Case 7: Hydranencephaly: Alfred B. Kurtz, MD Pamela T. Johnson, MD
Diunggah oleh
Kenzo Adhi WiranataDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Case 7: Hydranencephaly: Alfred B. Kurtz, MD Pamela T. Johnson, MD
Diunggah oleh
Kenzo Adhi WiranataHak Cipta:
Format Tersedia
Alfred B. Kurtz, MD Pamela T.
Johnson, MD
Case 7: Hydranencephaly1
a.
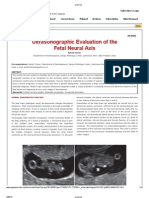
b. Figure 1. US images of the fetal head at 36 weeks gestation. (a) Transaxial image near the vertex demonstrates a discontinuous falx midline echo (curved arrow). There is no identifiable cortical mantel. (b) Transaxial image at the level of the normal thalami (T) again shows the disrupted falx midline echo (curved arrow). Normal hyperechoic choroid plexuses (straight arrows) are seen posterior to the thalami, and a small amount of occipital cortex remains, posterior to both. There again is no demonstrable cortical mantle (the echoes seen are artifactual). (c) Transaxial image slanted posteriorly to depict the posterior fossa demonstrates the midbrain (M) and the disrupted falx echo (curved arrow). The triangular posterior fossa with an intact cerebellum (straight arrows) and a normal cisterna magna (*) are seen.
c.
Index terms: Brain, abnormalities Brain, growth and development Fetal, abnormalities Diagnosis please Radiology 1999; 210:419422
1
HISTORY
A 22-year-old pregnant woman, gravida 4 para 3 (with three normal children) presented at 34 gestational weeks for her first prenatal care visit. Ultrasonographic (US) images showed an enlarged head in a single, live fetus (Fig 1). One month later, the woman was delivered of a 4,870 g (10 lb 7 oz) boy by means of cesarean section. The newborn had a head circumference of 42 cm (normal 34.5 cm). Computed tomography (CT) of the newborns head was performed on day 2 (Fig 2).
419
From the Department of Radiology, Thomas Jefferson University Hospital, Gibbon Bldg 3350AB, 111 S 11th St, Philadelphia, PA 19107. Received February 17, 1998; revision requested March 18; revision received April 8; accepted June 10. Address reprint requests to A.B.K. RSNA, 1999
a.
c. Figure 2. CT scan of the newborns head, without use of intravenous contrast material. (a) Transaxial view near the vertex shows a disrupted falx (curved arrow). No normal cortical mantle remains. (b) Transaxial view at the level of the normal thalami (T) shows normal choroid plexuses (solid arrows) posteriorly. Some occipital cortex (open arrows) remains. (c) Transaxial view through the base shows a normal posterior fossa, including cerebellum.
b.
IMAGING FINDINGS
US scans (Fig 1) of the enlarged fetal head demonstrated a discontinuous falx midline echo and no identifiable cortical mantel. Normal hyperechoic choroid plexuses were seen posterior to normal thalami, and a small amount of occipital cortex remained, posterior to both. The midbrain was preserved and an image of the posterior fossa demonstrated an intact cerebellum and a normal cisterna magna. Two days after delivery, CT of the newborns head was performed without intravenous contrast material (Fig 2). A disrupted falx was noted. No normal cortical mantle except some occipital cortex could be identified. At the level of the normal thalami, normal choroid plexuses were seen posteriorly. The posterior fossa including the cerebellum was normal.
