Quantum Chemistry Quantum Chemistry
Diunggah oleh
3334333Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Quantum Chemistry Quantum Chemistry
Diunggah oleh
3334333Hak Cipta:
Format Tersedia
QuantumChemistry Quantum Chemistry
Introduction Introduction
Statistical Averaging
Electromagnetic Spectrum Electromagnetic Spectrum
Black body Radiation
NC State Universityy
Overview Overview
Quantum Mechanics
Failure of classical physics p y
Wave equation
Rotational, vibrational and translational motion
Electronic structure
Spectroscopy
Statistical Mechanics
Partition function
Average energy
Entropy
Macroscopic viewof equilibrium Macroscopic view of equilibrium
For a chemical reaction in which X is the
reactant and Y is the product
X Y X Y
with free energy change AG
o
the equilibrium with free energy change AG
o
, the equilibrium
constant is
K =e
AG
o
/RT
=
[Y]
[X]
Microscopic viewof equilibrium Microscopic view of equilibrium
For a system where there are two energy
states, which we can call X and Y
YY
X
AE
X
with energy difference AE, the ratio of
population is
N
population is
where N and N are the numbers of
e
AE/kT
=
N
Y
N
X
where N
Y
and N
X
are the numbers of
particles in each state.
Boltzmanns constant Boltzmanns constant
Note that we k instead of R in the exponent.
k
B
is called Boltzmanns constant and it is
related to R by:
R
k =
R
N
A
B
where N
A
is Avagadros number.
k
B
T has units of J oules and is a measure of
the thermal energy of a molecule
RT has units of J oules/mole and is a measure
of the thermal energy of a mole
Microscopic probability Microscopic probability
When we look at the world in terms of energy
levels we can see that the probability of
being in a given level depends on temperature.
We define the probability of being in level j
as
number of molecules in state j
or
P
l
=
number of molecules in state j
total number of molecules
j
P
l
=
N
j
N +N
j
N
X
+N
Y
i
j
j
Microscopic probability Microscopic probability
We can calculate the probability of levels A
and B
P =
N
X
=
1
=
1
P
X
=
N
X
+N
Y
=
1 +N
Y
/N
X
=
1 +e
AE/kT
N N /N
AE/kT
P
Y
=
N
Y
N
X
+N
Y
=
N
Y
/N
X
1 +N
Y
/N
X
=
e
AE/kT
1 +e
AE/kT
Of course, P
X
+ P
Y
= 1
Average energy for
a two level system
We defined the relative energy of the two We defined the relative energy of the two
states as AE = E
Y
E
X
.
We can (arbitrarily) set the energy of state We can (arbitrarily) set the energy of state
X to zero, E
X
= 0.
Then the energy of state Y is E =AE Then the energy of state Y is E
Y
= AE.
The average energy of the system is:
<E >= P
j
E
j
E
i =1
=P
X
E
X
+P
Y
E
Y
e
AE/kT
E
e
AE/kT
AE =
e
1 +e
AE/kT
E
Y
=
e
1 +e
AE/kT
AE
Extension to many levels y
If we have a system with an infinite number
f ll d l l of equally spaced energy levels we can
calculate the probability and energy in the
same way.
jAE/kT
AE
P
j
=
e
jAE/kT
1 +e
AE/kT
++e
2AE/kT
++e
3AE/kT
+...
<E >= PE
E
AE
<E >= P
j
E
j
E
i =1
=
e
AE/kT
AE +2e
2AE/kT
AE +2e
3AE/kT
AE +...
1
AE/kT 2AE/kT 3AE/kT
3
1 +e
AE/kT
++e
2AE/kT
++e
3AE/kT
+...
Partition function for
l dd f l l ladder of energy levels
The denominator gives the average number of levels g g
that are accessible at a given temperature.
We can simplify it as follows:
Q =1 +e
AE/kT
+e
2AE/kT
+e
3AE/kT
+...
Let x =e
AE/kT
, then Q =1 +x +x
2
+x
3
+... , Q
Q 1 =x +x
2
+x
3
+x
3
+...
and
xQ =x +x
2
+x
3
+x
3
+...
since Q 1 =xQ
Q =
1
1 x
=
1
1 e
AE/kT
Calculating the energy
It is not obvious how to obtain the energy for this
system. We can consider the general formula and y g
make the substitution | = 1/kT.
<E >= P
j
E
j
E
<E > P
j
E
j
E
i =1
=
e
|AE
AE +2e
2|AE
AE +2e
3|AE
AE +...
1 +e
|AE
++e
2|AE
++e
3|AE
+
3
1 +e ++e ++e +...
=
cQ/c|
Q
For the energy ladder we have
1 e
|AE
e
|AE
AE
AE
<E >=
1 e
|AE
2
=
AE
e
|AE
1
High temperature limit
As the temperature approaches infinity all of the
levels become equally populated. The average
energy can be calculated assuming | = 1/kT << 1.
