Solid ST Solid Sta ATE TE: y Z Z y 1/y 1/z
Diunggah oleh
Uday Prakash SahuJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Solid ST Solid Sta ATE TE: y Z Z y 1/y 1/z
Diunggah oleh
Uday Prakash SahuHak Cipta:
Format Tersedia
SOLID STA STATE
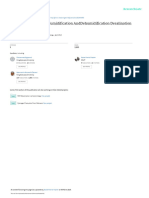
Q.1 The most efficient packing of similar spheres is obtained in(A) The simple cubic system and the body centered cubic system (B) The simple cubic system and the hexagonal close packed system. (C) The face centered cubic system and the hexagonal close packed system. (D) The body centered cubic system and the face centered cubic system. Q.2 If the ratio of co-ordination no. P to that of Q be Y : Z, then the formula of the solid is(A) PyQz (B) PzQy (C) P1/yQ1/z (D) None
Q.3 Xenon crystalizes in face centre cubic lattice and the edge of the unit cell is 620 pm, then radius of xenon atom is(A) 438.5 pm (B) 219.20 pm (C) 536.94 pm (D) 265.5 pm
Q.4 The arrangement of Cl ions in CsCl structure is(A) hcp (B) fcc (C) bcc (D) Simple cubic
Q.5 In closest packing of A type of atoms (radius, rA), the radius of atom B that can be fitted into Octahedral void is(A) 0.155 rA Q.6 In -WCl6 (i) All Cl ions are present in cubic close packing (ii) W occupies, 1/6th of the octahedral holes (iii) W occupies 1/3rd of the tetrahedral holes. (iv) All Cl ions are present in all octahedral (A) i, ii (B) i, iii (C) ii, iii (D) iv, i (B) 0.125 rA (C) 0.414 rA (D) 0.732 rA
Q.7 The edge length of a cube is 400 pm. Its body diagonal would be(A) 600 pm (B) 566 pm (C) 693 pm (D) 500 pm
Q.8 The mass of unit cell of CaF2 (fluorite structure) corresponds to(A) Mass of 8Ca++ ions & 4F ions(B) Mass of 4Ca++ ions & 8F ions (C) Mass of 4Ca++ ions & 4F ions(D) Mass of 1Ca++ ions & 2F ions Q.9 Close packing is maximum in the Crystal which is(A) Simple cube (B) bcc (C) fcc (D) None
Q.10 An ionic compound AB has ZnS type of structure, if the radius A+ is 22.5 pm, then the ideal radius of B is(A) 54.35 pm (B) 100 pm (C) 145.16 pm (D) None
ANSWER KEY
Q.No. Ans. 1 C 2 B 3 B Solid State 4 5 6 D C A 7 C 8 B 9 C 10 B
SOLUTIONS (SOLID STA STATE)
Sol.1 The hexagonal close packing and the face-centred cubic system have the closest packing as their packing efficiency is 74% and thus (C) represent the correct solution. Sol.2 Since the ratio of the co-ordination no. P to that of Q is Y : Z. i.e. P is surrounded by Y atoms of Q and Q is surrounded by Z atoms of P i.e. no. of atoms of P is Z & no. of atoms of Q is Y So, the formula is PZ QY
(B)
Sol.3 For fcc lattice 4r = 2 a where a = 620 pm
1 2 2
or, r =
1 2 2
620 pm = 219.20 pm
(B)
Sol.4 Arrangement of atoms or, ions in the corner of the unit cell is simple cubic. So in body centered cubic arrangement, Cl ions are arranged in the corner of the cube. So, it is a simple cubic.
(D)
Sol.5 For octahedral void
rB = 0.414 rA
or rB = 0.414 rA
(C)
Sol.6 In -WCl6, Cl ions are arranged in cubic close packing, so, there is only one Cl ion in 1-unit cell. So, the formula can be written W1/
6
Cl, i.e.
1 of the octahedral hole is filled by W. . 6
(A), (B)
Sol.7 Since in body center cubic, the body diagonal = =
3 400 pm = 692.82 pm
3 a.
= 693 pm
(C)
Sol.8 In CaF2 (Calcium fluorite) structure 1-unit cell contains 4-Ca2+ ions and 8F ions. So, mass of unit cell of CaF2 = mass of 4 Ca2+ ions + mass of 8F ions
(B)
Sol.9 The close packing in the crystal is 0.52, 0.68 and 0.74 for simple cubic, body centered cubic, and face-centered cubic respectively. i.e. the close packing is maximum in fcc.