DISCUSSION
Hydranencephaly is a rare, isolated abnormality occurring in less than 1 per 10,000 births worldwide (13). It is the most severe form of bilateral cerebral cortical destruction. The differential diagnosis includes bilaterally symmetric schizencephaly
420 Radiology February 1999
(a less severe destructive process), severe hydrocephalus, and alobar holoprosencephaly (a developmental anomaly). Hydranencephaly occurs after the brain and ventricles have fully formed, usually in the second trimester. The brain destruction is complete or almost complete in a bilateral internal carotid artery distribution, with the cerebral hemispheres replaced by fluid covered with leptomeninges and dura. During the destructive phase, unusual masses of hemorrhage and soft tissue may be seen (4). Because the ventricles have already been formed, the falx cerebri is present. The cerebellum, midbrain, thalami, basal ganglia, choroid plexus, and portions of the occipital lobes, all fed by the posterior circulation, are typically preserved. With most of the cerebral cortex absent, the fetal head would be expected to be small. Although this may occur, the head is more often normal or increased in size because the choroid plexuses within the lateral ventricles continue to produce cerebral spinal fluid that is not adequately absorbed. This causes increased pressure, which may expand the head and lead to rupture of the falx cerebri. Both of these findings were present in this case. While the pathogenesis of hydranencephaly is thought to be a vascular accident, this cannot always be confirmed because
Kurtz and Johnson
Figure 3. Moderate to severe hydrocephalus secondary to aqueductal stenosis. A transaxial US scan of a fetus at 30 weeks gestation demonstrates an enlarged fetal head with thinned but present temporoparietal cortical mantle (arrows) along the posterolateral aspect of the calvaria. The third ventricle (*) is also dilated between the thalami. Although the temporoparietal cortical mantle is also present anteriorly, it cannot be appreciated because of reverberation artifacts.
Figure 4. Alobar holoprosencephaly in a fetus at 22 weeks gestation. Coronal US image of a small fetal head shows fused thalami (T). A monoventricle (V) is identified without a normal falx echo. L left, R right.
internal carotid arteries are not always occluded at autopsy (1). Intrauterine infections, particularly toxoplasmosis and viral infections (enterovirus, adenovirus, parvovirus, cytomegalic, herpes simplex, Epstein-Barr, and respiratory syncytial viruses), have been implicated in a number of cases. Toxic exposures and cocaine abuse have been reported, and hydranencephaly has been described in rare syndromes (5). In monochorionic twin pregnancies, death of one twin in the second trimester may cause a vascular exchange to the living twin through the placental circulation, leading to hydranencephaly in the surviving fetus (6). Hydranencephaly may, on first impression, mimic severe hydrocephalus (dilated lateral ventricles)(2). Depending on the level of obstruction, concomitant dilatation of the third and fourth ventricles may be seen. The incidence of hydrocephalus approaches 1 in 1,000 births. Although there are many causes, the most common is an Arnold-Chiari type II malformation secondary to a spina bifida. The most severe cases, however, are usually secondary to aqueductal stenosis. Hydrocephalus is often not an isolated anomaly and can be associated with other intracranial abnormalities, multiple anomaly syndromes, and abnormal karyotype (7). With hydrocephalus, as with hydranencephaly, the head is normal to enlarged with an identifiable falx cerebri, which may be disrupted in severe cases. Unlike in hydranencephaly, an intact rim of cortex is always present even in the most severe forms of hydrocephalus (Fig 3). It may, however, be difficult to identify prenatally. In aqueductal stenosis, a dilated third ventricle can often also be identified. A porencephalic cyst is a focal area of cortical destruction (1,2). When caused by middle cerebral artery infarctions, porencephalic cysts appear as bilateral fluid-filled clefts that communicate with the ventricles and is called schizencephaly. Unlike in hydranencephaly, both the frontal and parietooccipital cortex are preserved. The falx cerebri is also preserved. Abnormal brain growth and development may result, dependVolume 210 Number 2
ing on the timing of the occlusions. The fetal head can be either normal or enlarged. Holoprosencephaly is a developmental anomaly resulting from absent or incomplete diverticulation of the forebrain (prosencephalon) and occurs in 1 in 16,000 live births worldwide. Alobar, its most severe form, shows no separation of the ventricles, an absent falx, and partial fusion of the thalami (Fig 4). The head is often considerably smaller than the body, and there are often additional and marked abnormalities. There are important reasons to differentiate hydranencephaly from hydrocephalus; these reasons relate to prognosis and management (8,9). Children with hydrocephalus, without chromosomal or other structural abnormalities, have an unpredictable prognosis. With proper ventricular shunt after birth, mentation may in some cases be normal. In contradistinction, hydranencephaly has an irretrievably poor prognosis, with only brain stem function remaining. Although most hydranencephalic children survive birth, they often die soon after. Rarely, these children may linger into their teenage years. If the fetal head is enlarged, the differentiation of hydranencephaly from hydrocephaly has added importance because an enlarged head may not be able to be delivered vaginally. If hydranencephaly were definitively diagnosed in utero, cephalocentesis could be offered to decompress the fetal head, thus allowing a vaginal delivery. While this may damage the fetal head further, it will not change the outcome and will importantly spare the mother an unnecessary operation. On the other hand, if hydrocephalus were present, particularly without the presence of other anomalies, a cesarean section must be seriously considered.
