Rectovaginal Fistula: History of The Procedure
Diunggah oleh
Romi Mauliza FauziJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Rectovaginal Fistula: History of The Procedure
Diunggah oleh
Romi Mauliza FauziHak Cipta:
Format Tersedia
Rectovaginal Fistula
History of the Procedure For thousands of years, women simply tolerated the distressing symptoms generated by rectovaginal fistulas (RVFs). This is no longer necessary because most RVFs can be surgically corrected via a number of approaches. A small percentage, however, cannot be corrected because of patient comorbidity or disease-related factors; in such cases, patients can be helped only by fecal diversion.[1] Problem A rectovaginal fistula (RVF) is an epithelial-lined tract between the rectum and vagina. This article discusses only acquired RVFs. Most RVFs are located at or just above the dentate line. Fistulas below the dentate line are not true RVFs but are instead anovaginal fistulas; these require different treatment than do RVFs. Epidemiology Frequency Among reported series, the frequency with which rectovaginal fistulas (RVFs) occur varies according to etiology. RVFs are classified on the basis of location, size, and etiology, each of which affects the treatment plan and prognosis. Low RVFs, which are located between the lower third of the rectum and the lower half of the vagina, are closest to the anus and can be corrected with a perineal approach. High fistulas, which occur between the middle third of the rectum and the posterior vaginal fornix, require a transabdominal approach for repair. RVFs may vary greatly in size, but most are less than 2 cm in diameter. Small-sized fistulas are less than 0.5 cm in diameter, medium-sized fistulas are 0.5-2.5 cm, and large-sized fistulas exceed 2.5 cm. Etiology The most common etiology for rectovaginal fistula (RVF) of traumatic origin, and probably for all RVFs, is obstetric injury.[2, 3] Other etiologies for RVF include radiation injury,[4] inflammatory bowel disease ([IBD], most often Crohn disease[5]), operative trauma, infectious etiologies, and neoplasm. Pathophysiology Several traumatic causes of rectovaginal fistula (RVF) exist. Perineal lacerations during childbirth, especially those due to episioproctotomy, predispose patients to RVFs. Perineal lacerations are more common in primigravidas, in precipitous births, or when forceps or vacuum extraction is used. Failure to recognize and correctly repair perineal lacerations, or secondary infection of perineal lacerations, further increases the chance of RVF. Prolonged labor with pressure on the rectovaginal septum can produce necrosis and result in RVF. Vaginal or rectal operative procedures, especially those performed near the dentate line, may cause RVFs. The stapled hemorrhoidopexy and STARR (stapled transanal rectal resection and TRANSTAR (transanal stapled resection) have had increasing complications of RVFs.[6] Pelvic operations can be complicated by the development of a high RVF. Traumatic injury (penetrating or blunt) and forceful coitus also have produced RVFs.
Crohn disease[5] and, less often, ulcerative colitis have been associated with RVFs. The fistula may arise primarily or, more often, in relation to a perirectal abscess and/or fistula, manifesting as complicated perianal sepsis. Radiation used in the treatment of pelvic malignancies may result in RVF.[4]Fistulas that occur during such therapy usually result from tumor regression. Most other fistulas become apparent 6 months to 2 years after completion of treatment. Diabetes, hypertension, smoking, and previous abdominal or pelvic surgery increase the risk of fistula formation. The use of biopsy to differentiate radiation-related change at the fistula from a recurrent tumor is imperative, because neoplasms (primary, recurrent, metastatic) can produce RVFs. A variety of infectious conditions can produce RVF. The most common are perirectal abscess/fistula and diverticulitis. Less commonly, tuberculosis, lymphogranuloma venereum, and Bartholin gland abscess can cause RVFs. Presentation The clinical presentation of rectal vaginal fistula (RVF) varies little. A few patients are asymptomatic, but most report the passage of flatus or stool through the vagina, which is understandably distressing. Patients may also experience vaginitis or cystitis. At times, a foul-smelling vaginal discharge develops, but frank stool per vagina usually occurs only when the patient has diarrhea. The clinical picture may include fecal incontinence due to associated anal sphincter damage or bloody, mucus-rich diarrhea caused by the underlying clinical etiology. Indications Because the symptoms of rectovaginal fistula are so distressing, surgical therapy is almost always indicated. Exceptions include patients who are moribund or those with prohibitive risks for the proposed anesthesia and surgery. Note that surgical therapy means repair in most cases. Some patients, however, are better served by a diverting stoma than by an ill-advised repair attempt. Relevant Anatomy The rectovaginal septum is the thin septum separating the anterior rectal wall and the posterior vaginal wall. The caudal portion of the septum is the perineal body. The anal sphincters are located in the posterior portion of the perineal body. The transverse perinei muscle traverses the perineal body and is often used in anal sphincteroplasty and rectovaginal fistula repair. The dentate line is the grossly visible demarcation between the squamous anal epithelium and the transitional-columnar epithelium of the rectum. The anal glands open into the bases of the anal crypts at this location. The lowest extent of the peritoneal cavity in the female lies in the pelvis and may be anterior to the cervix uteri and/or posterior to it. The occupation of this space by the small bowel is called an enterocele; when the space is occupied by the sigmoid colon, this is termed a sigmoidocele. Laboratory Studies Laboratory studies (eg, complete blood cell [CBC] count, blood cultures, electrolytes, blood urea nitrogen [BUN], creatinine, type and screen) are obtained to assess for sepsis, which is extremely rare in fistulas between the GI and female genital tracts. Laboratory studies are also helpful in the establishment of preoperative baselines.
