the equation obtained
y=0.033 + 0.03a 1
m= 0.033 mol / ml . min
a) Activity of the - glucosidase
0.033 x 50= 1.65 mumol / min
i)
= 1.65mumol/min
0.1 mg/ml x 0.1ml
= 165 units/mg protein
ii)
= 1.65 mumol/min
1ml of enzyme
= 1.65 units/ml of enzyme
b) Initial rate of reaction 0.033 mumol/ mL.min
S vs t Graph
1.2
y = 0.033x + 0.0391
1
0.8
0.6
0.4
0.2
0
0
10
15
20
25
30
35
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.2
a) Michaelis-Menten approach
The rate of product formation.
d[p]
dt
Since the enzyme is preferred,
Make E as the subject,
Since forward reaction = backward reaction.
( )
ubstitute
into
:
[(
(
Make
) (
)( )
)] ( )
)( )
)( )
as a subject:
(
)( )
)( )
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
(
)( )
)( )
[(
(
(
(
ub
into
)( )
)( )
)( )
)( )( )
)( )( )
as a subject,
(
)(
( )( ))
)
(
into
)( )( )
)( )( )
)(
)
(
ub
)( )
)
Make
)( )]
( )( ))
)( )( )
( )
( )( )
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
d[p]
dt
Since
)( )( )
)
( )
( )( )
)( )( )
)
( )
( )( )
)
( )( )
)
( )
( )( )
b) Since [ ]
[ ]
d[p]
dt
( )( )
( )( )
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.3
(a) E+S k1
(ES)1
(ES)1 k3 (ES)2
(ES)2 k2 E+P
V=
[ ]
= k5 [ES] 2
[E0] = [E] + [ES] + [ES]2
[E] = [E0]-[ES]-[ES]2
k2 = [E] [S]
k1 [ES]1
k2 [ES]1 = [Eo] [S] [ES]1 [S]
k1
[ES]1 ( k2 + [S] ) = [E0] [S] [ES]2 [S]
k1
[ES]1 = [E0] [S] [ES]2 [S]
k2 + [S]
k1
k4 = [ES]1
k3
[ES]2
k4 [ES]2 = [E0] [S] [ES]2 [S]
k3
k2 + [S]
k1
[ES]2 ( k2 k4 + k4 [S] ) = [E0] [S] [ES]2 [S]
k1k3
k3
[ES]2 ( k2 k4 + k4 [S] + [S] )= [E0] [S]
k1 k3
k3
[ES]2 = [E0] [S]
k2 k4 + k4 [S] + [S]
k1 k3
k3
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
V= d [P] = k5 [E0] [S]
dt
k2 k4 + k4 [S] + [S]
k1 k3
k3
=
Vm [S]
k2 k4 + k4 [S] + [S]
k1 k3
k3
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.4
a) Michaelis-Menten approach
The rate of product formation.
d[p]
dt
Since the enzyme is preferred,
Make E as the subject,
Since forward reaction = backward reaction.
( )
ubstitute
into
:
[(
Make
) (
)( )
)] ( )
)( )
)( )
as a subject:
(
)( )
)( )
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
(
)( )
)( )
[(
(
(
(
ub
into
)( )
)( )
)( )
)( )( )
)( )( )
as a subject,
(
)(
( )( ))
)
(
into
)( )( )
)( )( )
)(
)
(
ub
)( )
)
Make
)( )]
( )( ))
)( )( )
( )
( )( )
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
d[p]
dt
Since
)( )( )
)
( )
( )( )
)( )( )
)
( )
( )( )
)
( )( )
)
( )
( )( )
b) Briggs-Haldane approach
The rate of product formation,
d(p)
dt
Since the enzyme is preferred,
Make as a subject,
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Substrate consumption,
d(
( )( )
dt
d(
dt
ubstitute
into
)(
)(
)(
)( )
( )
( )
( )
( )
ubstitute
)( )
( )( )
)( )
( )(
( ))
( )
( )
( )(
( )
( )(
( )(
(
( ))
into
)( )
:
(
)(
)(
)(
( )
)( )
( )(
(
( ) ( )
( )
( ) ( )(
(
( )
( ))
)
( ))
( )
)( )
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
)(
)(
( )
( ) ( )
( ) ( )
(
ubstitute
)(
( ))
( ) ( )(
( )
)(
( )
( )
( ))
( ) ( )
( ) ( )
(
( ) ( )
( )
)(
( )
( ))
into
d(p)
dt
( ) ( )
(
( ) ( )
( )
)(
( )
( ))
v
d(p)
dt
v
(
( ) ( )
( ) ( )
( )
)(
( )
( ))
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.5
Lineweaver- Burk Plot
x-intercept= - 1
km
y-intercept= 1/ V more
Equation obtained y= 0.0172 x + 3.6342
y-intercept = 3.6342= 1/ V max
V max = 0.275
x-intercept , y= 0
0.0172x + 3.6342=0
0.0172x = -3.6342
x= -211.291
x= -1/km
km = 1/211.291
= 0.00473
Longmuir Plot
Equation obtained y= 3.3133x + 0.0191
1/Vm = m = 3.3133
Vm=0.302
y-intercept= km/Vm = 0.0191
Km = 0,0191x 0.302
= 0.00577
Eadie-Hofstee Plot
Equation obtained y= -0.0043x + 0.2645
-Km = m = -3.3133
Km=0.302
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
y-intercept= Vm = 0.2645
Non-Linear Regression Procedure
From the graph, Vm=0.2
Vmax = 0.1,
Km=0.0032
Data for Graph plot :
Langmuir Plot
s
s/v
0.0032 0.028829
0.0049 0.033108
0.0062 0.043357
0.008 0.048193
0.0095
0.0475