References 1. Filly RA. Ultrasound evaluation of the fetal neural axis. In: Callen PW, ed. Ultrasonography in obstetrics and gynecology. 3rd ed. Philadelphia, Pa: Saunders, 1994; 199218. 2. Nyberg DA, Pretorius DH. Cerebral malformations. In: Nyberg DA, Mahony BS, Pretorius DH, eds. Diagnostic ultrasound of fetal anomalies: text and atlas. Chicago, Ill: Year Book Medical, 1990; 98121. 3. Chervenak FA, Isaacson GC, Campbell S. Hydranencephaly. In: Chervenak FA, Isaacson GC, Campbell S, eds. Ultrasound in obstetrics and gynecology. Vol 3. Boston, Mass: Little, Brown, 1993; 11871188.
Hydranencephaly 421
4.
Greene MF, Benacerraf B, Crawford JM. Hydranencephaly: US appearance during in utero evolution. Radiology 1985; 156:779780. 5. Hoyme HE, Higginbottom MC, Jones KL. Vascular etiology of disruptive structural defects in monozygotic twins. Pediatrics 1981; 67:288291. 6. Rais-Bahrami K, Naqvi M. Hydranencephaly and maternal cocaine use: a case report. Clin Pediatr 1990; 29:729730. 7. Nicolaides KH, Snijders RJM, Gosden CM, Berry C, Campbell S. Ultrasonographically detectable markers of fetal chromosomal abnormalities. Lancet 1992; 340:704707.
8.
Sutton LN, Bruce DA, Schut L. Hydranencephaly versus maximal hydrocephalus: an important clinical distinction. Neurosurgery 1980; 6:3438. 9. Iinuma K, Handa I, Kojima A, Hayamizu S, Karahashi M. Hydranencephaly and maximal hydrocephalus: usefulness of electrophysiological studies for their differentiation. J Child Neurol 1989; 4:114117.
Our congratulations to the 139 individuals who submitted the most likely diagnosis (hydranencephaly) for Diagnosis Please, Case 7. Their names and locations, as submitted, are as follows:
Gholamali Afshang, MD, Tinley Park, Ill S. I. Al-Agha, MD, Gaza, Israel S. Manucher Alavi, MD, Richmond, Va David R. Anderson, MD, Richmond, Va Roger L. Antonelli, MD, Dayton, Ohio Majed Ashour, MD, Dhahran, Saudi Arabia A. Rhett Austin, MD, Kingsport, Tenn Edward L. Baker, MD, San Francisco, Calif Kenneth Baliga, Rockford, Ill Zubin N. Balsara, MD, Fort Smith, Ark Cynthia A. Barone, DO, Shrewsbury, NJ John Bennett, MDCM, FRCPC, London, Ontario, Canada Steven L. Bezinque, DO, Williamsville, NY Tom Bonk, MD, Seattle, Wash Nikos P. Bontozoglou, MD, Athens, Greece Eric L. Bressler, MD, Minnetonka, Minn Steve Burbidge, MD, St Louis Park, Minn Joseph W. Burke, MD, Huntingdon, Pa Can Cevikol, Antalya, Turkey Ercument Ciftci, MD, Houston, Tex Frederick U. Conard III, MD, Hartford, Conn Philippe A. Coquel, MD, Cran-Gevrier, France Mark T. DiMarcangelo, DO, MSc, Cherry Hill, NJ Vinay Duddalwar, Aberdeen, United Kingdom Peter English, FRACR, Hong Kong, China Keith D. Epperson, MD, Milwaukee, Wis Kate A. Feinstein, MD, Chicago, Ill Laura Zindell Fenton, MD, Denver, Colo Sylvia H. Ford, MD, Green Bay, Wis Jonathan Foss, MD, St Louis, Mo Michael A. Foster, MD, Cherry Hills Village, Colo Mary C. Frates, MD, Boston, Mass Jeffrey Friedland, Glendale, Colo Arnold C. Friedman, MD, New York, NY Stuart A. Fruman, MD, Vienna, Va Akira Fujikawa, Tokyo, Japan Douglas Gardner, MD, Windsor, Ontario, Canada Ronald B. J. Glass, MD, New