(C)
Sol.10 Since ionic compound AB has ZnS type of structure, therefore it has tetrahedral holes, for which,
radius of cation = 0.225 radius of anion
r+ = 0.225 r r+ = 0.225 r
Hence, r = 100 pm
(B)
Anda mungkin juga menyukai
- Solid StateDokumen16 halamanSolid StatePrahasBelum ada peringkat
- ENGINEERING CATALYST SOLID STATE HOMEWORK CHEMISTRYDokumen11 halamanENGINEERING CATALYST SOLID STATE HOMEWORK CHEMISTRYSerious BlackBelum ada peringkat
- Solid State MCQDokumen7 halamanSolid State MCQshikha nathBelum ada peringkat
- (Xii) Solid State (Assignment)Dokumen10 halaman(Xii) Solid State (Assignment)sitaramroyalBelum ada peringkat
- Solid StateDokumen4 halamanSolid StateGadde Gopala KrishnaBelum ada peringkat
- Solid State Structure PropertiesDokumen6 halamanSolid State Structure PropertiesAditi MahajanBelum ada peringkat
- Solid State PDFDokumen4 halamanSolid State PDFGadde Gopala KrishnaBelum ada peringkat
- Question BankDokumen3 halamanQuestion Bankkarangupta26795Belum ada peringkat
- JEE Chemistry Exercises on Solid State Structures and PropertiesDokumen16 halamanJEE Chemistry Exercises on Solid State Structures and PropertiesKrishna BajpaiBelum ada peringkat
- Solid State-1Dokumen12 halamanSolid State-1Ayush KumarBelum ada peringkat
- Solid StateDokumen2 halamanSolid StateKamal KishoreBelum ada peringkat
- 3 - 2-Solid State (Level)Dokumen21 halaman3 - 2-Solid State (Level)Sachin GargBelum ada peringkat
- Solid State Made BY KeshavPandey EngineerDokumen6 halamanSolid State Made BY KeshavPandey EngineerVibhansh BhatiaBelum ada peringkat
- Chem Academy: Exercise - IDokumen11 halamanChem Academy: Exercise - IHamit RanaBelum ada peringkat
- Ch-27.2 Crystalline Materials - Detects in Crystalline MaterialsDokumen102 halamanCh-27.2 Crystalline Materials - Detects in Crystalline MaterialsasjfgauojfgfBelum ada peringkat
- Solid State Physics MCQsDokumen7 halamanSolid State Physics MCQsAhsan MoinBelum ada peringkat
- Important Questions 2016Dokumen57 halamanImportant Questions 2016Anonymous t9LFhvF100% (1)
- Common unit cells and crystal structuresDokumen99 halamanCommon unit cells and crystal structuresasjfgauojfgfBelum ada peringkat
- Ch-27.2 Crystalline Materials & Detects in Crystalline MaterialsDokumen93 halamanCh-27.2 Crystalline Materials & Detects in Crystalline MaterialsSmruti Ranjan PattanayakBelum ada peringkat
- Document From Vipin SinghDokumen5 halamanDocument From Vipin SinghShashwatBelum ada peringkat
- EditedDokumen70 halamanEditedVimal PrasadBelum ada peringkat
- 295 4 Solid State Practice ProblemsDokumen11 halaman295 4 Solid State Practice ProblemsArijit SinghBelum ada peringkat
- SS 1Dokumen7 halamanSS 1xanshahBelum ada peringkat
- NEET Material Solid StateDokumen26 halamanNEET Material Solid StateApex Institute100% (7)
- This Test Contains A Total of 15 Objective Type Questions. Each Question Carries 1 Mark. There Is NO NEGATIVE MarkingDokumen9 halamanThis Test Contains A Total of 15 Objective Type Questions. Each Question Carries 1 Mark. There Is NO NEGATIVE MarkingvarunkohliinBelum ada peringkat
- 01 Solid State EDokumen1 halaman01 Solid State EKunalSinghBelum ada peringkat
- Class 12 Chemistry - Solid State - McqsDokumen22 halamanClass 12 Chemistry - Solid State - McqsDivyam GargBelum ada peringkat
- The Solid State Class 12 MCQs Questions With AnswersDokumen19 halamanThe Solid State Class 12 MCQs Questions With AnswersRohit Chavariya100% (1)
- Solid StateDokumen2 halamanSolid StateRajat KaliaBelum ada peringkat
- (PP) 11th Paper PDFDokumen8 halaman(PP) 11th Paper PDFChemistry classes by Dr.AshokBelum ada peringkat
- Solid State (Exercise) - Copy ExportDokumen10 halamanSolid State (Exercise) - Copy ExportVishu PatryBelum ada peringkat