Lineweaver-Burk Plot
1/s
1/v
312.5
9.009009
204.0816 6.756757
161.2903 6.993007
125
6.024096
105.2632
5
Eadie-Hofstee Plot
v/s
v
34.6875
0.111
30.20408 0.148
23.06452 0.143
20.75
0.166
21.05263
0.2
Non-Linear Regression Plot
S
v
0.0032
0.111
0.0049
0.148
0.0062
0.143
0.008
0.166
0.0095
0.2
Kinetic Parameters
Vmax
Km
Langmuir
0.2750
0.0047
Lineweaver-Burk
0.0191
0.0057
Eadie-Hofstee
0.2645
0.0043
Non-Linear Regression 0.2000
0.0032
Type of Plot
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Langmuir Plot
0.06
y = 3.3133x + 0.0191
0.05
0.04
0.03
0.02
0.01
0
0
0.002
0.004
0.006
0.008
0.01
Lineweaver Burk Plot
10
9
8
7
6
5
4
3
2
1
0
y = 0.0172x + 3.6342
50
100
150
200
250
300
350
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Eadie-Hofstee Plot
0.25
0.2
0.15
y = -0.0043x + 0.2645
0.1
0.05
0
0
10
15
20
25
30
35
40
Non-Linear Regression Procedure
0.25
0.2
0.15
0.1
0.05
0
0
0.002
0.004
0.006
0.008
0.01
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.6
Rate of product formation
( )
Enzyme is preserved,
d( )
dt
(
Assumptions:
[ ]small,
neg igib e
( )( )
)(
)(
(
(
into
))( )
)
( )
(
(
)
)
( ) )
( )
( )
(
( )
d(p)
dt
(
(
( )
into
( )
(
( )( )
(
)
Substitute
v
( )( )
Substitute equation
(
()
( )
( )
)
)
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Dividing
[(
with the value of
( )
( )
]/
v s
( )
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.7
a) FCs0 - FCs + rSv = V
For Batch reactor F=0
rSv = V
[ ]
[ ]
[
]
[
= 60mol/m3.min
b) Equation obtained y = 6.3852x + 59.571
m = Vmax
= 6.3852
y- intercept = - Km = 59.571
Km = - 59.571
c) FCs0 - FCs + rSv = 0
FCs0 - FCs = - rSv = rpv
FCs0 - FCs =
F = 0.0001m3/min
V = 0.0003m3
( FCSo - FCs ) (Km + Cs)
= Vmax CsV
2
FCSo Km + FCSo Cs - FKm Cs FCs = Vmax CsV
(0.0001 (300)(200) + 0.0001(300)Cs 0.001(200)Cs 0.001Cs2 = 100 (0.0003)Cs )
6 + 0.03Cs 0.02Cs 0.001Cs2 = 0.03Cs
0.0001Cs2 + 0.02Cs 6 = 0
Cs=165mol/m3
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Data :
Cs t t/ln(Cso/Cs) (Cso-Cs)/ln(Cso/Cs)
1 1
0.175322
52.42135
5 5
1.221197
72.0506
10 10
2.940141
85.26409
20 20
7.385387
103.3954
Graph :
(Cso-Cs)/ln(Cso/Cs)
120
y = 6.3852x + 59.571
100
80
(Cso-Cs)/ln(Cso/Cs)
60
Linear ((CsoCs)/ln(Cso/Cs))
40
20
0
0
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.8
a) Km =0.01 mol/L
Cso = 3.4 x 10 -4 mol/L
Cs = 0.9 x 3.4 x 10-4
= 3.06 x 10-4 mol/L
t= 5minutes
[ ]
[ ]
( 3.4x 10-4 3.06 x 10-4) = Vmax (3.06 x 10-4)
S
0.01 + (3.06 x 10-4)
6.8 x 10-6 = Vmax ( 0.03)
Vmax = 2.27 x 10-4 mol/L-min
b) 6.8 x 10-6 x 15 = 1.02 x 10-4 mol/L
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.9
Km = 0.03mol/L
rmax = 13mol/L min 60 = 780mol/L hr
F=10L/Hr
Cs=10mol/L
F=10L/Hr
Cs=0.5mol/L
CSTR
a) V = ?
CSTR @ Stead State
FCs0 - FCs + rSv = 0
F (Cs0 - Cs ) = rpv
(
10 (10 0.5) =
V = 0.129 liter
b) Plug - Flow @ Stead State
Km ln
+ (Cs0 - Cs )
0.03 ln
+ (10 - 0.5 ) = 780t
= rmax t
9.95899 = 780t
t = 0.0123hr
t = V/F = 0.0123
V = 0.0123 10
= 0.123liter
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.10
Km = 10g/L
rmax = 7g/L.min
a)
F=0.5L/min
Cs0=50g/L
1L
F=0.5L/min
Cs1=?g/L
1L
F=0.5L/min
Cs2=? g/L
CSTR @ Steady State
FCs0 - FCs + rSv = 0
F (Cs0 - Cs ) = rpv
0.5 (50 Cs1) =
)
s
(1)
(25-0.5Cs1)(10+ Cs1)=7Cs1
250+25Cs1-5Cs1-0.5Cs12=7Cs1
0.5Cs12-13Cs1-250=0
Cs1=38.86g/L
0.5 (38.86 Cs2) =
)
s
(1)
(19.43-0.5 Cs2)(10+ Cs2)=7 Cs2
194.3+19.43Cs2-5Cs2-0.5Cs22=7Cs2
0.5Cs22-7.43Cs2-194.3 =0
Cs2=28.49g/L
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
b)
F=0.5L/min
Cs0=50g/L
2L
F=0.5L/min
Cs1=?g/L
CSTR @ Steady State
FCs0 - FCs + rSv = 0
F (Cs0 - Cs ) = rpv
0.5 (50 Cs1) =
)
s
(2)
(25-0.5Cs1)(10+ Cs1)=14Cs1
250+25Cs1-5Cs1-0.5Cs12=14Cs1
0.5Cs12-6Cs1-250=0
Cs1=29.15g/L
Since in the Cs in two reactor system is less than Cs in one reactor system, therefore two reactor
system is more efficient than one reactor system as it indicates more substrates have been
consumed to form products.