York, NY Ishikawa Goemon, Shiga, Japan Jacob A. Goldenberg, MD, Fargo, ND Dulce Gomez-Santos, MD, Madrid, Spain Devang Gor, DMRD, Costa Mesa, Calif Daniel S. Gordon MD, MAJ USA MC, Sanford, NC Dr. Rajesh Gothi, New Delhi, India Athanassios D. Gouliamos, Athens, Greece D. Joseph Grunz, MD, Creve Coeur, Mo Mark Guelfguat, Flushing, NY Dr Arunima Gupta, Ludhiana, India Sunita Gupta, MD, Francistown, Botswana David C. Harrison, MD, Cambridge, Mass Rufus W. Head, MD, North Bridgton, Me Maureen Heldmann, MD, Shreveport, La Elizabeth Hingsbergen, MD, Richmond, Va Carlos Holguera Blazquez, MD, Madrid, Spain Lowrey H. Holthaus, MD, Richmond, Va Kamil Karaali, Antalya, Turkey Aake Karlsson Douglas S. Katz, MD, Mineola, NY Ji Chang Kim, MD, Taejon, Korea Mitchell Klein, MD, Milwaukee, Wis Arlene M. Klink, MD, Bronx, NY John D. Knudtson, MD, Wichita, Kan Craig D. Korbin, MD, Weston, Mass Dawna J Kramer, MD, Seattle, Wash Dr. Renee G. Kulkarni, New Delhi, India Yu-Ting Kuo, MD, Taiwan, ROC Dong Lin Kwak, MD, Roanoke, Va Kathleen M. Lazzarini, MD, Branford, Conn Jong Beum Lee, MD, Seoul, Korea
Ronaldo Lessa, Jr, MD, Recife, Brazil Julio Loureiro, MD, Buenos Aires, Argentina David R. Ludwig, MD, Amherst, NY Anto nio Jose Madureira, MD, Porto, Portugal Gildo Matta, MD, Cagliari, Italy James M. McAfee, MD, West Linn, Ore Jeffrey J. McClure, MD, Grand Rapids, Mich Steven Medwid, MD, Fall River, Mass Edward Menges, MD, Aptos, Calif Frank H. Miller, MD, Chicago, Ill Manabu Minami, MD, Tokyo, Japan Hidetoshi Miyake, MD, Oita, Japan Sergio J. Moguillansky, MD, Rio Negro, Argentina Silvia Moguillansky, Buenos Aires, Argentina Eduardo Mondello, Buenos Aires, Argentina Albert Nizzero, MD, Sudbury, Ontario, Canada Aksel Ongre, Arendal, Norway Mahesh R. Patel, MD, Brookline, Mass Narendrakumar Patel, MD, Newburgh, NY Eduardo Pavon Tinoco MD, Oaxaca, Mexico Tim L. Pendergrass, MD, Fairchild AFB, Wash Dr Roberto E. Perez Gautrin, Sonora, Mexico Marvin W. Petry, MD, Chicago, Ill Pedro J. S. Pinto, MD, Gondomar, Portugal John Plotke, MD, Naperville, Ill Gary Podolny, MD, Park City, Utah Carlos H. Previgliano, MD, Salta, Argentina Anita Price, MD, Mineola, NY Shawn P. Quillin, MD, Charlotte, NC M. R. Ramakrishnan, MD, Big Stone Gap, Va Oswaldo A. Ramos, MD, Estado Trujillo, Venezuela Lorenz (Larry) Ramseyer, MD, Enid, Okla Enrique Remartinez Escobar, Melilla, Spain Marco A. Rocha Mello, MD, Sao Paulo, Brazil Javier Rodriguez Lucero, MD, Santa Fe, Argentina Derek J. Roebuck, FRACR, Hong Kong, China Stuart A. Royal, MD, Birmingham, Ala Dr Eduardo Sanchez Heras Paul S. Schaefer, MD Steven M. Schultz, MD, Fort Worth, Tex Anthony J. Scuderi, MD Hassan Semaan, Toledo, Ohio A. Utku Senol, Antalya, Turkey Waldo Sepulveda, MD, Santiago, Chile Matt Shapiro, MD, Boxborough, Mass Yoshihisa Shimanuki, MD, Yamagata, Japan L.H. Sie, MD, Beverwijk, the Netherlands James F. Smith, Columbia, Mo Eric R. Sover, DO, Brookfield, Wis Joanne B. Speigle, MD, Richmond, Va Michael S. Stecker, MD, Iowa City, Iowa Marius Stellmann, MD, Rotenburg, Germany Simon Strauss, MBChB, Kfar Shmaryahu, Israel Jeffrey Y. Sue, MD, Honolulu, Hawaii