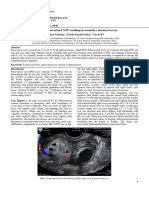
Imaging Studies Ancillary studies may illustrate a rectovaginal fistula (RVF) that is elusive on physical examination.[7] Barium enema can demonstrate RVF or the more common sigmoid-vaginal cuff fistula observed in diverticulitis. Computed tomography (CT) scanning often shows perifistular inflammation, identifying the responsible digestive organ. Other Tests Physical examination is essential. This usually confirms the diagnosis of rectovaginal fistula (RVF) and affords much information regarding its size and location, the function of the sphincters, and the possibility of IBD or local neoplasm. (Anal sphincter disruptions are commonly seen in association with RVFs of obstetric origin. Sphincter function should be evaluated prior to any repair.) Office examination usually consists of a rectovaginal examination (visual and palpation) and proctosigmoidoscopy. The fistula opening may be seen as a small dimple or pit and occasionally can be gently probed for confirmation. The suspicion of Crohn disease should be high if there is any other abnormality of the rectal mucosa or a previous or currently coexisting fistula-in-ano. Failure to recognize Crohn disease can lead to inappropriate operative intervention and can worsen the patient's situation. Placing a vaginal tampon, instilling methylene blue into the rectum, and examining the tampon after 15-20 minutes can often establish the presence of RVF. If the tampon is unstained, another part of the GI tract may be involved. Endorectal and transvaginal ultrasonography may be used to help identify low fistulas. Diagnostic Procedures Flexible endoscopy (sigmoidoscopy or colonoscopy) is used to fully evaluate the possibility of treatment varies according to the diagnosis, endoscopy with biopsies must precede any operative approach to the fistula, when IBD is in the differential diagnosis. Histologic Findings Histology is most important in the evaluation of possible IBD. Neither a diagnosis of ulcerative colitis nor of Crohn disease completely excludes operative repair of a rectovaginal fistula (RVF), but operative planning is altered, as is the prognosis. If the rectum is grossly normal in Crohn disease, the prognosis of RVF repair is fair. When the rectum is abnormal, prognosis is considerably worse. The histopathology of any fistula considered suggestive of primary or recurrent neoplasm is of the utmost importance. Medical Therapy Use local care, drainage of abscesses, and directed antibiotic therapy to treat acute rectovaginal fistulas (RVFs) of traumatic origin (including those caused by obstetric[2, 3] and operative trauma), RVFs complicated by secondary infection, and fistulas of infectious origin. Allow tissues to heal for 6-12 weeks. Dietary modification and supplemental fiber can greatly diminish symptoms during this period. Many fistulas resulting from obstetric or operative trauma heal completely, requiring no further therapy. When the fistula persists after this period of treatment and the tissues become uninflamed and supple, repair may be considered.
Perform a biopsy on any area suggestive of neoplasm. Treat neoplasms as appropriate. In this setting, very symptomatic fistulas may prompt the physician and patient to consider a diverting colostomy for patient comfort. Otherwise, fecal diversion is rarely used with RVFs.[1] If the evaluation is consistent with the diagnosis of IBD, institute appropriate medical therapy. Repair of an RVF can be performed while the patient is on steroids, with the understanding that the risk of failure is increased. Even after initial failed repair attempts, some patients with Crohn disease can maintain RVF repair while on antimetabolites, such as 6-mercaptopurine or azathioprine (Imuran). Clinical use of infliximab (Remicade)[8] suggests that few fistulas heal completely, but most patients are dramatically improved symptomatically. Predictors of failure requiring fecal diversion have been identified and include significant colonic involvement and the presence of anal stricture.[1] The development of carcinoma has been described in Crohn fistulas.[9] RVFs originating from radiation therapy are very difficult to treat surgically,[4] and medical therapy is often initially recommended in this setting. Diet and fiber are the mainstays of therapy. Surgical Therapy See Intraoperative Details. Preoperative Details Complete mechanical bowel preparation is essential for the transabdominal repair of rectovaginal fistula (RVF) and is also recommended for local repairs. The practice of including poorly absorbed oral antibiotics in the bowel preparation is under scrutiny. New data suggest that intravenous antibiotics administered in a manner that provides appropriate tissue levels at the beginning of the operative procedure are sufficient for prophylaxis. The author recommends that prophylactic intravenous antibiotics be administered preoperatively for all patients undergoing RVF repairs, transabdominal or local. Although diverting colostomy was used in the past, the overwhelming majority of RVFs are now repaired without this procedure being performed beforehand. Cleanse the vaginal lumen with an antiseptic solution, such as Betadine. Insert a catheter into the urinary bladder. If a transabdominal procedure is planned, perform standard preoperative cardiopulmonary evaluation as appropriate. Prophylaxis against venous thromboembolism is essential and may include the use of fractionated or unfractionated heparin, as well as the employment of sequential compression devices. If the pelvis has been irradiated or previously operated upon, the use of ureteral catheters may aid in dissection. A laparoscopic approach has been described.[10] Intraoperative Details Local repair methods Transanal advancement flap repair[11] The best results have been reported with this type of repair. General, regional, or local anesthesia may be used. The patient is placed in the prone, flexed position with a hip roll in place; the buttocks are taped apart for exposure. The fistula is identified using the operating anoscope. A flap is outlined, extending at least 4 cm cephalad to the fistula, with the base of the flap twice the width of the apex to allow adequate blood supply to the flap tip. Local
anesthetic with epinephrine is injected submucosally to facilitate raising the flap and to diminish bleeding. The flap, consisting of mucosa and submucosa, is raised; some surgeons include circular muscle as well. Meticulous hemostasis is imperative. The fistula tract is curetted gently. Circular muscle is closed over the fistula. The tip of the flap, which includes the fistula opening, is excised. The flap is sutured in place with simple interrupted, absorbable sutures, effectively closing the rectal opening of the fistula. The vaginal side of the fistula is left open for drainage. This approach separates the suture line from the fistula site and interposes healthy muscle between the rectal and vaginal walls. Proponents point out that the relatively high pressure within the rectum serves to buttress the repair, in contrast to a transvaginal repair, in which the intrarectal pressure is more prone to disrupt the repair. If indicated, sphincteroplasty can be performed concomitantly.[12] Transvaginal inversion repair The vaginal mucosa is circumferentially elevated, exposing the fistula. Two or 3 concentric pursestring sutures are used to invert the fistula into the rectal lumen. The vaginal mucosa is reapproximated. This approach is suitable only for small, low fistulas in otherwise healthy tissues with an intact perineal body. It is rarely performed today. Bioprosthetic repair Bioprosthetic interposition graft is placed by making a transverse incision over the midportion of the perineal body with dissection through the subcutaneous tissue. The fistula tract is transected. The dissection is continued 2 cm proximal to the transected fistula tract and laterally. The fistula openings are closed with 3/0 interrupted, absorbable sutures. The graft needs an overlap of 2 cm on all sides of the rectal and vaginal mucosal closures. A bioprosthetic plug is placed through the rectal opening and out the vaginal opening. The excess plug is trimmed and secured on the rectal side with 2/0 absorbable suture. Conversion to complete perineal laceration with layer closure[11] The fistulous tract is laid open in the midline, essentially creating a cloaca. Closure in layers follows, identical to the classic obstetric repair of a fourth-degree perineal laceration. This method is described in the gynecologic literature; it is rarely employed by colorectal surgeons because of concerns of juxtaposed suture lines. Simple fistulotomy This procedure works well for true anovaginal fistulas, in which no sphincter is involved in the tract. If the technique is used to treat an RVF, however, partial or total fecal incontinence results. Transabdominal approaches Transabdominal approaches are generally used for high RVFs when the fistula originates from a neoplasm, from radiation, or, occasionally, from IBD. They are also used if concomitant disease (eg, diverticulitis) warrants an abdominal approach. Fistula division and closure without bowel resection This is the simplest abdominal approach. The rectovaginal septum is dissected, the fistula is divided, and the rectum and vagina are closed primarily without bowel resection. Interposition of healthy tissue, such as omentum, may be used to buttress the repair and separate the suture lines. Good results have been reported when the fistula is not large and the tissues available for closure are healthy.