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.11
a) k1 [E] [S] = k2 [ES]
[ES] = k2
k1 [E] [S]
k1 [E] [S]
k2
k3 [E] [P] = k4 [EP]
[EP] = k3
k4 [E] [P]
k5 [ES][P] = k6 [ESP]
[ESP] = k5 [ES] [P]
k6
k7 [EP] [S] = k8 [ EPS ]
[EPS] = k7 [EP] [ S ]
K8
= k7 k3 [ S ]
k8 k4 [E] [P]
From,
[ESP] = k5 [P] k2
k6k1 [E] [S]
[E0] = [E] + [ES] + [EP] + [ESP] + [EPS]
[E0] = [ES] + [ESP] + [E] + [EP] + [EPS]
[E0] = [ES] + [ESP] + [E] + [EP] +
][ ]
[ ]
[E0] = [ES] + [ESP] + [E] + [EP] + (
[ ][ ]
[E0] = [ES] + [ESP] + [E] +
[ ]
[E0] = [ES] + [ESP] + [E] [
[E0] = [ES] +
[E0] = [ES] {1 +
][ ]
][
[ ]
[ ]
+(
[ ]
(
[ ]
[
[ ]
)]
[ ]
(
[ ]
)]}
)]
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
[ES] =
V=
[ ]
b)
Given:
[ ]
[ ]
KSP =
KSP =
[ESP] =
[ ]
[ ]
[ ]
)]
[ ]
)]
KPS =
[ ][ ][ ]
[
]
KPS =
[ ][ ][ ]
[
]
[ ][ ][ ]
[EPS] =
[ ][ ][ ]
][ ]
]
][ ]
]
[ESP] = [EPS]
KS KSP = KP KPS
=
[ ]
=
=
[
[ ]
[ ]
[ ]
]
[ ]
[ ]
[ ]
]
]
[ ]
[ ]
c)
Ks=Kps
Kp=Ksp
[ESP]=[EPS]
[ ]
(
( )
[
[ ]
[ ][ ]
)[
[ ]
][ ]
]
[ ]
[ ]
[ ]
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
[
][ ]
[ ])
[ ](
[ ]
[ ]
[ ]
[ ])
[ ]
[ ]
[ ])
(
(
Compare with
[ ]
[ ]
Hence, Vmax = (
Km=
d)
[ ])
[ ]
(
[ ])
[ ]
[ ]
[ ]
[ ]
*
[
[ ]
[ ]
[ ]
[ ]
[ ]
[ ]
[ ]
[ ]
[ ]
n(
[ ]
)
[ ]
[ ]
+
[ ] [ ]]
[ ] [ ]
[ ]
[ ]
[ ]
n(
[ ]
)
[ ]
n([ ] [ ])
(
[ ]
[ ]
)
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Y = mx+c
[ ]
)
]
n ([
Y=
M=
[
X= (
] [ ]
C=
So we can plot a graph of
[ ]
)
]
n ([
vs (
] [ ]
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.14
Rate:
rp = k9CES +k10CEIS1 +k10CEIS2
---- 1
Enzyme balance:
CEo = CE + CES
---- 2
CEo = CEIS1 + CES + CE
---- 3
CEo = CEIS2 + CEI + CE
---- 4
The equilibrium reaction equations are as follows:
CE Cs / CES = k2/k1
---- 5
CECI / CEI = K4/K3
---- 7
CESCI /CEIS1 = K6/K5
---- 6
CEICS / CEIS2 = K8/K7
---- 8
By rearranging Equation 5,
CE = (k2/k1) Cs CES
From Equation 2,
CEo = [(k2/k1)CE + 1] CES
CES = CEo /[( k2/k1)CS +1]
---- 9
By rearranging Equation 6,
CES = [(K6/K5)CI ] CEIS1
From Equation 3,
CEo = CEIS +CES + (k2/k1) Cs CES
= {CEIS1 + [1 + (k2/k1) Cs]( K6/K5)CI }CEIS1
= {1 + [1 + (K2/K1) Cs ]( K6/K5)CI } CEIS1
CEIS1 = CEo/ {1 + [1 + (k2/k1) Cs ]( K6/K5)CI }
By rearranging Equation 7,
CE = (K4/K3) CEI
By rearranging Equation 8,
CEI = K8/K7CS CEIS2
---- 10
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
From Equation 4,
CEo = CEIS2 + CEI + [(K4/K3)CI]CEI
= CEIS2 + [1 + (K4/K3)CI ]CEI
= CEIS2 + [1 + (K4/K3)CI ]( K8/K7)CS CEIS2
CEo = {1 + [1 + (K4/K3)CI ]( K8/K7)CS } CEIS2
CEIS2 = CEo / {1 + [1 + (K4/K3)CI ]( K8/K7)CS }
---- 11
From Equation 1, since rp = k9CES +k10CEIS1 +k10CEIS2,
By substituting Equation 9, 10 & 11 into Equation 1,
Therefore,
rp = k9 CEo /[( k2/k1)CS +1] + k10 CEo/ {1 + [1 + (k2/k1) Cs ]( K6/K5)CI } + k10 CEo / {1 + [1 + (K4/K3)CI
]( K8/K7)CS }
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.15
a) Based on the graphs
The y-intercept in Lineweaver Burk plot is almost the same.