<E >=
AE
|AE
=
AE
1 |AE 1
=
1
|
=kT
Thus, kT is a classical energy for an averaged
E
e
|AE
1
1 |AE+.... 1 |
kT
Thus, kT is a classical energy for an averaged
system at high temperature. In units of joules/mole
<E >=RT
The energy of a gas is related to its PV
m
product
(V i th l l ) th
<E >=RT
(V
m
is the molar volume) thus,
PV
m
=RT
The wavelength and the frequency
f l t ti di ti of electromagnetic radiation
The wavelength is the distance between the
peaks in a traveling wave.
I l i l h i li ht i th t t l In classical physics light is a wave that travels
with velocity c. The frequency is v = c/.
The wavenumber v =v /c The
~
~
The wavenumber v v /c. The
wavenumber has units of cm
-1
.
~
The electromagnetic spectrum
increasing
v decreasing
The wavelength and frequency are inversely related
g
The wavelength and frequency are inversely related.
Frequency and wavelength are
inversely related inversely related
decreasing
v increasing
From the
Brittanica Encyclopedia
The electric field vector
The electric field vector is most important for
spectroscopy. The oscillation of the electric vector
i t t ith th h l d t d interacts with the charge clouds on atoms and
molecules to give rise to an oscillating dipole.
It is this oscillating dipole that gives rise to It is this oscillating dipole that gives rise to
spectroscopic transitions.
Poynting vector
Electric vector
Two extreme view of an oscillating dipole
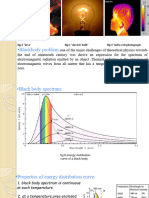
Black body Radiation Black body Radiation
An ideal emitter of radiation is called a black
body.
O f f Observation: that peak of the energy of
emission shifts to shorter wavelengths as the
temperature is increased temperature is increased.
Wien displacement law: Wien displacement law:
max
T = 2.88 x 10
6
nm-K.
Dilemma for Classical Physics Dilemma for Classical Physics
The maximum in energy for the black body
spectrumis not explained by classical physics spectrum is not explained by classical physics.
The cavity modes of the black body are
predicted to be predicted to be
=
8t k
B
T
4
Wh i th di t d it Thi f ti
4
Where is the radiant energy density. This function
increases without bound as 0. This law is known
as the Rayleigh-J eans law.
UV Catastrophe UV Catastrophe
=
8tk
B
T
The Planck Distribution Law The Planck Distribution Law
Planck assumed quantization of cavity
modes: E = nhv. (n=0,1,2..)
The constant h determines that only those
modes with an energy specified by the
i t i b it d precise amounts given can be excited.
The population of the levels will favor lower
( t b ) d energy (quantum number) modes over
higher energies.
Planck Assumption implies that average p p g
energy is temperature dependent
In classical physics the average energy in an
energy level is < E > = kT.
Quantized levels imply that the average energy in
each oscillator is < E > = hv/(e
hv/kT
- 1).
Si l it thi Since c = v we can also write this as
< E > = hc/(e
hc/kT
- 1).
To obtain the Planck formula simply replace kT To obtain the Planck formula simply replace kT
by the < E > = hc/ (e
hc/kT
- 1)
expression implied by quantization. expression implied by quantization.
Plancks Innovation
Classical radiation
All frequencies
Quantized radiation
Only frequencies nhv All frequencies
are possible
Only frequencies nhv
are allowed
Mathematical Form of
the Planck Law
The energy levels will be populated according to
a thermal weighting. The higher levels will be
l l t d th th l l l less populated than the lower levels.
In the Planck theory the energy density
becomes: becomes:
=
8t hc 1
=
5
e
hc / k
B
T
1
UV Catastrophe UV Catastrophe
=
8tk
B
T
Resolution of UV Catastrophe
8 h 1
=
8thc
5
1
e
hc / k
B
T
1
=
8tk
B
T
Planck Distribution Law Planck Distribution Law
Consistency with Experiment Consistency with Experiment
The temperature behavior of the Rayleigh- The temperature behavior of the Rayleigh
J eans law is recovered because
e
hc/kT
1 ~ hc/kT as T
The integral of the total energy is proportional
to T
4
which gives the Stefan-Boltzmann law,
W = oT
4
.
W is the flux or energy/area.
Th Wi di l t l i d f The Wien displacement law is recovered from
differentiation of . Setting d/d = 0 gives
the maximumin the distribution law. the maximum in the distribution law.
Wien displacement law
0
c
c 8t hc 1
0 =
c
c
=
c
c
8t hc
5
1
e
hc / k
B
T
1
Stefan-Boltzmann law
8 h 1
W = d
0
=
8t hc
5
1
e
hc / k
B
T
1
d
0
=oT
4
Consequences of Planck Law Consequences of Planck Law
Classical physics fails to describe black-
body radiation. A model that includes y
quantized modes of electromagnetic
radiation succeeds.