Bowel resection When tissues are abnormal because of irradiation, inflammation, or neoplasm, the repair is doomed to failure unless the abnormal tissues are resected. Preserve functional anal sphincters whenever possible by use of a low anterior resection, a coloanal anastomosis technique, or a pull-through; the last alternative has the poorest results with respect to continence. Rarely, abdominoperineal resection may be necessary for symptom control in the setting of radiation damage or neoplasm. An alternative, particularly in cases of poor operative risks or with patients whose survival is limited, is simple fecal diversion with a loop ileostomy or colostomy. Ancillary procedures A host of supplementary procedures have been described to augment bowel resection in the difficult pelvis. These include local flaps, such as the bulbocavernosus flap, and a variety of muscle, fascial, and musculocutaneous flaps for repair of large pelvic defects. A variety of graft procedures also have been described.[13] All of these procedures have the goal of interposing healthy tissue between vaginal and rectal repairs. These are well described in the plastic surgery literature. Postoperative Details Local repairs Pay attention to the patient's bowel habits. Constipation or diarrhea can disrupt a repair. The goal is a soft, formed, deformable stool. The patient is carefully counseled regarding diet, copious fluid intake, and the use of stool softeners. The use of bulking agents immediately after repair is at the discretion of the surgeon and is a matter of individual preference rather than of scientifically proven practice. The use of oral antibiotics also varies. The author prefers that patients use an oral broad-spectrum antibiotic for 3-5 days postoperatively, take 1 tablespoon of mineral oil orally twice daily for 2 weeks postoperatively, and avoid bulking agents for 2 weeks postoperatively. The patients need to refrain from sexual activity or any physical activity more strenuous than a slow walk for 3 weeks. Abdominal repairs Postoperative care is identical to the care administered to all patients who have undergone major laparotomy with bowel resection and anastomosis. Postoperative gastric decompression is performed selectively, expecting that 15-20% of patients require cessation of oral intake or gastric decompression for symptomatic postoperative ileus. Most patients can be offered sips of clear liquids on the first postoperative day. Early ambulation is beneficial in many ways. Continue perioperative prophylaxis for thromboembolic events until the patient is ambulating well. Follow-up Patients are seen 2 weeks after discharge for evaluation of wounds and bowel habits. In the absence of recurrent fistula symptoms or other specific indications, no follow-up investigation, aside from physical examination, is required. Specific signs and symptoms are investigated appropriately. For example, fever, diarrhea, and low abdominal pain indicating an abscess are evaluated by a CT scan of the abdomen and pelvis. In this setting, physical examination may be difficult because of patient discomfort. Complications
Complications of local repairs Bleeding is rarely encountered postoperatively, probably because of careful intraoperative hemostasis. If bleeding occurs beneath the flap, fistula recurrence is common. Infection is a feared complication, because it almost invariably results in a failed repair. However, good data on the incidence of infection after local repair are few. Of course, repairs may fail in the absence of infection as well (see Outcome and Prognosis). Rarely, postoperative pain precipitates urinary retention. Complications of transabdominal repairs These may include the usual complications of any laparotomy with bowel resection, including fistula recurrence. The most common complications are bleeding and wound infection, each with an incidence of less than 2-5% in reasonable-risk candidates. Pelvic abscess occurs in 5-7% of patients. Data from the United States and Europe suggest that anastomotic leaks occur more often than is clinically recognized. However, because intervention is indicated only in clinically evident leaks, routine postoperative anastomotic evaluation is not warranted. Outcome and Prognosis Local repair methods Transanal advancement flap repair This approach to rectovaginal fistula (RVF) repair is very safe. Results are good to excellent, with success reported in 77-100% of patients in various series. Reports have noted the importance of preoperative assessment of anal sphincter integrity. Sphincter repair is easily performed simultaneously and increases the success rate of RVF repair. Vaginal childbirth after RVF repair is not associated with increased risk of RVF recurrence. However, if a sphincter repair is performed along with the RVF repair, many surgeons recommend cesarean delivery for subsequent pregnancies in order to avoid disruption of the sphincteroplasty. Transvaginal inversion repair and conversion to complete perineal laceration with layer closure Results from these approaches can be acceptable in selected cases, as noted above (see Intraoperative Details). Bioprosthetic repair This is a new technique for RVF repair. Early experience indicates that it produces results that are equal or superior to those of advancement flap repair.[14] The new button fistula plug has been successful in 58% of rectovaginal and ileal pouch-vaginal fistulas.[15] Simple fistulotomy As noted, this is suitable for true anovaginal fistulas only, which incorporate no sphincter muscle whatsoever. Application of this approach to RVF results in incontinence. Transabdominal approaches With approximation of healthy tissue in the absence of inflammation, infection, or tension, transabdominal repairs yield good long-term results. Always consider the morbidities of major abdominal surgery and any coexistent morbidities related to the patient's history. Patients with fistulas due to radiation may have added morbidities associated with other irradiated tissues. These morbidities include (1) cystitis; (2) ureteral complications, including