Y-intercept => 3.8266; 3.6342
Whereas in Langmuir Plot
Two equations obtained
Y = 2.9883x + 0.0489
Y = 3.3133x + 0.0191
When y=0
X=
X=
X = -0.016
X = -0.005
In Line weaver Burk Plot and Langmuir Plot both indicates its a competitive inhibitor
Data :
Lineweaver
1/s
1/Vo
312.5
9.009009
204.0816 6.756757
161.2903 6.993007
125
6.024096
105.2632
5
Langmuir
s
s/Vo
0.0032 0.028829
0.0049 0.033108
0.0062 0.043357
0.008
0.048193
0.0095
0.0475
1/Vi
16.94915
14.08451
10.98901
9.009009
8
S/Vi
0.054237
0.069014
0.068132
0.072072
0.076
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Lineweaver-Burk Plot
20
18
16
14
12
10
8
6
4
2
0
y = 0.0439x + 3.8266
1/Vo
1/Vi
y = 0.0172x + 3.6342
Linear (1/Vo)
Linear (1/Vi)
100
200
300
400
Langmuir Plot
0.09
0.08
y = 2.9883x + 0.0489
0.07
0.06
s/Vo
y = 3.3133x + 0.0191
0.05
S/Vi
0.04
Linear (s/Vo)
0.03
Linear (S/Vi)
0.02
0.01
0
0
0.002
0.004
0.006
b) Y-intercept = 1/Vmax = 0.00489
Vmax = 1/0.00489 = 204.5 mol /L.min
Km/Vmax = 2.9883
Km=2.9883*204.5
=611mol/L
0.008
0.01
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.16
(a) E + S
ES + S
k5 E + P
(E0 ) = (E) +(ES) + (ESS)
(E) = (E0 ) (ES) (ESS) -------V=
=
(
( )
= k5 (ES) ------
)( )
)
) = (ES)(S)/ (k4 / k3)
K2 / k1 = (E)(S) / (ES)
K2/k1 (ES) = (E0)(S) (ES)(S)
(ES)((k2/k1) + (S)) = (E0)(S) (ES)(S)2 /
(ES)( (k2/k1) + (S)( ) ) =
(E0)(S) (ES)(S)2
(ES) ( (k2/k1) + (S)( ) + (S)2 ) =
(ES) =
(E0)(S)
(E0)(S) / (k2/k1) + (S)( ) + (S)2 --------
3
V=
=
( )
k5 (E0) (S) / (k2/k1) + (S)( ) + (S)2
Vm(S) / (k2/k1) + (S)( ) + (S)2
(b) At low substrate concentration,
1/ Vm = 3.1209
Vm = 0.3204
Km/Vm = 106.07
Km / 0.3204 = 106.7
K1m = 33.98
At high substrate concentration,
1/ Vm = 3.0574
Vm = 0.3271
1/ K1. Vm = 0.0032
1/ Km(3.0574) = 0.0032
Km = 102mol/L
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.17
V= 5L
Cso = 100 mmol/L
F = 1 L/hr
Cs = 10m mol/L
a) F (s0 FCs = rp V
1(100-10) = rp (5)
Rp = 18 m mol/ L.min
b) Find rp for each F and s
Equation obtained y= 0.0391x + 0.1641
M= 1/Vmax = 0.0391
Vmax= 25.57 m mol/L.min
Km/ Vm = 0.1641
Km= 4.197
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.18
[SO]1 = 0.1 mol/L
[S0]2 = 0.3mol/L
[ ][ ]
[
]
[ ][
]
]
[ ][
[ ][
]
]
[
[ ][
[
]
]
[ ][ ]
[
]
V1 =
= k5[ES1]
=3.5 [ES1] --V2 =
= k6[ES2]
=2.8 [ES2] ---
[E0] = [E] + [ES1] + [ES2]
[ ][
[E0] = [E] + [ES1] +
[E0] = [ES1] + [E] (1+
[E0] = [ES1] +
[E0] = [ES1] {1 +
[
[ES1] =
[
[
]
(1+
(1+
]
(
)}
[E0] = 0.05 mol/L
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Vmt = [S1]0 [S2] + K ln
[ ]
[ ]
3.5 [ES1] t = 0.1 [S1] +0.0714 ln [
3.5 [ES1] t + [S1] = 0.1 +0.0714 ln 0.1 - 0.0714 ln [S1]
3.5 [ES1] t + [S1] + 0.0644 = -0.0714 ln [S1]
ln[S1] =
[S1] =
---
[E0] = [E] + [ES1] + [ES2]
[E0] = [E] +
[ ][
[E0] = [E] (1+
[E0] =
[
]
[E0] = [E2] [
+ [ES2]
(1+
)+ [ES2]
[
(1+
Vmt = [S1]0 [S2] + KMln
)+ [ES2]
]
)+ 1]
]
2.8[ES1] t = 0.3 [S2] + 0.2207ln [
2.8[ES1] t + [S2] = 0.3 + 0.2207ln 0.3 0.2207ln [S2]
2.8[ES1] t + [S2] 0.0343 = 0.2207ln [S2]
ln[S2] =
[S2] = e
---
As [S1] increases, [ES1] also increases as in eq.3. [P1] also increases as in eq.1. This also occurs in
[S2]. As [S1] increases, [ES1] also increases as in eq.4. [P2] also increases as in eq.2
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.19
Data :
s
6.7
3.5
1.7
s/v
22.33333
14
10.625
Langmuir Plot
25
y = 2.3722x + 6.2429
20
15
10
5
0
0
1/Vm = 2.3722
Vmax = 0.4215 mumol/L.min
Km/Vm = 6.2429
Km = 6.2429(0.4215)
=2.63mumol/L
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Solution 2.20
a. Write the kinetic model.