Plancks constant h = 6.626 x 10
-34
J s is
a fundamental constant the determines a fundamental constant the determines
the scale of energy quantization.
The Sun is a Black Body The Sun is a Black Body
The sun and stars are black bodies The sun and stars are black bodies
The peak of the emission spectrum
depends on temperature as indicated depends on temperature as indicated
by the Wien displacement law.
What is the radiant power of the sun?
Use the Stefan-Boltzman law
W= o T
4
(W is the flux or power per unit area) ( p p )
o = 5.6704 10
-8
kg s
-3
K
-4
(Watts/m
2
/K
4
)
Assuming that the temperature at the surface is 5500 K
and the diameter is 1 4 x 10
6
km and the diameter is 1.4 x 10 km.
The way that the Sun's diameter is measured is The way that the Sun s diameter is measured is
by taking angular diameter measurements and then
translating them to linear diameter measurements.
The angular diameter of the Sun can be measured using
a telescope during a total solar eclipse or by timing a telescope during a total solar eclipse or by timing
Mercury when it is in transit in front of the Sun. The first series
of measurements were taken in the early 1700's by Jean Picard in Paris, France.
What is the radiant power of the sun?
First calculate the area and then the flux (power).
The area is A = 4tR
2
A = 4(3.1416)(7 x 10
8
)
2
A=6 16 x 10
18
m
2
A = 6.16 x 10 m
The flux is
W T
4
W= o T
4
W = 5.6704 10
-8
(5500)
4
W = 5.19 x 10
7
Watts/m
2
The total power is
P =WA=(5 19 x 10
7
Watts/m
2
)(6 16 x 10
18
m
2
) P = WA= (5.19 x 10 Watts/m)(6.16 x 10 m )
P = 3.2 x 10
26
Watts
What is the radiant power at the surface
of the earth?
We use the distance from We use the distance from
the earth to the sun
to obtain the
fl t th th flux at the earth.
The earth is
R
e
= 1.5 x 10
8
km
from the sun.
The area
irradiated is
A 4 R
2
A
e
= 4tR
e
2
A
e
= 2.83 x 10
23
m
2
What is the radiant flux at the surface
The flux in space above the earth is called the insolation
of the earth?
The flux in space above the earth is called the insolation.
The insolation is the power coming from the sun divided
by the total area at the radius of the earth.
W P/A (3 2 10
26
W tt )/(2 83 10
23 2
) W
e
= P/A
e
= (3.2 x 10
26
Watts)/(2.83 x 10
23
m
2
)
W
e
= 1.13 x 10
3
Watts/m
2
This is very close to the measured
value for radiation in space
above the earth above the earth.
How much energy does the earth absorb?
The earth has a cross-sectional area of The earth has a cross sectional area of
A
c
= tR
earth
2
A
c
= (3.1416)(1.3 x 10
7
m)
2
A 5 3 10
14 2
A
c
= 5.3 x 10
14
m
2
P
abs
= W
e
A
c abs e c
P
abs
= (1.13 x 10
3
Watts/m
2
)(5.3 x 10
14
m
2
)
P
abs
= 6 x 10
17
Watts
What is the temperature at the surface
P
b
=P
it
=6 x 10
17
Watts
of the earth?
P
abs
P
emit
6 x 10 Watts
P
emit
= o T
earth
4
A
earth
[A
earth
= 4t R
earth
2
= 2.1 x 10
15
m
2
]
T
earth
= (P
emit
/o A
earth
)
1/4
T (6 10
17
/5 6704 10
8
/2 1 10
15
)
1/4
T
earth
= (6 x 10
17
/5.6704 10
-8
/2.1 x 10
15
)
1/4
T
earth
= 266 K
This is close, but it is a little frosty.
Why is this?
P.S. What is 266 K in
o
C?
What is 266 K in
o
F?
What is the temperature at the surface
P
b
=P
it
=6 x 10
17
Watts
of the earth?
P
abs
P
emit
6 x 10 Watts
P
emit
= o T
earth
4
A
earth
[A
earth
= 4t R
earth
2
= 2.1 x 10
15
m
2
]
T
earth
= (P
emit
/o A
earth
)
1/4
T (6 10
17
/5 6704 10
8
/2 1 10
15
)
1/4
T
earth
= (6 x 10
17
/5.6704 10
-8
/2.1 x 10
15
)
1/4
T
earth
= 266 K
This is close, but it is a little frosty.
Why is this?
We ignored the fact that
the earth has an atmosphere!
Th t h fl t b k The atmosphere reflects back
some of the radiated heat.
What is the role of the atmosphere?
1. Some molecules in the atmosphere absorb incident light.
Ozone absorbs UV light and prevents harmful radiation g p
from reaching the surface of the earth.