stricture and obstruction; (3) vascular injury, including stenosis and occlusion; (4) small bowel injury, including stricture, malabsorption, and obstruction; (5) neurologic complications; and (6) bony complications, including necrosis and fractures. Prognosis of recurrent RVFs Recurrence of an RVF confers a poorer prognosis for future repair attempts.[16]Rectal sleeve advancement had an overall healing rate of 75% for persistent rectovaginal fistulas.[17] Recurrence is influenced by the etiology of the fistula and by its complexity. Fistulas of obstetric origin and fistulas that are considered simple (rather than complex) fare better after repeated repair attempts. Future and Controversies Crohn disease Rectovaginal fistulas (RVFs) associated with Crohn disease are difficult to manage.[5, 18] When symptoms are few, operative intervention may not be indicated. Conversely, severely symptomatic patients may require proctectomy. Patients with relatively normal rectal mucosa and an RVF are good candidates for an endorectal advancement flap. In this specific setting, outcome is good, although it is not as good as in patients without Crohn disease. An endorectal advancement flap is considered the preferred technique for local RVF repair in patients with Crohn disease and a relatively normal rectum. A multivariable logistic regression model identified immunomodulators as being associated with successful healing and smoking and steroid usage as being associated with failure.[19] Bricker patch The on-lay Bricker patch also has been used to repair RVFs, chiefly those produced by radiation. Briefly summarized, the rectosigmoid colon is mobilized transabdominally, and the RVF is exposed. The rectosigmoid is divided above the fistula. The proximal end is brought out as an end sigmoid colostomy. The distal rectosigmoid is turned down, and the open end is anastomosed to the debrided edge of the rectal opening of the fistula, essentially creating an internal loop with drainage through the anus. When healing of the inferior-patched rectum can be demonstrated radiologically several months later, continuity of the colon is reestablished by anastomosis of the colostomy to the apex of the patch loop in an end-to-side fashion. Advantages to this procedure may include less difficulty than with resection approaches; therefore, less morbidity of hemorrhage and organ injury occurs. Disadvantages include the radiation-damaged rectum being left in place and in use, with the possibility of further morbidity, including bleeding and stricture. Although situations exist in which this approach may be preferable to a resection approach, the author believes that resection of the radiation-damaged bowel provides the best long-term results in patients who are reasonable operative candidates. References 1. 2. 3. Galandiuk S, Kimberling J, Al-Mishlab TG, et al. Perianal Crohn disease: predictors of need for permanent diversion. Ann Surg. May 2005;241(5):796-801; discussion 801-2. Bangser M. Obstetric fistula and stigma. Lancet. Feb 11 2006;367(9509):535-6. Browning A, Menber B. Women with obstetric fistula in Ethiopia: a 6-month follow up after surgical treatment. BJOG. Nov 2008;115(12):1564-9.
4. 5. 6.
Bricker EM, Johnston WD. Repair of postirradiation rectovaginal fistula and stricture. Surg Gynecol Obstet. Apr 1979;148(4):499-506. Cohen JL, Stricker JW, Schoetz DJ, et al. Rectovaginal fistula in Crohn's disease. Dis Colon Rectum. Oct 1989;32(10):825-8. Giordano P, Gravante G, Sorge R, Ovens L, Nastro P. Long-term outcomes of stapled hemorrhoidopexy vs conventional hemorrhoidectomy: a meta-analysis of randomized controlled trials. Arch Surg. Mar 2009;144(3):266-72. Shobeiri SA, Quiroz L, Nihira M. Rectovaginal fistulography: a technique for the identification of recurrent elusive fistulas. Int Urogynecol J Pelvic Floor Dysfunct. Jan 22 2009;. Gonzalez-Lama Y, Abreu L, Vera MI, et al. Long-term oral tacrolimus therapy in refractory to infliximab fistulizing Crohn's disease: a pilot study. Inflamm Bowel Dis. Jan 2005;11(1):8-15. Laurent S, Barbeaux A, Detroz B, et al. Development of adenocarcinoma in chronic fistula in Crohn's disease. Acta Gastroenterol Belg. Jan-Mar 2005;68(1):98-100.
7.
8.
9.
10. Kumaran SS, Palanivelu C, Kavalakat AJ, et al. Laparoscopic repair of high rectovaginal fistula: is it technically feasible?. BMC Surg. 2005;5:20. 11. Casadesus D, Villasana L, Sanchez IM, et al. Treatment of rectovaginal fistula: a 5-year review. Aust N Z J Obstet Gynaecol. Feb 2006;46(1):49-51. 12. Khanduja KS, Yamashita HJ, Wise WE Jr. Delayed repair of obstetric injuries of the anorectum and vagina. A stratified surgical approach. Dis Colon Rectum. Apr 1994;37(4):344-9. 13. Jasonni VM, La Marca A, Manenti A. Rectovaginal fistula repair using fascia graft of autologous abdominal muscles. Int J Gynaecol Obstet. Jan 2006;92(1):85-6. 14. Ellis CN. Outcomes after repair of rectovaginal fistulas using bioprosthetics. Dis Colon Rectum. Jul 2008;51(7):1084-8. 15. Gonsalves S, Sagar P, Lengyel J, Morrison C, Dunham R. Assessment of the efficacy of the rectovaginal button fistula plug for the treatment of ileal pouch-vaginal and rectovaginal fistulas. Dis Colon Rectum. Nov 2009;52(11):1877-81. 16. Ulrich D, Roos J, Jakse G, et al. Gracilis muscle interposition for the treatment of rectourethral and rectovaginal fistulas: a retrospective analysis of 35 cases. J Plast Reconstr Aesthet Surg. Jan 20 2009;. 17. Schouten WR, Oom DM. Rectal sleeve advancement for the treatment of persistent rectovaginal fistulas.Tech Coloproctol. Dec 2009;13(4):289-94. 18. Loffler T, Welsch T, Muhl S, et al. Long-term success rate after surgical treatment of anorectal and rectovaginal fistulas in Crohn's disease. Int J Colorectal Dis. Jan 27 2009;. 19. El-Gazzaz G, Hull T, Mignanelli E, Hammel J, Gurland B, Zutshi M. Analysis of function and predictors of failure in women undergoing repair of Crohn's related rectovaginal fistula. J Gastrointest Surg. May 2010;14(5):824-9. 20. Burke C. Rectovaginal fistulas. Clin J Oncol Nurs. Jun 2005;9(3):295-7. 21. Fry RD, Kodner IJ. Rectovaginal fistula. Surg Annu. 1995;27:113-31.