Since the Michaelis constant KM is not affected by the presence of the inhibitor (which has shown on
the given table); then this enzyme reaction is noncompetitive inhibition reaction.
Kinetic Model:
k 1, k 2
E S
ES
k 3, k 4
E I
EI
k 5, k 6
EI S
EIS
k 7 ,k 8
ES I
ESI
k9
ES
EP
b. Derive the rate equation. State the assumptions.
Assumptions:
The dissociation constant for the first equilibrium reaction is the same as that of the third
equilibrium reaction.
The dissociation constant for the second equilibrium reaction is the same as that of the
fourth equilibrium reaction.
The two equilibrium reactions,
k
k2
K S 6 K IS
k1
k5
k
k4
K I 8 K SI
k3
k7
If the slower reaction, the product formation step, determines the rate of reaction according to
Michaelis-Menten assumption, the rate can be expressed as:
rP k 9 [ ES ]
(1)
The enzyme balance gives
[ E0 ] [ E ] [ ES ] [ EI ] [ ESI ]
(2)
k 9 [ ES ]
rP
[ E0 ] [ E ] [ ES ] [ EI ] [ ESI ]
(3)
Divide (1) by (2),
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Applied Law of mass action,
Ks
K 2 [ E ][S ]
[ E ][S ]
[ ES ]
K1
[ ES ]
KS
(4)
KI
K 4 [ E ][ I ]
[ E ][ I ]
[ EI ]
K3
[ EI ]
KI
(5)
KI
k8 [ ES ][ I ]
[ ES ][ I ]
[ ESI ]
k7
[ ESI ]
KI
(6)
Substitute (4), (5), (6) into (3),
[ E ][ S ]
KS
rP
[ E ][ S ] [ E ][ I ] [ ES ][ I ]
[ E0 ]
[E]
KS
KI
KI
k9
[ E ][ S ]
KS
rP
[ E ][ S ] [ E ][ I ] [ E ][ S ][ I ]
[ E0 ]
[E]
KS
KI
KS KI
k9
Eliminate [E],
[S ]
KS
rP
[ S ] [ I ] [ S ][ I ]
[ E0 ]k 9
1
KS KI KS KI
Substitute rPmax [ E0 ]k 9
rP
rPmax
[S ]
KS
[ S ] [ I ] [ S ][ I ]
1
KS KI KS KI
Multiply numerator and denominator by Ks,
rP
rPmax
[S ]
K [ I ] [ S ][ I ]
K S [S ] S
KI
KI
BK10110302 V.PRASARNTH RAAJ VEERA RAO BIOPROCESS
Rearranging,
rP
rPmax
[S ]
K [I ]
[ S ][ I ]
KS S
[S ]
KI
KI
rP
rPmax
[S ]
K S (1
[I ]
[I ]
) [ S ](1
)
KI
KI
Anda mungkin juga menyukai
- Reviewer For Chemical Engineering Licensure Examination 3 Edition Solutions ManualDokumen56 halamanReviewer For Chemical Engineering Licensure Examination 3 Edition Solutions ManualSherry Anne Ynciong Panganiban100% (4)
- Enzyme KinecticsDokumen25 halamanEnzyme KinecticsRhia80% (5)
- Optimize ethanol production from yeast in a continuous fermenterDokumen17 halamanOptimize ethanol production from yeast in a continuous fermenterrhia81% (16)
- Cell Kinetics and Fermenter Design Solution ManualDokumen5 halamanCell Kinetics and Fermenter Design Solution ManualMarthy DayagBelum ada peringkat
- Biochemical Engineering Sample ProblemsDokumen5 halamanBiochemical Engineering Sample ProblemsVan Vesper Dulliyao100% (1)
- Cell Kinetics Chapter 6Dokumen56 halamanCell Kinetics Chapter 6Sanjay Kumar73% (11)
- JJDokumen4 halamanJJEureca ParraBelum ada peringkat
- BIochem AssDokumen10 halamanBIochem AssJomhel Callueng50% (2)
- Biochem Enzyme KineticsDokumen53 halamanBiochem Enzyme KineticsJayvee Francisco67% (3)
- Sepa and PartechDokumen5 halamanSepa and Partechdiana bunagan0% (1)
- Bioche ProblemsDokumen5 halamanBioche ProblemsTimothy Jones100% (1)
- Computer ApplicationsDokumen8 halamanComputer Applicationsapi-3728602100% (1)
- Separation Process Compilation of Problem SetDokumen60 halamanSeparation Process Compilation of Problem SetKaye Fabros100% (5)
- LEC LE 3 Part 2 Stoichiometry of Growth and Product FormationDokumen27 halamanLEC LE 3 Part 2 Stoichiometry of Growth and Product FormationRaymond Fuentes100% (1)
- Cell Kinetics and Fermenter DesignDokumen116 halamanCell Kinetics and Fermenter DesignCriselda Oliva CarinoBelum ada peringkat
- Orca - Share - Media1575204849829 2Dokumen113 halamanOrca - Share - Media1575204849829 2Lily Antonette AgustinBelum ada peringkat
- CN3121 Homework Questions and Solutions (Chap1)Dokumen3 halamanCN3121 Homework Questions and Solutions (Chap1)Weng Hong WeiBelum ada peringkat
- Sample Problem #2Dokumen2 halamanSample Problem #2Dozdi67% (3)
- Problems in Mass TransferDokumen3 halamanProblems in Mass TransferAngelica Joyce BenitoBelum ada peringkat