2 Molecules can also absorb emitted or radiated light 2. Molecules can also absorb emitted or radiated light.
What is the wavelength of such light? It can be obtained
from the Wien displacement law.
max
T = 2.88 x 10
6
nm-K
Thus for the sun withT =5500 K =523 nm Thus, for the sun with T = 5500 K,
max
= 523 nm
For the earth with T = 266 K,
max
= 10,800 nm = 10.8 m
f The suns emission is peaked in the visible region of the
Electromagnetic spectrum and the earth emits in the infrared.
Absorption by gases in the atmosphere
P
&
O
f
i
g
4
.
2
)
5500
o
K
266
o
K
s
s
i
o
n
b
y
(
s
o
u
r
c
e
:
P
a
n
d
e
m
i
s
b
s
o
r
p
t
i
o
n
s
e
s
l
e
c
t
i
v
e
a
b
p
h
e
r
i
c
g
a
3
.
5
S
e
l
a
t
m
o
s
p
Electronic Vibrational Rotational
The Wa e Particle D alit The Wave-Particle Duality
Th f t th t th D B li l th The fact that the DeBroglie wavelength
explains the quantization of the
h d t i h l hydrogen atom is a phenomenal
success.
Other wave-like behavior of particles
includes electron diffraction.
Particle-like behavior of waves is shown
in the photoelectric effect p
Photoelectric Effect
Electrons are ejected
froma metal surface by
e
-
from a metal surface by
absorption of a photon.
Depends on frequency,
hv
e
p q y
not on intensity.
Threshold frequency
corresponds to hv =u
Metal Surface
Kinetic
Energy
corresponds to hv
0
= u
u is the work function.
It is essentially equal to
hv
hv
u
u
gy
y q
the ionization potential
of the metal.
Insufficient energy
for photoejection
Photoejection occurs
Photoelectric Effect Photoelectric Effect
The kinetic energy of the ejected particle is given The kinetic energy of the ejected particle is given
by:
1/2 mv
2
= hv - u
The threshold energy is u, the work function.
This demonstrates the particle-like behavior of This demonstrates the particle like behavior of
photons.
A wave-like behavior would be indicated if the a e e be a o oud be dcated t e
intensity produced the effect.
Debroglie relation g
Light waves behave like particles in the
h t l t i ff t photoelectric effect.
Particles can also behave like waves. For
l l t diff t ( t f example, electrons can diffract (a property of
light waves).
Debroglie proposed a relationship between Debroglie proposed a relationship between
particle momentum and wavelength:
p =h/ p = h/
A light particle is called a photon. The
momentumis p =E/c Since c =v and E =hv momentum is p E/c. Since c v and E hv
this also gives p = h/.
Properties of exponentials Properties of exponentials
10
A
10
B
10
A+B
10
A
10
B
= 10
A+B
10
A
/10
B
=10
A-B
10
A
/10
B
= 10
A B
I f ti i l ith Inverse function is logarithm
l (10
A
) A log
10
(10
A
) = A
10
log
10
(B)
B 10
log
10
(B)
= B
Exponential to the base e Exponential to the base e
e
A
e
B
= e
A+B
e
A
/e
B
= e
A-B
Inverse function
is natural logarithm
ln(e
A
) = A
e
ln(B)
= B
Converting from
one base to another
10
A
= e
x
What is x?
A
ln(10
A
) = ln(e
x
) Take natural ln
of both sides
Aln(10) =x Solvefor x Aln(10) x Solve for x
x=23025A x = 2.3025A
Derivative: rate of change Derivative: rate of change
A c Ay cy
Ax cx
=
Slope Infinitesimal
Rate of change
Derivative: rate of change Derivative: rate of change
Example function: parabola
Derivative: rate of change Derivative: rate of change
Slope = - 80
x = - 40
Derivative: rate of change Derivative: rate of change
Slope = - 40
x = - 20
Derivative: rate of change Derivative: rate of change
Slope = 0
x = 0
Derivative: rate of change Derivative: rate of change
Slope = 40
x = 20
Derivative: rate of change Derivative: rate of change
Slope = 80
x = 40
What is the pattern? Slope = 2x
Derivative: rate of change Derivative: rate of change
Plot the slope as the green points.
Derivative: rate of change Derivative: rate of change
It is indeed a line with slope 2x.
Derivatives and Integrals
of the logarithm
Derivative dln(x)/dx = 1/x.
Integral definition of natural logarithm:
da
x
exp(x) is inverse of ln(x).
ln(x) =
da
a
1
exp(x) is inverse of ln(x).
The integral is the inverse of the derivative The integral is the inverse of the derivative.