22. Hilger WS, Cornella JL. Rectovaginal fistula after posterior intravaginal slingplasty and polypropylene mesh augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. Jan 2006;17(1):89-92. 23. Husain A, Johnson K, Glowacki CA, et al. Surgical management of complex obstetric fistula in Eritrea. J Womens Health (Larchmt). Nov 2005;14(9):839-44. 24. MacRae HM, McLeod RS, Cohen Z. Treatment of rectovaginal fistulas that has failed previous repair attempts. Dis Colon Rectum. Sep 1995;38(9):921-5. 25. Miklos JR, Kohli N. Rectovaginal fistula repair utilizing a cadaveric dermal allograft. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10(6):405-6. 26. Moore RD, Miklos JR, Kohli N. Rectovaginal fistula repair using a porcine dermal graft. Obstet Gynecol. Nov 2004;104(5 Pt 2):1165-7. 27. Nowacki MP. Ten years of experience with Parks' coloanal sleeve anastomosis for the treatment of post-irradiation rectovaginal fistula. Eur J Surg Oncol. Dec 1991;17(6):5636. 28. Steichen FM, Barber HK, Loubeau JM, et al. Bricker-Johnston sigmoid colon graft for repair of postradiation rectovaginal fistula and stricture performed with mechanical sutures. Dis Colon Rectum. Jun 1992;35(6):599-603. 29. Tsang CB, Madoff RD, Wong WD. Anal sphincter integrity and function influences outcome in rectovaginal fistula repair. Dis Colon Rectum. Sep 1998;41(9):1141-6. 30. Tsang CB, Rothenberger DA. Rectovaginal fistulas. Therapeutic options. Surg Clin North Am. Feb 1997;77(1):95-114.
Anda mungkin juga menyukai
- Rectovaginal and Anovaginal Fistulas - UpToDateDokumen39 halamanRectovaginal and Anovaginal Fistulas - UpToDateROzi BarriosBelum ada peringkat
- Genital FistulaeDokumen27 halamanGenital Fistulaeapi-3705046100% (1)
- Lower Genital Tract Precancer: Colposcopy, Pathology and TreatmentDari EverandLower Genital Tract Precancer: Colposcopy, Pathology and TreatmentBelum ada peringkat
- Rectovaginal Fistulae: Bidhan Das, MD Michael Snyder, MDDokumen7 halamanRectovaginal Fistulae: Bidhan Das, MD Michael Snyder, MDNovaBelum ada peringkat
- VVF Clinical Presentation 1Dokumen24 halamanVVF Clinical Presentation 1api-370504683% (6)
- VVF Clinical Presentation 1Dokumen24 halamanVVF Clinical Presentation 1georgeloto12Belum ada peringkat
- Genital FistulaeDokumen15 halamanGenital Fistulaesangeetha francisBelum ada peringkat
- 5 Congenital Malformation (Continue)Dokumen4 halaman5 Congenital Malformation (Continue)Mohamed Al-zichrawyBelum ada peringkat
- Vesicoureteral Reflux: Done By: Khalid Al-Qudsi Faisal Burghal Supervised By: Dr. Osama Bani HaniDokumen27 halamanVesicoureteral Reflux: Done By: Khalid Al-Qudsi Faisal Burghal Supervised By: Dr. Osama Bani HaniRashed ShatnawiBelum ada peringkat
- EsophagusDokumen168 halamanEsophagusnancy voraBelum ada peringkat
- Operative Considerations For Rectovaginal Fistulas: Kevin R Kniery, Eric K Johnson, Scott R SteeleDokumen6 halamanOperative Considerations For Rectovaginal Fistulas: Kevin R Kniery, Eric K Johnson, Scott R SteeleEmillyVidyaAgathaRelmasiraBelum ada peringkat
- Presentation VVFDokumen60 halamanPresentation VVFMairmon KhanBelum ada peringkat
- Intussusception 161007042729 PDFDokumen44 halamanIntussusception 161007042729 PDFDina MarselinaBelum ada peringkat
- Genital Injury, VVF, RVFDokumen65 halamanGenital Injury, VVF, RVFDevuchandana RBelum ada peringkat
- Ectopic Pregnancy: by Amielia Mazwa Rafidah Obstetric and Gynecology DepartmentDokumen43 halamanEctopic Pregnancy: by Amielia Mazwa Rafidah Obstetric and Gynecology DepartmentAlrick AsentistaBelum ada peringkat
- Female ReproductiveDokumen13 halamanFemale Reproductiveta CBelum ada peringkat
- Obstetric Emergencies: First - Trimester Pregnancy EmergenciesDokumen14 halamanObstetric Emergencies: First - Trimester Pregnancy EmergenciesdianBelum ada peringkat
- Absceso y Fistula AnalDokumen24 halamanAbsceso y Fistula AnalSinue PumaBelum ada peringkat
- Ectopic PregnancyDokumen14 halamanEctopic PregnancytaufiqBelum ada peringkat
- Ruiz, P. - SGD and NCP On Imperforate AnusDokumen8 halamanRuiz, P. - SGD and NCP On Imperforate AnusPatricia Dianne RuizBelum ada peringkat
- 15b Ectopicpregnancy 090507104012 Phpapp02 130825152041 Phpapp01Dokumen48 halaman15b Ectopicpregnancy 090507104012 Phpapp02 130825152041 Phpapp01Erwynsonsaut SimanjuntakBelum ada peringkat
- Clinical Practice Guideline For The Management of Anorectal Abscess Fistula-In-Ano and Rectovaginal FistulaDokumen17 halamanClinical Practice Guideline For The Management of Anorectal Abscess Fistula-In-Ano and Rectovaginal FistulaAlivia HanumBelum ada peringkat
- Laparoscopic Exploration in Pediatric Surgery EmergenciesDokumen6 halamanLaparoscopic Exploration in Pediatric Surgery EmergenciesFathiah HusainBelum ada peringkat
- The Management of Genitourinary Fistula in The Third MillenniumDokumen9 halamanThe Management of Genitourinary Fistula in The Third MillenniumDikiArmaDuhaAlaydrusBelum ada peringkat
- Appendix Part 2Dokumen7 halamanAppendix Part 2Abdullah EssaBelum ada peringkat
- Atlas of Vesicovaginal FistulaDokumen7 halamanAtlas of Vesicovaginal FistulaYodi SoebadiBelum ada peringkat