- ALPS 2331 Chemistry Assignment PaperDokumen7 halamanALPS 2331 Chemistry Assignment PaperAyushBelum ada peringkat
- Solid State Revision SheetDokumen6 halamanSolid State Revision SheetRumaysa -Belum ada peringkat
- Day-4 Solid StateDokumen4 halamanDay-4 Solid StatepriyanshuBelum ada peringkat
- Solid State & LS-QuizDokumen4 halamanSolid State & LS-QuizSNEHAL ANANDBelum ada peringkat
- S 3Dokumen3 halamanS 3Jitendra KumarBelum ada peringkat
- Ch-27.2 Crystalline Materials - Detects in Crystalline MaterialsDokumen92 halamanCh-27.2 Crystalline Materials - Detects in Crystalline MaterialsManojBelum ada peringkat
- Solid State: Chemistry DPP 3 by Garima Verma (Chemistry Faculty) - Referral Code: "Cgvmam"Dokumen4 halamanSolid State: Chemistry DPP 3 by Garima Verma (Chemistry Faculty) - Referral Code: "Cgvmam"Tanisha SubudhiBelum ada peringkat
- Solid StateDokumen5 halamanSolid StateGadde Gopala KrishnaBelum ada peringkat
- Solid State 1Dokumen6 halamanSolid State 1bibhas_samantaBelum ada peringkat
- DPP 01 Solid StateDokumen14 halamanDPP 01 Solid Stateanupamgupta112Belum ada peringkat
- Colligative Properties & Crystal StructuresDokumen8 halamanColligative Properties & Crystal Structuresdasari karthikBelum ada peringkat
- ChemistryDokumen3 halamanChemistryuniquestarBelum ada peringkat
- NCERT Exemplar Class 11 Chemistry Chapter 11: Top 15 MCQs on p-Block ElementsDokumen20 halamanNCERT Exemplar Class 11 Chemistry Chapter 11: Top 15 MCQs on p-Block ElementsAnidhya TiwariBelum ada peringkat
- FCC Crystal Structure and PropertiesDokumen4 halamanFCC Crystal Structure and PropertiespewBelum ada peringkat
- Revision Structure of Atom J Classification of ElementsDokumen3 halamanRevision Structure of Atom J Classification of ElementsDebbie SarahBelum ada peringkat
- Solid State 1Dokumen20 halamanSolid State 1Kamal Jit DhimanBelum ada peringkat
- +2PB Solidstate OBJ 01Dokumen2 halaman+2PB Solidstate OBJ 01Rajat KaliaBelum ada peringkat
- Practise ProblemsDokumen10 halamanPractise ProblemsJoshuaBelum ada peringkat
- Solid State: Objective Type Questions Multiple Choice QuestionsDokumen5 halamanSolid State: Objective Type Questions Multiple Choice QuestionsSnehashis BoseBelum ada peringkat
- Term 1 Mcqs Series Solid StateDokumen108 halamanTerm 1 Mcqs Series Solid StateshubhamBelum ada peringkat
- PhysicsDokumen34 halamanPhysicsKoustav Pal70% (10)
- Chemistry - Solid State MCQ Jee Neet NewDokumen49 halamanChemistry - Solid State MCQ Jee Neet NewadarshBelum ada peringkat
- Solid State Worksheet and SolutionsDokumen5 halamanSolid State Worksheet and Solutionsaryanpw905Belum ada peringkat
- Master Iit Academy: 2s Electron in Li IsDokumen5 halamanMaster Iit Academy: 2s Electron in Li IsSesha Sai KumarBelum ada peringkat
- FCC structure propertiesDokumen61 halamanFCC structure propertiesStephen RaiderBelum ada peringkat
- Chem Test ProbDokumen8 halamanChem Test ProbJill RagaBelum ada peringkat
- Crystal StructureDokumen4 halamanCrystal StructureUnemployed EngineerBelum ada peringkat
- Theoretical Solid State Physics: International Series in Natural Philosophy, Volume 1Dari EverandTheoretical Solid State Physics: International Series in Natural Philosophy, Volume 1Penilaian: 1 dari 5 bintang1/5 (1)
- 9th Maths Ch-03 Coordinate Geometry FinalDokumen20 halaman9th Maths Ch-03 Coordinate Geometry FinalUday Prakash SahuBelum ada peringkat
- Circular Motion Practice QuestionsDokumen28 halamanCircular Motion Practice QuestionsUday Prakash SahuBelum ada peringkat
- Akash Neet Question Bank PDFDokumen440 halamanAkash Neet Question Bank PDFUday Prakash Sahu75% (4)