- Sample Problem #11Dokumen6 halamanSample Problem #11Dozdi100% (4)
- Balmes, Patricia R. - Enzyme KineticsDokumen11 halamanBalmes, Patricia R. - Enzyme KineticsGlecie RasBelum ada peringkat
- Gas AbDokumen14 halamanGas AbJackielyn EugenioBelum ada peringkat
- Stoichiometry of Microbial Growth and Product FormationDokumen10 halamanStoichiometry of Microbial Growth and Product FormationfayeBelum ada peringkat
- 2 Cell Kinetics and Fermenter Design Part 2 DiscussionDokumen4 halaman2 Cell Kinetics and Fermenter Design Part 2 DiscussionEzekielBelum ada peringkat
- CHE 511A Compilation 2 3 M F PDFDokumen164 halamanCHE 511A Compilation 2 3 M F PDFMaame Efua NeizerBelum ada peringkat
- Kinetics (Gjjkkkgty)Dokumen5 halamanKinetics (Gjjkkkgty)Chrysler Kane DepnagBelum ada peringkat
- Separation Process Compilation of Problem SetDokumen55 halamanSeparation Process Compilation of Problem Setjames100% (1)
- ChE514A Cell Kinetics and Fermenter DesignDokumen116 halamanChE514A Cell Kinetics and Fermenter DesignAnonymous 0zrCNQ55% (11)
- 5 Enzyme KineticsDokumen39 halaman5 Enzyme KineticsEbook Download100% (1)
- Shuler KargiDokumen15 halamanShuler Kargisureandhraindia67% (6)
- Evaporator Heat and Mass Transfer CalculationsDokumen16 halamanEvaporator Heat and Mass Transfer CalculationsArlene DeppBelum ada peringkat
- Chapter 8: Sterilization: Che 514A: Biochemical EngineeringDokumen9 halamanChapter 8: Sterilization: Che 514A: Biochemical EngineeringEzekielBelum ada peringkat
- BIochem AssDokumen5 halamanBIochem AssCheng PasionBelum ada peringkat
- Soln KG Inert KG 5 - 1: Sample Problem #1Dokumen1 halamanSoln KG Inert KG 5 - 1: Sample Problem #1DozdiBelum ada peringkat
- Extraction Problem Solving DrillDokumen2 halamanExtraction Problem Solving Drilledmark icalina40% (5)
- Liquid Liquid ExtractionDokumen50 halamanLiquid Liquid ExtractionArrianne Jaye Mata86% (14)
- Sample Problem #6Dokumen4 halamanSample Problem #6Dozdi100% (2)
- Problems in Biochemical EngineeringDokumen22 halamanProblems in Biochemical EngineeringArrianne Jaye Mata50% (4)
- Lecture 26-Problems On Cell Growth KineticsDokumen15 halamanLecture 26-Problems On Cell Growth KineticsHemanth Peddavenkatappa GariBelum ada peringkat
- CHEMICAL ENGINEERING INSTRUMENTATION AND PROCESS CONTROL PROBLEMSDokumen4 halamanCHEMICAL ENGINEERING INSTRUMENTATION AND PROCESS CONTROL PROBLEMSMarco SarmientoBelum ada peringkat
- Gas Solubility in Aqueous SolutionDokumen93 halamanGas Solubility in Aqueous SolutionArgie Adduru73% (11)
- ChE127 NUNEZ Assignment1Dokumen1 halamanChE127 NUNEZ Assignment1John Patrick Sanay NunezBelum ada peringkat
- ScreeningDokumen30 halamanScreeningPatricia de Leon100% (2)
- DocxDokumen2 halamanDocxDiana BunaganBelum ada peringkat
- Day 2 200 ItemsDokumen25 halamanDay 2 200 ItemsRobert DelfinBelum ada peringkat
- مسائل وحلول ماسDokumen31 halamanمسائل وحلول ماسHmid Aljbre100% (4)
- Michaelis-Menten kinetics of enzyme reactionDokumen4 halamanMichaelis-Menten kinetics of enzyme reactionVan Vesper DulliyaoBelum ada peringkat
- Test 1 Book Notes Examples-1 PDFDokumen65 halamanTest 1 Book Notes Examples-1 PDFRalph Castino87% (15)
- COMPIDokumen129 halamanCOMPIJeanne Roselle Dulatre CortezBelum ada peringkat
- 14-Plant Design-Ans Key-Master FileDokumen22 halaman14-Plant Design-Ans Key-Master FilePaul Philip LabitoriaBelum ada peringkat
- Physical Chemistry: Answer KeyDokumen15 halamanPhysical Chemistry: Answer Keyvishal110085Belum ada peringkat
- Iit Jee 2012 Pet4 Solns p2Dokumen22 halamanIit Jee 2012 Pet4 Solns p2Ishita AggarwalBelum ada peringkat
- E 199 SolDokumen10 halamanE 199 SolDavid Alemán SánchezBelum ada peringkat
- Introduction to Bioprocess Engineering Revision Questions and AnswersDokumen3 halamanIntroduction to Bioprocess Engineering Revision Questions and AnswersGia BooBelum ada peringkat
- Orca Share Media1521362143835Dokumen122 halamanOrca Share Media1521362143835Ana Lorraine DalilisBelum ada peringkat