- DD AppDokumen3 halamanDD AppVicky XieBelum ada peringkat
- Articulo HC CG IIrDokumen6 halamanArticulo HC CG IIrKelly F RuizBelum ada peringkat
- Ectopic Pregnancy22Dokumen43 halamanEctopic Pregnancy22Sunil YadavBelum ada peringkat
- Vesicoureteral RefluxDokumen5 halamanVesicoureteral RefluxHUSAMBelum ada peringkat
- Borderline Paratubal Cyst: A Case ReportDokumen10 halamanBorderline Paratubal Cyst: A Case ReportqisthiaufaBelum ada peringkat
- Rectal Prolapse: Scott D. Goldstein, M.D. and Pinckney J. Maxwell IV, M.DDokumen7 halamanRectal Prolapse: Scott D. Goldstein, M.D. and Pinckney J. Maxwell IV, M.Dade-djufrieBelum ada peringkat
- Abdominal Approach To Vesicovaginal FistulaDokumen12 halamanAbdominal Approach To Vesicovaginal FistulaPoto SucioBelum ada peringkat
- IJGMP - Medicine - Repair of Long Standing Radiation Induced Vesico - Prashant DinkarDokumen6 halamanIJGMP - Medicine - Repair of Long Standing Radiation Induced Vesico - Prashant Dinkariaset123Belum ada peringkat
- Anorectal Abscess: Principles of Internal Medicine, 18E. New York, Ny: Mcgraw-Hill 2012Dokumen7 halamanAnorectal Abscess: Principles of Internal Medicine, 18E. New York, Ny: Mcgraw-Hill 2012Irene SohBelum ada peringkat
- 2 5 21 861 PDFDokumen3 halaman2 5 21 861 PDFAhmed ZidanBelum ada peringkat
- Diagnostic LaparosDokumen8 halamanDiagnostic Laparosdrhiwaomer100% (3)
- Hysterosalpingography A Re - Emerging Study With CDokumen7 halamanHysterosalpingography A Re - Emerging Study With CTuyul Yelsi 1Belum ada peringkat
- Vesicovaginal FistulaDokumen6 halamanVesicovaginal FistulaMaiza TusiminBelum ada peringkat
- Preinvasive Disease of TheDokumen18 halamanPreinvasive Disease of TheSondang Natalia SiallaganBelum ada peringkat
- ONCOLOGICAL GYNECOLOGY - Malignant Tumors of The Vagina and Modalities of TreatmentDokumen9 halamanONCOLOGICAL GYNECOLOGY - Malignant Tumors of The Vagina and Modalities of TreatmentVesna AntovskaBelum ada peringkat
- Buccal Mucosa Urethroplasty For Adult Urethral Strictures - PMCDokumen13 halamanBuccal Mucosa Urethroplasty For Adult Urethral Strictures - PMCEllya Syahfitri 2108125983Belum ada peringkat
- Cloacal MalformationsDokumen6 halamanCloacal MalformationsRayhanun MardhatillahBelum ada peringkat
- Bab Ii Tinjauan PustakaDokumen7 halamanBab Ii Tinjauan PustakaDMdewiBelum ada peringkat
- Hymen ImperforataDokumen17 halamanHymen ImperforataLinda AuroraBelum ada peringkat
- Pelvic Inflammatory Disease: Bibo Yuan M.D.,PH.DDokumen51 halamanPelvic Inflammatory Disease: Bibo Yuan M.D.,PH.DNorman AjxBelum ada peringkat
- Fernandez 2021Dokumen24 halamanFernandez 2021Waseem MoukhtarBelum ada peringkat
- Inguinal and Scrotal Disorders.Dokumen11 halamanInguinal and Scrotal Disorders.Pablo GuamanBelum ada peringkat
- Anorectal MalformationsDokumen19 halamanAnorectal MalformationsJumrotun Ni'mahBelum ada peringkat
- Figo 2012 VaginaDokumen3 halamanFigo 2012 VaginaEunike_oisBelum ada peringkat
- Anal AtresiaDokumen13 halamanAnal AtresiaAhmad IhsanBelum ada peringkat
- Best Practice & Research Clinical Obstetrics and GynaecologyDokumen12 halamanBest Practice & Research Clinical Obstetrics and GynaecologyMarely GuemesBelum ada peringkat
- AppendicitisDokumen36 halamanAppendicitisPetro MyronovBelum ada peringkat
- Colonic ObstructionDokumen4 halamanColonic ObstructionKristine AlejandroBelum ada peringkat
- Anal Abscess and FistulaDokumen12 halamanAnal Abscess and FistulaGustavoZapataBelum ada peringkat
- Urinary Fistula - EAU-Guidelines-on-Non-neurogenic-Female-LUTS-2022 - 2022-05-12-115954 - KVPRDokumen7 halamanUrinary Fistula - EAU-Guidelines-on-Non-neurogenic-Female-LUTS-2022 - 2022-05-12-115954 - KVPRBayu HernawanBelum ada peringkat
- 1 CervixDokumen12 halaman1 Cervixzianab aliBelum ada peringkat
- 2.obstruction of The Urinary TractDokumen22 halaman2.obstruction of The Urinary TractAkli JahBelum ada peringkat
- TOEFL and Confersation Discussion ClassDokumen2 halamanTOEFL and Confersation Discussion ClassRomi Mauliza FauziBelum ada peringkat
- Jhptump A Anifitriya 755 1 Artikel RDokumen12 halamanJhptump A Anifitriya 755 1 Artikel RRomi Mauliza FauziBelum ada peringkat
- BR J Ophthalmol-2014-Chan-79-81 PDFDokumen4 halamanBR J Ophthalmol-2014-Chan-79-81 PDFRomi Mauliza FauziBelum ada peringkat
- Follow Up Balance CairanDokumen3 halamanFollow Up Balance CairanRomi Mauliza FauziBelum ada peringkat
- Rectovaginal FistulaDokumen8 halamanRectovaginal FistulaRomi Mauliza FauziBelum ada peringkat
- Master Data Uji ValiditasDokumen2 halamanMaster Data Uji ValiditasRomi Mauliza FauziBelum ada peringkat
- New Jurnal RomiDokumen4 halamanNew Jurnal RomiRomi Mauliza FauziBelum ada peringkat
- Maternal and Child Mortality Is A Measure of The Soundness of A NationDokumen1 halamanMaternal and Child Mortality Is A Measure of The Soundness of A NationRomi Mauliza FauziBelum ada peringkat
- J Neurol Neurosurg Psychiatry 2011 Jankovic 1300 3Dokumen5 halamanJ Neurol Neurosurg Psychiatry 2011 Jankovic 1300 3Romi Mauliza FauziBelum ada peringkat