Plot of the natural logarithm Plot of the natural logarithm
Slope of the tangent line at 1/4 Slope of the tangent line at 1/4
The derivative is the slope The derivative is the slope
x=1/4 , slope = 4
Slope of the tangent line at 1/2 Slope of the tangent line at 1/2
x=1/2 , slope = 2
Slope of the tangent line at 1 Slope of the tangent line at 1
x=1 , slope = 1
Slope of the tangent line at 2 Slope of the tangent line at 2
x=2 , slope = 1/2
Plot of the slopes of the tangents Plot of the slopes of the tangents
Plot of slope vs. x
Derivative dln(x)/dx =1/x Derivative dln(x)/dx = 1/x
f(x) = 1/x
g(x) = ln(x)
Integral of da/a:
the area under the curve
l (2)
da
2
ln(2) =
da
a
1
g(x) = ln(x)
f(a) = 1/a
Integral of da/a:
th ti f th the negative of the
area under the curve
ln(1/2) =
da
1/2
=
da
1
ln(1/2)
a
1
a
1/2
g(x) = ln(x)
f(a) = 1/a
Derivation of the Schrdinger Equation g q
The Schrdinger equation is a wave equation.
J ust as you might imagine the solution of such an equation J ust as you might imagine the solution of such an equation
in free space is a wave. Mathematically we can express a
wave as a sine or cosine function. These functions are
ll f ll d h oscillating functions. We will derive the wave equation in
free space starting with one of its solutions: sin(x).
Before we begin it is important to realize that bound states
may provide different solutions of the wave equation than
those we find for free space Bound states include those we find for free space. Bound states include
rotational and vibrational states as well as atomic wave
functions. These are important cases that will be treated
h f d t l d t di f th i i f once we have fundamental understanding of the origin of
the wave equation or Schrdinger equation.
The derivative
The derivative of a function is the instantaneous rate of change.
The derivative of a function is the slope The derivative of a function is the slope.
We can demonstrate the derivative graphically.
W id th f ti f( ) i ( ) h b l We consider the function f(x) = sin(x) shown below.
The derivative of sin(x) ( )
The derivative of a function is the instantaneous rate of change.
The derivative of a function is the slope The derivative of a function is the slope.
At sin(0) the slope is 1 as shown by the blue line.
The derivative of sin(x) ( )
The derivative of a function is the instantaneous rate of change.
The derivative of a function is the slope The derivative of a function is the slope.
At sin(t/4) the slope is 1/\2 as shown by the blue line.
The derivative of sin(x) ( )
The derivative of a function is the instantaneous rate of change.
The derivative of a function is the slope The derivative of a function is the slope.
At sin(t/2) the slope is 0 as shown by the blue line.
The derivative of sin(x) ( )
The derivative of a function is the instantaneous rate of change.
The derivative of a function is the slope The derivative of a function is the slope.
At sin(3t/4) the slope is -1/\2 as shown by the blue line.
The derivative of sin(x) ( )
The derivative of a function is the instantaneous rate of change.
The derivative of a function is the slope The derivative of a function is the slope.
At sin(3t/4) the slope is -1/\2 as shown by the blue line.
Th l f ll li h f l d bl k The slopes of all lines thus far are plotted as black squares.
The derivative of sin(x) ( )
The derivative of a function is the instantaneous rate of change.
The derivative of a function is the slope The derivative of a function is the slope.
At sin(t) the slope is -1 as shown by the blue line.
Th l f ll li h f l d bl k The slopes of all lines thus far are plotted as black squares.
The derivative of sin(x) ( )
The derivative of a function is the instantaneous rate of change.
The derivative of a function is the slope The derivative of a function is the slope.
At sin(5t/4) the slope is -1/\2 as shown by the blue line.
Th l f ll li h f l d bl k The slopes of all lines thus far are plotted as black squares.
The derivative of sin(x) ( )
The derivative of a function is the instantaneous rate of change.
The derivative of a function is the slope The derivative of a function is the slope.
At sin(5t/4) the slope is -1/\2 as shown by the blue line.
Th l f ll li h f l d bl k The slopes of all lines thus far are plotted as black squares.
The derivative of sin(x) ( )
The derivative of a function is the instantaneous rate of change.
The derivative of a function is the slope The derivative of a function is the slope.
We see from of the black squares (slopes) that the
d i i f i ( ) i ( ) derivative of sin(x) is cos(x).
The derivative of sin(x) ( )
d
sin(x) = cos(x)
d
dx
The derivative of cos(x) ( )
d
cos(x) =-sin(x)
d
dx
The second derivative of sin(x) ( )
d
d
sin(x) =-sin(x)
d
dx
d
dx
The second derivative of sin(x) ( )
d
2
sin(x) =-sin(x)
d
2
dx
2
Sin(x) is an eigenfunction ( ) g
If we define as an operator G then we have:
d
2
dx
2
-
sin(x) =sin(x)
d
2
-
dx
2
sin(x) sin(x)
dx
2
which can be written as:
Gsin(x) =sin(x)
This is a simple example of an operator equation that
is closely related to the Schrdinger equation. is closely related to the Schrdinger equation.
Sin(kx) is also an eigenfunction ( ) g
We can make the problem more general by including
a constant k This constant is called a wavevector a constant k. This constant is called a wavevector.