Christopher Sweet, MD, Clarkston, Mich Koyama Takashi, Kyoto, Japan J. Takasugi, Mercer Island, Wash Oscar Tenreiro Picon, MD, Maracay, Venezuela J. Keith Thompson, MD, Richmond, Va Joseph Z. H. Toutounji, MD, Beirut, Lebanon Carlos Triana Rodriguez, Santefe de Bogota, Colombia Herminia Tyminski, MD, Manama, Bahrain T. E. G. van Zanten, MD, Haarlem, the Netherlands Andrew L. Wagner, MD, Durham, NC Chien-Kuo Wang, MD, Taiwan, ROC Edward W. Williams, FRCR, Cayman Islands, British West Indies Joseph T. Wroblicka, MD, Iowa City, Iowa Masanobu Yasuda, MD, Kanagawa, Japan Dr. Zhang Youbin, Gaborone, Botswana
422 Radiology February 1999
Kurtz and Johnson
Anda mungkin juga menyukai
- Neural Tube DefectsDokumen12 halamanNeural Tube Defectsdaniel_1592Belum ada peringkat
- The Myth of Autism: How a Misunderstood Epidemic Is Destroying Our Children, Expanded and Revised EditionDari EverandThe Myth of Autism: How a Misunderstood Epidemic Is Destroying Our Children, Expanded and Revised EditionPenilaian: 2.5 dari 5 bintang2.5/5 (3)
- Emergency in DermatologyDokumen47 halamanEmergency in DermatologyKenzo Adhi WiranataBelum ada peringkat
- Differential Diagnosis of Intracranial AnomaliesDokumen4 halamanDifferential Diagnosis of Intracranial AnomaliesFitrianidilaBelum ada peringkat
- HydrancephalyDokumen3 halamanHydrancephalyinammetBelum ada peringkat
- Hydranencephaly: Case NoteDokumen4 halamanHydranencephaly: Case NoteAndinaBelum ada peringkat
- Hydranencephaly: A Rare Cause of An Enlarging Head Size in An InfantDokumen3 halamanHydranencephaly: A Rare Cause of An Enlarging Head Size in An InfantdyahBelum ada peringkat
- Ultrasonographic Evaluation of The Fetal Neural Axis: JournalsDokumen10 halamanUltrasonographic Evaluation of The Fetal Neural Axis: Journalsdgina8800Belum ada peringkat
- Sonography of Congenital Midline Brain MalformationsDokumen15 halamanSonography of Congenital Midline Brain MalformationsJohana MartakBelum ada peringkat
- 51187-Article Text-75903-1-10-20100216Dokumen2 halaman51187-Article Text-75903-1-10-20100216Diana Indah 1608111531Belum ada peringkat
- Alobar Holoprosencephaly Is A Subtype ofDokumen2 halamanAlobar Holoprosencephaly Is A Subtype ofYustina Nada Jon PutriBelum ada peringkat
- Exencephaly-Anencephaly Sequence: Consult SeriesDokumen4 halamanExencephaly-Anencephaly Sequence: Consult SeriesMegaSariDewiBelum ada peringkat
- Blake's Pouch Cyst: An Entity Within The Dandy-Walker ContinuumDokumen6 halamanBlake's Pouch Cyst: An Entity Within The Dandy-Walker ContinuumJessica GilesBelum ada peringkat
- The Essential Identity of The Klippel-Feil Syndrome and IniencephalyDokumen16 halamanThe Essential Identity of The Klippel-Feil Syndrome and IniencephalyIsolda Alanna RlBelum ada peringkat
- Labrune 1997Dokumen6 halamanLabrune 1997dad dzd adaBelum ada peringkat
- Cephalohematoma, CaputDokumen5 halamanCephalohematoma, CaputSusana TorresBelum ada peringkat
- Govaert2009 Prenatal StrokeDokumen17 halamanGovaert2009 Prenatal StrokeModou NianeBelum ada peringkat
- Case ReportDokumen6 halamanCase ReportJellie MendozaBelum ada peringkat