- Solutions Manual Urban Drainage 2ndedDokumen17 halamanSolutions Manual Urban Drainage 2ndedJamesBonn OlingaBelum ada peringkat
- Derive The Integrated Rate Equation Half-LifeDokumen7 halamanDerive The Integrated Rate Equation Half-Lifeumut2000Belum ada peringkat
- Problem Set - Enzymes From LehningerDokumen11 halamanProblem Set - Enzymes From LehningervioletbrownBelum ada peringkat
- Department of Chemistry Faculty of Mathematics and Science State University of Padang 2014Dokumen9 halamanDepartment of Chemistry Faculty of Mathematics and Science State University of Padang 2014Anelin OsiriknaBelum ada peringkat
- Chem II AP PacketDokumen4 halamanChem II AP PacketAmanda Rose DalyBelum ada peringkat
- CarDokumen1 halamanCarVPrasarnth RaajBelum ada peringkat
- 308 - November 2015 Version 29Dokumen19 halaman308 - November 2015 Version 29VPrasarnth RaajBelum ada peringkat
- Mach CC App Form Jun16 Eng 20160629Dokumen6 halamanMach CC App Form Jun16 Eng 20160629ZulIzzamreeZolkepliBelum ada peringkat
- Hyundai AccentDokumen10 halamanHyundai AccentVPrasarnth RaajBelum ada peringkat
- Prototype Development To Test EOR Methods: Joana - Sanches@ist - Utl.ptDokumen10 halamanPrototype Development To Test EOR Methods: Joana - Sanches@ist - Utl.ptVPrasarnth RaajBelum ada peringkat
- ResignationDokumen1 halamanResignationAlan KhorBelum ada peringkat
- HP PDS Con EngDokumen3 halamanHP PDS Con EngVPrasarnth RaajBelum ada peringkat
- Finaldissertation NoRestrictionDokumen120 halamanFinaldissertation NoRestrictionVPrasarnth RaajBelum ada peringkat
- C19115I2901Dokumen1 halamanC19115I2901VPrasarnth RaajBelum ada peringkat
- Windscreen Report Claim FormDokumen3 halamanWindscreen Report Claim FormVPrasarnth RaajBelum ada peringkat
- Job satisfaction in human resource managementDokumen102 halamanJob satisfaction in human resource managementMed ElbanadiBelum ada peringkat
- LCADokumen1 halamanLCAVPrasarnth RaajBelum ada peringkat
- Proton On The Road Price After Gst-20160215Dokumen1 halamanProton On The Road Price After Gst-20160215VPrasarnth RaajBelum ada peringkat
- Map To CBJ OfficeDokumen1 halamanMap To CBJ OfficeVPrasarnth RaajBelum ada peringkat
- Basic Well Log Analysis - Introduction - Oct2013Dokumen35 halamanBasic Well Log Analysis - Introduction - Oct2013Jorge PirelaBelum ada peringkat
- 4 TH SOGCEDirectoryDokumen40 halaman4 TH SOGCEDirectoryVPrasarnth RaajBelum ada peringkat
- Bioprocess QuizDokumen4 halamanBioprocess QuizVPrasarnth RaajBelum ada peringkat
- 2011 AccentDokumen8 halaman2011 AccentVPrasarnth RaajBelum ada peringkat
- Milestone 5 Outline KC40203 2012Dokumen1 halamanMilestone 5 Outline KC40203 2012VPrasarnth RaajBelum ada peringkat
- Petroleum Assignment - UOP Q-Max Cumene Process (FULL)Dokumen436 halamanPetroleum Assignment - UOP Q-Max Cumene Process (FULL)VPrasarnth Raaj100% (12)
- Pchardware StartecDokumen1 halamanPchardware StartecVPrasarnth RaajBelum ada peringkat
- Jadual Waktu MOG Semester 2, Sesi 2014.2015Dokumen1 halamanJadual Waktu MOG Semester 2, Sesi 2014.2015VPrasarnth RaajBelum ada peringkat
- All It DesktopDokumen4 halamanAll It DesktopNatasya WillmoreBelum ada peringkat
- Petroleum Home Assignment OutlineDokumen3 halamanPetroleum Home Assignment OutlineVPrasarnth RaajBelum ada peringkat
- List of Tables, Figures & Executive SummaryDokumen4 halamanList of Tables, Figures & Executive SummaryVPrasarnth RaajBelum ada peringkat
- British PetroleumDokumen10 halamanBritish PetroleumVPrasarnth RaajBelum ada peringkat
- Rajni and ManagementDokumen12 halamanRajni and ManagementVPrasarnth RaajBelum ada peringkat
- Packed Tower AbsorberDokumen58 halamanPacked Tower AbsorberCelestino Montiel MaldonadoBelum ada peringkat
- Comparison of Different Collection Efficiency Models For Venturi ScrubbersDokumen10 halamanComparison of Different Collection Efficiency Models For Venturi ScrubbersPassmore DubeBelum ada peringkat