- J Neurol Neurosurg Psychiatry 2012 Kiernan 1 2Dokumen3 halamanJ Neurol Neurosurg Psychiatry 2012 Kiernan 1 2Romi Mauliza FauziBelum ada peringkat
- Headache Disorders in A Tertiary Care Headache Outpatient Clinic in AustriaDokumen1 halamanHeadache Disorders in A Tertiary Care Headache Outpatient Clinic in AustriaRomi Mauliza FauziBelum ada peringkat
- J Neurol Neurosurg Psychiatry 2003 Fuller Ii20 4Dokumen6 halamanJ Neurol Neurosurg Psychiatry 2003 Fuller Ii20 4Romi Mauliza FauziBelum ada peringkat
- The Galveston Orientation and Amnesia TestDokumen1 halamanThe Galveston Orientation and Amnesia TestRomi Mauliza Fauzi100% (1)
- Berita Buku Apr Jun08Dokumen4 halamanBerita Buku Apr Jun08Muhammad Rizki AidillahBelum ada peringkat
- Ling Kung AnDokumen3 halamanLing Kung AnRomi Mauliza FauziBelum ada peringkat
- Hubungan Karakteristik Dengan DepresiDokumen5 halamanHubungan Karakteristik Dengan DepresiChaterine KnowickyBelum ada peringkat
- InformasiDokumen3 halamanInformasiRomi Mauliza FauziBelum ada peringkat
- KedokteranDokumen24 halamanKedokteranRomi Mauliza FauziBelum ada peringkat
- Neoplasms of The Biliary TractDokumen186 halamanNeoplasms of The Biliary Tracthgr58patologiaBelum ada peringkat
- Metaplastic (Chronic) Atrophic Gastritis - UpToDate PDFDokumen16 halamanMetaplastic (Chronic) Atrophic Gastritis - UpToDate PDFDinaBelum ada peringkat
- AppendicitisDokumen38 halamanAppendicitisEmaan Tanveer100% (1)
- Nasogastric Tube FeedingDokumen7 halamanNasogastric Tube FeedingVina Empiales100% (1)
- Temporal Sphenoidal Line: T4 Gall BladderDokumen3 halamanTemporal Sphenoidal Line: T4 Gall Bladdertaichi7Belum ada peringkat
- Lactose Intolerance - Symptoms, Causes and Precautions - Dr. Samrat JankarDokumen4 halamanLactose Intolerance - Symptoms, Causes and Precautions - Dr. Samrat JankarDr. Samrat JankarBelum ada peringkat
- PEDIA ER FormularyDokumen1 halamanPEDIA ER FormularyAnonymous ic2CDkFBelum ada peringkat
- Gallstone Disease and Acute CholecystitisDokumen21 halamanGallstone Disease and Acute CholecystitisTanyaNganBelum ada peringkat
- Running A Colorectal Surgery ServiceDokumen21 halamanRunning A Colorectal Surgery ServicebloodsphereBelum ada peringkat
- Canine Gastric Pathology A ReviewDokumen29 halamanCanine Gastric Pathology A ReviewRachel AutranBelum ada peringkat
- Intestinal PermeabilityDokumen6 halamanIntestinal PermeabilitysakuraleeshaoranBelum ada peringkat
- Hirschsprung Disease Case Study: Maecy P. Tarinay BSN 4-1Dokumen5 halamanHirschsprung Disease Case Study: Maecy P. Tarinay BSN 4-1Maecy OdegaardBelum ada peringkat
- 11 AppendixDokumen15 halaman11 AppendixOmar MohammedBelum ada peringkat
- LPRDokumen6 halamanLPRGoranJankovicBelum ada peringkat
- Bariatric SurgeryDokumen71 halamanBariatric Surgeryanalourdes87Belum ada peringkat
- Nursing Care Plan - FormatDokumen6 halamanNursing Care Plan - FormatKyle VargasBelum ada peringkat
- The Digestive System and Body MetabolismDokumen3 halamanThe Digestive System and Body MetabolismJonash MoralesBelum ada peringkat
- PeritonitisDokumen34 halamanPeritonitisabrar_zaidiBelum ada peringkat
- Detailed Lesson PlanDokumen6 halamanDetailed Lesson Planジョイス エンジェル67% (3)
- History Taking SurgeryDokumen8 halamanHistory Taking SurgeryKingston HoBelum ada peringkat
- Bahasa Inggris (SOAL)Dokumen13 halamanBahasa Inggris (SOAL)niana hiddlestonBelum ada peringkat
- Sialadenosis in A Patient With Alcoholic Fatty Liv PDFDokumen3 halamanSialadenosis in A Patient With Alcoholic Fatty Liv PDFPutri AmaliaBelum ada peringkat
- Laporan PasienDokumen248 halamanLaporan Pasiennasita kristianaBelum ada peringkat
- Dyspepsia: Group 3ADokumen14 halamanDyspepsia: Group 3AAradhanaRamchandaniBelum ada peringkat
- Gastro Intestinal Disorders:: Kawasaki DiseaseDokumen6 halamanGastro Intestinal Disorders:: Kawasaki DiseaseJoanna TaylanBelum ada peringkat
- Ganguan PankreasDokumen31 halamanGanguan PankreasMeilia Ayu SuariBelum ada peringkat
- Gastroenterology EsophagusDokumen2 halamanGastroenterology EsophagusJayanthBelum ada peringkat
- Histology of LiverDokumen9 halamanHistology of LiverSunil BasnetBelum ada peringkat
- Herbivore Means Plant Eater: Difference Between Digestive Tract of Herbivores Vs CarnivoresDokumen10 halamanHerbivore Means Plant Eater: Difference Between Digestive Tract of Herbivores Vs CarnivoresariyatiBelum ada peringkat
- NCMB 316 Assignment NCP Part 2Dokumen3 halamanNCMB 316 Assignment NCP Part 2I'm a PepegaBelum ada peringkat
- Glucose Revolution: The Life-Changing Power of Balancing Your Blood SugarDari EverandGlucose Revolution: The Life-Changing Power of Balancing Your Blood SugarPenilaian: 5 dari 5 bintang5/5 (351)
- Forever Strong: A New, Science-Based Strategy for Aging WellDari EverandForever Strong: A New, Science-Based Strategy for Aging WellBelum ada peringkat
- The Diabetes Code: Prevent and Reverse Type 2 Diabetes NaturallyDari EverandThe Diabetes Code: Prevent and Reverse Type 2 Diabetes NaturallyPenilaian: 4.5 dari 5 bintang4.5/5 (3)
- The Beck Diet Solution Weight Loss Workbook: The 6-Week Plan to Train Your Brain to Think Like a Thin PersonDari EverandThe Beck Diet Solution Weight Loss Workbook: The 6-Week Plan to Train Your Brain to Think Like a Thin PersonPenilaian: 3.5 dari 5 bintang3.5/5 (33)