- Congenital Malformations of The BrainDokumen57 halamanCongenital Malformations of The Brainmanisha paikarayBelum ada peringkat
- Essentials of Neurology 2Dokumen153 halamanEssentials of Neurology 2prahul2588100% (7)
- Sato Neuro Cases After CourseDokumen81 halamanSato Neuro Cases After CourseghassanBelum ada peringkat
- Embryology of The Hind GutDokumen10 halamanEmbryology of The Hind Gutproject-247758Belum ada peringkat
- Cranium BifidaDokumen7 halamanCranium BifidafebriantaraBelum ada peringkat
- Cornell A 2003Dokumen13 halamanCornell A 2003dnazaryBelum ada peringkat
- Robinson 2006Dokumen1 halamanRobinson 2006Alejandro TpnsBelum ada peringkat
- The Cisterna Magna SeptaDokumen13 halamanThe Cisterna Magna SeptaFrancisco A. Villegas-LópezBelum ada peringkat
- HydrocephalusDokumen8 halamanHydrocephalusJean Albine CatipanBelum ada peringkat
- Disrafismo Spinal OcultaDokumen4 halamanDisrafismo Spinal OcultaCarlos CalderwoodBelum ada peringkat
- Neural Tube DefectsDokumen15 halamanNeural Tube DefectsadriricaldeBelum ada peringkat
- Congenitalhydrocephalus: Chelsie M. EsteyDokumen13 halamanCongenitalhydrocephalus: Chelsie M. Esteyfalon papalangiBelum ada peringkat
- Antenatal Fetal Face and Neck IMAGINGDokumen102 halamanAntenatal Fetal Face and Neck IMAGINGSUVEC100% (1)
- Antenatal Fetal Face and Neck IMAGINGDokumen102 halamanAntenatal Fetal Face and Neck IMAGINGSUVECBelum ada peringkat
- DiscursoDokumen21 halamanDiscursoLeslye SimbañaBelum ada peringkat
- Stippled Epiphyses in The Newborn and in Infants (Synonyms: Chondrodystrophia Calcificans Congenita, Dysplasia Epiphysialis Punctata)Dokumen18 halamanStippled Epiphyses in The Newborn and in Infants (Synonyms: Chondrodystrophia Calcificans Congenita, Dysplasia Epiphysialis Punctata)Prateek Kumar PandaBelum ada peringkat
- 9w HoloprosencephalyDokumen4 halaman9w HoloprosencephalyVũ Quang ĐăngBelum ada peringkat
- Journal of Diagnostic Medical Sonography 2010 Moore 286 9Dokumen4 halamanJournal of Diagnostic Medical Sonography 2010 Moore 286 9Hanarisha Putri AzkiaBelum ada peringkat
- CP ResearchDokumen13 halamanCP ResearchReinaJazzIslaBelum ada peringkat
- HydrocephalusDokumen40 halamanHydrocephalusAstrid Sabirin100% (1)
- Notes in Pediatrics. 2 Ed. 2001/02 Senior & Junior Peds RotationsDokumen18 halamanNotes in Pediatrics. 2 Ed. 2001/02 Senior & Junior Peds Rotationsopis08100% (1)
- Children With Spina BifidaDokumen14 halamanChildren With Spina Bifidafabsscribdworks100% (1)
- Antenatal Diagnosis ofDokumen6 halamanAntenatal Diagnosis ofnskhldBelum ada peringkat
- 3I. Scoiuosis: AnomaliesDokumen7 halaman3I. Scoiuosis: AnomaliesValentina OprisanBelum ada peringkat
- HSS Clinical Pronostic ConsiderationDokumen4 halamanHSS Clinical Pronostic ConsiderationReyes Ivan García CuevasBelum ada peringkat
- Hydra Nence Pha LyDokumen10 halamanHydra Nence Pha LyAya SalBelum ada peringkat
- Hydrocephalus UpdatesDokumen65 halamanHydrocephalus Updatescddinchimm100% (1)
- ArticleDokumen6 halamanArticleحمزہ محبBelum ada peringkat
- Sibs With Anencephaly, Anophthalmia, Clefts, Omphalocele, and Polydactyly: Hydrolethalus orDokumen4 halamanSibs With Anencephaly, Anophthalmia, Clefts, Omphalocele, and Polydactyly: Hydrolethalus orSarly FebrianaBelum ada peringkat