It determines the period of the sin function. Now we must
take the derivative of the sin function and also the
sin(kx) kcos(kx)
d
function kx inside the parentheses (chain rule).
sin(kx) =-kcos(kx)
dx
-
d
2
sin(kx) =k
2
sin(kx)
d
2
dx
2
-
Here we call the value k
2
the eigenvalue.
Sin(kx) is an eigenfunction ( ) g
of the Schrdinger equation
The example we are using here can easily be expressed
as the Schrodinger equation for wave in space. We only
have to add a constant. have to add a constant.
sin(kx) = sin(kx)
d
2
-
h
2 -
h
2
k
2 -
In this equation h is Plancks constant divided by 2t and
sin(kx) sin(kx)
dx
2
2m 2m
-
In this equation h is Plancks constant divided by 2t and
m is the mass of the particle that is traveling through space.
The eigenfunction is still sin(kx), but the eigenvalue in
this equation is actually the energy this equation is actually the energy.
The Schrdinger equation g q
Based on these considerations we can write a compact
formfor the Schrdinger equation form for the Schrdinger equation.
H+ =E+
d
2
d
2
-
h
2
2m
-
H =
Energy operator, Hamiltonian
dx
2
2m
h
2
k
2 -
E =
h
2
k
2
2m
Energy eigenvalue, Energy
+=sin(kx)
Wavefunction
The momentum
The momentum is related to the kinetic energy. Classically
The kinetic energy is:
E = mv
2
1
E = mv
2
The momentum is:
2
p = mv
So the classical relationship is: p
p
2
E =
2m 2m
If we compare this to the quantum mechanical energy:
h hk
E
h
2
k
2 -
we see that: p = hk
E =
2m
The general solution to the g
Schrdinger equation in free space
The preceding considerations are true in free space.
Since a cosine function has the same form as a sine
function, but is shifted in phase, the general solution function, but is shifted in phase, the general solution
is a linear combination of cosine and sine functions.
+ Asin(kx) +Bcos(kx)
Wavefunction
The coefficients A and B are arbitrary in free space.
+=Asin(kx) + Bcos(kx)
Wavefunction
However, if the wave equation is solved in the presence
of a potential then there will be boundary conditions. of a potential then there will be boundary conditions.
Anda mungkin juga menyukai
- KompaneetsDokumen8 halamanKompaneetsStamatis StathBelum ada peringkat
- Pocket PhysicsDokumen10 halamanPocket PhysicsFaraz KhanBelum ada peringkat
- Lecture5 PDFDokumen28 halamanLecture5 PDFDamon DixonBelum ada peringkat
- Microwave Infrared: SpectrosDokumen66 halamanMicrowave Infrared: SpectrosPrathamesh Dash100% (2)
- Slides EC574 Part I F2013Dokumen175 halamanSlides EC574 Part I F2013Lauren StevensonBelum ada peringkat
- CHEM-UA 127: Advanced General Chemistry I: I. The Classical View of The UniverseDokumen10 halamanCHEM-UA 127: Advanced General Chemistry I: I. The Classical View of The Universehkalloli@gmail.comBelum ada peringkat
- Ari Lehto - On The Planck Scale and Structures of MatterDokumen9 halamanAri Lehto - On The Planck Scale and Structures of Matter939392Belum ada peringkat
- E R Williams - New Experimental Test of Coulomb-S LawDokumen40 halamanE R Williams - New Experimental Test of Coulomb-S LawNestor GasparBelum ada peringkat
- Fundamentals of Statistical Mechanics: EnsemblesDokumen10 halamanFundamentals of Statistical Mechanics: EnsemblesRUIVTOMBelum ada peringkat
- Physics HandbookDokumen10 halamanPhysics HandbookNational Physical Laboratory100% (1)
- Basic Quantum MechanicsDokumen38 halamanBasic Quantum MechanicsAlmas GulfBelum ada peringkat
- Quantum Mechanics PyEd 342Dokumen113 halamanQuantum Mechanics PyEd 342mesfint100% (3)
- CH103 - Part 1 Physical Chemistry: y y Introduction To BondingDokumen33 halamanCH103 - Part 1 Physical Chemistry: y y Introduction To BondingNimanyu JoshiBelum ada peringkat
- Black Body RadiationDokumen3 halamanBlack Body RadiationDelta MikeBelum ada peringkat
- Rydberg's Spectrum AnalysisDokumen50 halamanRydberg's Spectrum AnalysisUday Prakash SahuBelum ada peringkat
- Inorganic Chemistry 1semesterDokumen12 halamanInorganic Chemistry 1semestermanjunathu731Belum ada peringkat