- Summary of Mary Claire Haver's The Galveston DietDari EverandSummary of Mary Claire Haver's The Galveston DietPenilaian: 5 dari 5 bintang5/5 (1)
- Instant Loss On a Budget: Super-Affordable Recipes for the Health-Conscious CookDari EverandInstant Loss On a Budget: Super-Affordable Recipes for the Health-Conscious CookPenilaian: 3.5 dari 5 bintang3.5/5 (2)
- Grit & Grace: Train the Mind, Train the Body, Own Your LifeDari EverandGrit & Grace: Train the Mind, Train the Body, Own Your LifePenilaian: 4 dari 5 bintang4/5 (3)
- The Body Book: The Law of Hunger, the Science of Strength, and Other Ways to Love Your Amazing BodyDari EverandThe Body Book: The Law of Hunger, the Science of Strength, and Other Ways to Love Your Amazing BodyBelum ada peringkat
- Sugar Crush: How to Reduce Inflammation, Reverse Nerve Damage, and Reclaim Good HealthDari EverandSugar Crush: How to Reduce Inflammation, Reverse Nerve Damage, and Reclaim Good HealthPenilaian: 4 dari 5 bintang4/5 (6)
- Metabolism Revolution: Lose 14 Pounds in 14 Days and Keep It Off for LifeDari EverandMetabolism Revolution: Lose 14 Pounds in 14 Days and Keep It Off for LifeBelum ada peringkat
- Summary: Fast Like a Girl: A Woman’s Guide to Using the Healing Power of Fasting to Burn Fat, Boost Energy, and Balance Hormones: Key Takeaways, Summary and AnalysisDari EverandSummary: Fast Like a Girl: A Woman’s Guide to Using the Healing Power of Fasting to Burn Fat, Boost Energy, and Balance Hormones: Key Takeaways, Summary and AnalysisPenilaian: 3 dari 5 bintang3/5 (2)
- Gut: The Inside Story of Our Body's Most Underrated Organ (Revised Edition)Dari EverandGut: The Inside Story of Our Body's Most Underrated Organ (Revised Edition)Penilaian: 4 dari 5 bintang4/5 (378)
- Find Your Path: Honor Your Body, Fuel Your Soul, and Get Strong with the Fit52 LifeDari EverandFind Your Path: Honor Your Body, Fuel Your Soul, and Get Strong with the Fit52 LifePenilaian: 4 dari 5 bintang4/5 (3)
- The Diet Trap Solution: Train Your Brain to Lose Weight and Keep It Off for GoodDari EverandThe Diet Trap Solution: Train Your Brain to Lose Weight and Keep It Off for GoodBelum ada peringkat
- Body Love Every Day: Choose Your Life-Changing 21-Day Path to Food FreedomDari EverandBody Love Every Day: Choose Your Life-Changing 21-Day Path to Food FreedomPenilaian: 4 dari 5 bintang4/5 (1)
- The Arm: Inside the Billion-Dollar Mystery of the Most Valuable Commodity in SportsDari EverandThe Arm: Inside the Billion-Dollar Mystery of the Most Valuable Commodity in SportsPenilaian: 4 dari 5 bintang4/5 (49)
- Secrets From the Eating Lab: The Science of Weight Loss, the Myth of Willpower, and Why You Should Never Diet AgainDari EverandSecrets From the Eating Lab: The Science of Weight Loss, the Myth of Willpower, and Why You Should Never Diet AgainPenilaian: 3.5 dari 5 bintang3.5/5 (38)
- Molecules of Emotion: Why You Feel the Way You FeelDari EverandMolecules of Emotion: Why You Feel the Way You FeelPenilaian: 4 dari 5 bintang4/5 (128)
- The Food Lover's Cleanse: 140 Delicious, Nourishing Recipes That Will Tempt You Back into Healthful EatingDari EverandThe Food Lover's Cleanse: 140 Delicious, Nourishing Recipes That Will Tempt You Back into Healthful EatingPenilaian: 4 dari 5 bintang4/5 (3)
- Eat to Lose, Eat to Win: Your Grab-n-Go Action Plan for a Slimmer, Healthier YouDari EverandEat to Lose, Eat to Win: Your Grab-n-Go Action Plan for a Slimmer, Healthier YouBelum ada peringkat
- The Candida Cure: The 90-Day Program to Balance Your Gut, Beat Candida, and Restore Vibrant HealthDari EverandThe Candida Cure: The 90-Day Program to Balance Your Gut, Beat Candida, and Restore Vibrant HealthBelum ada peringkat
- The Volumetrics Eating Plan: Techniques and Recipes for Feeling Full on Fewer CaloriesDari EverandThe Volumetrics Eating Plan: Techniques and Recipes for Feeling Full on Fewer CaloriesBelum ada peringkat
- The End of Craving: Recovering the Lost Wisdom of Eating WellDari EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellPenilaian: 4.5 dari 5 bintang4.5/5 (81)
- Keto Friendly Recipes: Easy Keto For Busy PeopleDari EverandKeto Friendly Recipes: Easy Keto For Busy PeoplePenilaian: 3.5 dari 5 bintang3.5/5 (2)
- How to Be Well: The 6 Keys to a Happy and Healthy LifeDari EverandHow to Be Well: The 6 Keys to a Happy and Healthy LifePenilaian: 5 dari 5 bintang5/5 (1)
- How Not to Die by Michael Greger MD, Gene Stone - Book Summary: Discover the Foods Scientifically Proven to Prevent and Reverse DiseaseDari EverandHow Not to Die by Michael Greger MD, Gene Stone - Book Summary: Discover the Foods Scientifically Proven to Prevent and Reverse DiseasePenilaian: 4.5 dari 5 bintang4.5/5 (84)
- Glucose Goddess Method: A 4-Week Guide to Cutting Cravings, Getting Your Energy Back, and Feeling AmazingDari EverandGlucose Goddess Method: A 4-Week Guide to Cutting Cravings, Getting Your Energy Back, and Feeling AmazingPenilaian: 5 dari 5 bintang5/5 (61)