- BK10110302-Shuler Problems PDFDokumen13 halamanBK10110302-Shuler Problems PDFVPrasarnth Raaj100% (2)
- G16 - A21 - DAA 2723 WBPDokumen16 halamanG16 - A21 - DAA 2723 WBPanazraf 23MzBelum ada peringkat
- Journal - Development PlanDokumen11 halamanJournal - Development Planadeyemi kehindeBelum ada peringkat
- Inked Lives - Tattoos Identity and PowerDokumen88 halamanInked Lives - Tattoos Identity and Powerךו ני אלBelum ada peringkat
- SJG06 017 (11) GRACE01 by MengdeDokumen24 halamanSJG06 017 (11) GRACE01 by MengdeБахтияр АбдуразаковBelum ada peringkat
- Department of Education: Republic of The PhilippinesDokumen7 halamanDepartment of Education: Republic of The PhilippinesCristeta ToqueroBelum ada peringkat
- IodinDokumen7 halamanIodinnovi lianaBelum ada peringkat
- Challenges of Using Eclectic Approach for EFL BeginnersDokumen12 halamanChallenges of Using Eclectic Approach for EFL BeginnersRahil MahBelum ada peringkat
- Dinosaur Lesson by SlidesgoDokumen41 halamanDinosaur Lesson by SlidesgoDonalyn PaderesBelum ada peringkat
- Eng201 ch7 Accuracy Clarity Conciseness CoherenceDokumen2 halamanEng201 ch7 Accuracy Clarity Conciseness CoherenceAyesha MughalBelum ada peringkat
- Transcription M3S2Dokumen21 halamanTranscription M3S2GrowUP. AIBelum ada peringkat
- 3.1.a-Answer Sheet Perdev, Week 1, Quarter 1Dokumen5 halaman3.1.a-Answer Sheet Perdev, Week 1, Quarter 1Precious EspejoBelum ada peringkat
- Self Evaluation Rubric ShadowingDokumen1 halamanSelf Evaluation Rubric ShadowingJoaquín LaraBelum ada peringkat
- Kathmandu University Presentation on CSTR Simulation and Process ControlDokumen19 halamanKathmandu University Presentation on CSTR Simulation and Process Controlramesh pokhrelBelum ada peringkat
- StatisticsDokumen1 halamanStatisticsNathan Stuart The Retarded idiotBelum ada peringkat
- Determine pressure and amount of air in gas tank problemsDokumen3 halamanDetermine pressure and amount of air in gas tank problemsyeng botzBelum ada peringkat
- Online Workshop Analisa Gangguan PetirDokumen64 halamanOnline Workshop Analisa Gangguan PetirJumhanter hutagaolBelum ada peringkat
- Changes in AATCC Manual 2011Dokumen3 halamanChanges in AATCC Manual 2011rajeshpatkar39Belum ada peringkat
- O LCA Roadtesting 1.9.17 1Dokumen100 halamanO LCA Roadtesting 1.9.17 1Dhani PriyambodoBelum ada peringkat
- Optimal Use of Cullet in Hollow Block ProductionDokumen22 halamanOptimal Use of Cullet in Hollow Block ProductionKyle BravoBelum ada peringkat
- History Memoir - Anjuli DarjeeDokumen10 halamanHistory Memoir - Anjuli DarjeeTshering NamgyalBelum ada peringkat
- UP ACME - Chem 28 - 2nd LE SamplexDokumen4 halamanUP ACME - Chem 28 - 2nd LE SamplexDoom RefugeBelum ada peringkat
- Structural Welding Code-Steel: 1. General RequirementsDokumen3 halamanStructural Welding Code-Steel: 1. General Requirementssivagnanam sBelum ada peringkat
- Mt271 Lecture Notes 1Dokumen13 halamanMt271 Lecture Notes 1Ahmed MasoudBelum ada peringkat
- Sample Question Paper Class: Xii Session: 2021-22 Mathematics (Code-041) Term - 1Dokumen8 halamanSample Question Paper Class: Xii Session: 2021-22 Mathematics (Code-041) Term - 1MasterzimoBelum ada peringkat
- Self ReflectionDokumen2 halamanSelf Reflectionapi-575370301Belum ada peringkat
- ADB Procurement Framework UpdateDokumen59 halamanADB Procurement Framework UpdateAvisheak PalBelum ada peringkat
- Instant Download Ebook PDF Dynamics of Structures 5th Edition by Anil K Chopra PDF ScribdDokumen41 halamanInstant Download Ebook PDF Dynamics of Structures 5th Edition by Anil K Chopra PDF Scribdlisa.vanwagner713100% (44)
- Osman Jayasinghe Chemistry IIDokumen4 halamanOsman Jayasinghe Chemistry IIyasidu rashmikaBelum ada peringkat
- Nature and Scope of EconomicsDokumen6 halamanNature and Scope of EconomicsRenu AgrawalBelum ada peringkat
- Full Text o Module in Ge 14 M. NacinoDokumen94 halamanFull Text o Module in Ge 14 M. NacinoAryza NacinoBelum ada peringkat