- An Overview of Computational ChemistryDokumen19 halamanAn Overview of Computational ChemistryRavindramn7Belum ada peringkat
- 1 - Nature of Matter and Chemical ChangeDokumen6 halaman1 - Nature of Matter and Chemical ChangeSagi IbiBelum ada peringkat
- Chem. 266 Physical Chemistry III: Quantum MechanicsDokumen70 halamanChem. 266 Physical Chemistry III: Quantum MechanicsJohn Edward ZapaterBelum ada peringkat
- SFSU ElectronicStructure Lect 1Dokumen31 halamanSFSU ElectronicStructure Lect 1Sonik AlexBelum ada peringkat
- Boltzmann ConstantDokumen6 halamanBoltzmann ConstantuyenphuongdangBelum ada peringkat
- QM BasicsDokumen12 halamanQM Basicsh.anurag248Belum ada peringkat
- Chapter 3Dokumen29 halamanChapter 3820604jasonBelum ada peringkat
- PHY520 Introduction To Quantum Mechanics About Myself: Kwok-Wai NG Office: CP 385 Telephone: 7-1782 E-Mail: Office Hour: Mon 10:00-11:00Dokumen12 halamanPHY520 Introduction To Quantum Mechanics About Myself: Kwok-Wai NG Office: CP 385 Telephone: 7-1782 E-Mail: Office Hour: Mon 10:00-11:00MSUIITBelum ada peringkat
- The Atomic Spectrum of Hydrogen Orbitals and SPDF NotationDokumen33 halamanThe Atomic Spectrum of Hydrogen Orbitals and SPDF Notationdela2Belum ada peringkat
- SFI 5800 Spectroscopy General3Dokumen10 halamanSFI 5800 Spectroscopy General3Alejandra Ayulo CumpalliBelum ada peringkat
- Quantum ChemistryDokumen71 halamanQuantum Chemistrybinseung skzBelum ada peringkat
- 1 Introduction To Quantum Mechanics Unit IDokumen12 halaman1 Introduction To Quantum Mechanics Unit IyomamaBelum ada peringkat
- Final Exam 216 AFCDokumen8 halamanFinal Exam 216 AFCDré ColoradoBelum ada peringkat
- Inorganic Chemistry I-VIDokumen134 halamanInorganic Chemistry I-VIMurad AlDamen100% (3)
- Electromagnetics in Biomedicine: MEDE 2002Dokumen235 halamanElectromagnetics in Biomedicine: MEDE 2002Zero-hm WongBelum ada peringkat
- Fallsem2016-17 7570 RM001 14-Jul-2016 Phy1001 EthDokumen12 halamanFallsem2016-17 7570 RM001 14-Jul-2016 Phy1001 EthAnirudhBelum ada peringkat
- Chemistry Notes Part 1Dokumen59 halamanChemistry Notes Part 1SharleneBelum ada peringkat
- Time-Energy Uncertainty RelationDokumen14 halamanTime-Energy Uncertainty RelationAdith SaiBelum ada peringkat
- Stat Thermo L2Dokumen108 halamanStat Thermo L2mandalsuman20092001Belum ada peringkat
- CH 8 Atomic PhysicsDokumen34 halamanCH 8 Atomic PhysicsOsman AbdullahBelum ada peringkat
- Introduction To Statistical Mechanics and A Two-Dimensional Ising ModelDokumen50 halamanIntroduction To Statistical Mechanics and A Two-Dimensional Ising ModelTradingCLoudBelum ada peringkat
- Ensemble CanónicalDokumen8 halamanEnsemble CanónicalAlexis QuirozBelum ada peringkat
- MIT2 57S12 Lec Notes 2004 PDFDokumen177 halamanMIT2 57S12 Lec Notes 2004 PDFGerman ToledoBelum ada peringkat
- Electrons in Metals: Free Electron ModelDokumen12 halamanElectrons in Metals: Free Electron ModelSofeah FatimaBelum ada peringkat
- Theoretical Calculation of The Heat CapacityDokumen13 halamanTheoretical Calculation of The Heat Capacityprakush01975225403Belum ada peringkat
- The NaturalDokumen58 halamanThe NaturalIan BeardsleyBelum ada peringkat
- T2 Skript CH 1corrDokumen26 halamanT2 Skript CH 1corratowarBelum ada peringkat
- Photoelectric EffectDokumen3 halamanPhotoelectric EffectFilip Leonard100% (1)
- Morden PhysicsDokumen37 halamanMorden PhysicsSimilinga MnyongeBelum ada peringkat
- Unit 3 Quantum Physics PDFDokumen30 halamanUnit 3 Quantum Physics PDFprithwiraj chatterjeeBelum ada peringkat
- Quantum MechanicsDokumen6 halamanQuantum MechanicsRINA MORENOBelum ada peringkat
- Vibrations Phonons3Dokumen53 halamanVibrations Phonons3Kartik Dutta67% (3)
- Electronic Theory of ChemistryDokumen43 halamanElectronic Theory of ChemistryMaheshBelum ada peringkat
- Bohr's TheoryDokumen26 halamanBohr's TheorySukhwinder Singh GillBelum ada peringkat
- Gek1510 Topic3 QuantumPhysics IDokumen40 halamanGek1510 Topic3 QuantumPhysics Iliuxc000Belum ada peringkat