- Hydrocephalus AND Neural Tube DefectDokumen7 halamanHydrocephalus AND Neural Tube DefectTherese ArellanoBelum ada peringkat
- Hydrocephalus AND Neural Tube DefectDokumen7 halamanHydrocephalus AND Neural Tube DefectTherese ArellanoBelum ada peringkat
- Congenital LaryngomalaciaDokumen8 halamanCongenital LaryngomalaciaRettha SigiroBelum ada peringkat
- Manx Cat StudiesDokumen7 halamanManx Cat Studieslmary20074193Belum ada peringkat
- Dyscephalia Mandibulo-Oculo-FacialisDokumen5 halamanDyscephalia Mandibulo-Oculo-FacialisReyes Ivan García CuevasBelum ada peringkat
- Maller2019 Neonatal Head Ultrasound Part 2Dokumen12 halamanMaller2019 Neonatal Head Ultrasound Part 2Modou NianeBelum ada peringkat
- Large HeadDokumen2 halamanLarge HeadMedoLacieBelum ada peringkat
- Tetralogy of Fallot - StatPearls - NCBI BookshelfDokumen9 halamanTetralogy of Fallot - StatPearls - NCBI BookshelfElfrida AuliaBelum ada peringkat
- Neonatal Head Protocol 14Dokumen5 halamanNeonatal Head Protocol 14api-276847924Belum ada peringkat
- Incidence of The Bell'Clapper Deformity in An Autopsy SeriesDokumen3 halamanIncidence of The Bell'Clapper Deformity in An Autopsy SeriesOttofianus Hewick KalangiBelum ada peringkat
- MedulloblastomaDokumen2 halamanMedulloblastomaMohammadAwitBelum ada peringkat
- The Myth of Autism: How a Misunderstood Epidemic Is Destroying Our ChildrenDari EverandThe Myth of Autism: How a Misunderstood Epidemic Is Destroying Our ChildrenPenilaian: 2.5 dari 5 bintang2.5/5 (8)
- Textbook of Special Pathological Anatomy of Domestic AnimalsDari EverandTextbook of Special Pathological Anatomy of Domestic AnimalsBelum ada peringkat
- Cleft Lip and Palate Management: A Comprehensive AtlasDari EverandCleft Lip and Palate Management: A Comprehensive AtlasRicardo D. BennunBelum ada peringkat
- DHMOSH Public Health Unit COVID-19: Outbreak Reporting Form: InstructionsDokumen3 halamanDHMOSH Public Health Unit COVID-19: Outbreak Reporting Form: InstructionsKenzo Adhi WiranataBelum ada peringkat
- Covid 19 Facilities (Indo Fpu Minusca)Dokumen9 halamanCovid 19 Facilities (Indo Fpu Minusca)Kenzo Adhi WiranataBelum ada peringkat
- Use of Antibody Tests V6Dokumen9 halamanUse of Antibody Tests V6Kenzo Adhi WiranataBelum ada peringkat
- List of Staff Medical For Assisting Swab Sampling of Covid 19 For 39 CONTACT CASES OF INDO FPUDokumen1 halamanList of Staff Medical For Assisting Swab Sampling of Covid 19 For 39 CONTACT CASES OF INDO FPUKenzo Adhi WiranataBelum ada peringkat
- Standard Operating Procedure (SOP) Tasks Calendar PWS Name: Schedule of Tasks For The YearDokumen3 halamanStandard Operating Procedure (SOP) Tasks Calendar PWS Name: Schedule of Tasks For The YearKenzo Adhi WiranataBelum ada peringkat
- SocialDokumen9 halamanSocialKenzo Adhi WiranataBelum ada peringkat
- Conjunctival Tumours: 1. BenignDokumen12 halamanConjunctival Tumours: 1. BenignKenzo Adhi WiranataBelum ada peringkat
- Methyl ErgometrineDokumen1 halamanMethyl ErgometrineKenzo Adhi WiranataBelum ada peringkat