- Physics Notes Fbise FSC 2 CHAPTER - 17 ADVENT OF MODERN PHYSICSDokumen8 halamanPhysics Notes Fbise FSC 2 CHAPTER - 17 ADVENT OF MODERN PHYSICSflyfalcon100% (1)
- Ch24 QuantumDokumen18 halamanCh24 QuantumsumitsingwtfBelum ada peringkat
- CH2422 - Quantum ChemistryDokumen15 halamanCH2422 - Quantum ChemistryJohnBelum ada peringkat
- Quantum Field Theory RadiationDokumen5 halamanQuantum Field Theory RadiationieiediediedieBelum ada peringkat
- Ch1 Asm REVSM NptelDokumen19 halamanCh1 Asm REVSM NptelRajat KumarBelum ada peringkat
- Mathematical Solution Unifying the Four Fundamental Forces in NatureDari EverandMathematical Solution Unifying the Four Fundamental Forces in NatureBelum ada peringkat
- Advances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenDari EverandAdvances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenR. BrillBelum ada peringkat
- Probability and Statistics PDFDokumen124 halamanProbability and Statistics PDF3334333Belum ada peringkat
- Ar PDFDokumen3 halamanAr PDF3334333Belum ada peringkat
- Statistical PhysicsDokumen126 halamanStatistical Physics3334333100% (1)
- Electronics PDFDokumen165 halamanElectronics PDF3334333Belum ada peringkat
- Tilahun2007 Nuclear PhysicsDokumen112 halamanTilahun2007 Nuclear PhysicsAmanuelGelilaBelum ada peringkat
- Properties of Matter PDFDokumen80 halamanProperties of Matter PDF3334333Belum ada peringkat
- Solid State PhysicsDokumen118 halamanSolid State Physics3334333Belum ada peringkat
- Thermal Physics PDFDokumen96 halamanThermal Physics PDF3334333Belum ada peringkat
- Electricity and Magnetism PDFDokumen88 halamanElectricity and Magnetism PDF3334333Belum ada peringkat
- Probability and Statistics PDFDokumen124 halamanProbability and Statistics PDF3334333Belum ada peringkat
- Electricity and Magnetism PDFDokumen88 halamanElectricity and Magnetism PDF3334333Belum ada peringkat
- The Classical Framework: - S $ (J R Sti GS% RHDokumen16 halamanThe Classical Framework: - S $ (J R Sti GS% RH3334333Belum ada peringkat
- Thermal Physics PDFDokumen96 halamanThermal Physics PDF3334333Belum ada peringkat
- Numerical MethodsDokumen132 halamanNumerical MethodsDISCOVERYBelum ada peringkat
- 4000 Essential English Words 5 AnswerKeyDokumen30 halaman4000 Essential English Words 5 AnswerKey3334333100% (1)
- Understanding MechanicsDokumen405 halamanUnderstanding Mechanics3334333100% (4)
- Tilahun2007 Nuclear PhysicsDokumen112 halamanTilahun2007 Nuclear PhysicsAmanuelGelilaBelum ada peringkat
- Physical SciencesDokumen2 halamanPhysical Sciences3334333Belum ada peringkat
- Witn Species For PDFDokumen2 halamanWitn Species For PDF3334333Belum ada peringkat
- Statistical PhysicsDokumen126 halamanStatistical Physics3334333100% (1)
- 4000 Essential English Words6 AnswerKeyDokumen30 halaman4000 Essential English Words6 AnswerKeymairalaidiotaBelum ada peringkat
- 4000 - Essential - English - Words 4 - Answer Key PDFDokumen30 halaman4000 - Essential - English - Words 4 - Answer Key PDF3334333Belum ada peringkat
- Dataform General PDFDokumen2 halamanDataform General PDF3334333Belum ada peringkat
- Tensor AnalysisDokumen73 halamanTensor AnalysisDaniel Salinas-Arizmendi100% (2)
- 4000 - Essential - English - Words 4 - Answer Key PDFDokumen30 halaman4000 - Essential - English - Words 4 - Answer Key PDF3334333Belum ada peringkat
- Chemical Process Control A First Course With Matlab - P.C. Chau PDFDokumen255 halamanChemical Process Control A First Course With Matlab - P.C. Chau PDFAli NassarBelum ada peringkat
- Casio fx-82MSDokumen49 halamanCasio fx-82MSPéter GedeBelum ada peringkat
- Wang Yimin PDFDokumen87 halamanWang Yimin PDF3334333Belum ada peringkat
- Using Matlab For Solving Differential EquationsDokumen12 halamanUsing Matlab For Solving Differential EquationsdicoursfigureBelum ada peringkat
- Rambo Matthew P PDFDokumen63 halamanRambo Matthew P PDF3334333Belum ada peringkat