- Coordinate Geometry Booster For IIT JEE Main and Advanced PDFDokumen383 halamanCoordinate Geometry Booster For IIT JEE Main and Advanced PDFUday Prakash Sahu100% (5)
- Optics 10Dokumen24 halamanOptics 10Uday Prakash SahuBelum ada peringkat
- Acid Base RXNDokumen7 halamanAcid Base RXNUday Prakash SahuBelum ada peringkat
- Revised Internal Campus Bus ScheduleDokumen2 halamanRevised Internal Campus Bus ScheduleUday Prakash SahuBelum ada peringkat
- Chemistry Chapter 2 Multiple Choice QuestionsDokumen74 halamanChemistry Chapter 2 Multiple Choice QuestionsUday Prakash SahuBelum ada peringkat
- ToDokumen2 halamanToUday Prakash SahuBelum ada peringkat
- Reactions of Aldehydes and KetonesDokumen1 halamanReactions of Aldehydes and KetonesUday Prakash SahuBelum ada peringkat
- Drum Press, Temp CalDokumen22 halamanDrum Press, Temp CalUday Prakash SahuBelum ada peringkat
- Test Paper TwelfthDokumen1 halamanTest Paper TwelfthUday Prakash SahuBelum ada peringkat
- Rotary ActuatorDokumen20 halamanRotary ActuatorUday Prakash Sahu100% (1)
- Collision and Mean Free Path 40 CDokumen2 halamanCollision and Mean Free Path 40 CUday Prakash SahuBelum ada peringkat
- E2 EliminationDokumen2 halamanE2 EliminationUday Prakash SahuBelum ada peringkat
- Roadmap For Reactions of Alkanes, Alkenes, Alkynes, Alcohols & EthersDokumen5 halamanRoadmap For Reactions of Alkanes, Alkenes, Alkynes, Alcohols & EthersUday Prakash SahuBelum ada peringkat
- PaperDokumen3 halamanPaperUday Prakash SahuBelum ada peringkat
- Proforma For OBC NC CertificateDokumen1 halamanProforma For OBC NC CertificateUday Prakash SahuBelum ada peringkat
- Pages From 10 Ct-22Dokumen1 halamanPages From 10 Ct-22Uday Prakash SahuBelum ada peringkat
- CHEM Study Guide on Atomic StructureDokumen4 halamanCHEM Study Guide on Atomic StructureUday Prakash Sahu0% (1)
- Science Class-10 Sample PaperDokumen8 halamanScience Class-10 Sample PaperUday Prakash Sahu100% (1)
- Fluid MechanicsDokumen3 halamanFluid MechanicsUday Prakash SahuBelum ada peringkat
- 01 Lecture KMDokumen44 halaman01 Lecture KMUday Prakash SahuBelum ada peringkat
- Ambedkar Nagar: Prime Classes For IIT-JEE/PMT, Ambedkar NagarDokumen2 halamanAmbedkar Nagar: Prime Classes For IIT-JEE/PMT, Ambedkar NagarUday Prakash SahuBelum ada peringkat
- Ambedkar Nagar: Prime Classes For IIT-JEE/PMT, Ambedkar NagarDokumen2 halamanAmbedkar Nagar: Prime Classes For IIT-JEE/PMT, Ambedkar NagarUday Prakash SahuBelum ada peringkat
- Class 9 Oct 09Dokumen3 halamanClass 9 Oct 09Uday Prakash SahuBelum ada peringkat
- Bohr's Model QuestionsDokumen2 halamanBohr's Model QuestionsUday Prakash SahuBelum ada peringkat
- Compressed Air System Design ManualDokumen26 halamanCompressed Air System Design ManualBadrul HishamBelum ada peringkat
- Quiz - Compressors and Compressed Air SystemsDokumen3 halamanQuiz - Compressors and Compressed Air SystemsUday Prakash Sahu100% (1)
- Samudra ManthanDokumen4 halamanSamudra ManthanUday Prakash SahuBelum ada peringkat
- Chem16 LE3 SamplexDokumen3 halamanChem16 LE3 SamplexmariemfranciscoBelum ada peringkat
- Pressure Vessel Manual-M.asgaRZADEGANDokumen79 halamanPressure Vessel Manual-M.asgaRZADEGANH BBelum ada peringkat
- Guidelines For Term Paper: Course Code: PHY112 Course Title: Modern Physics and ElectronicsDokumen9 halamanGuidelines For Term Paper: Course Code: PHY112 Course Title: Modern Physics and ElectronicsAnuj AroraBelum ada peringkat
- Assignment 1Dokumen3 halamanAssignment 1Yeleti FamilyBelum ada peringkat
- A Review On Grease Lubrication in Rolling BearingsDokumen12 halamanA Review On Grease Lubrication in Rolling BearingsRepositorio MantenimientoBelum ada peringkat
- Products For Polyurethanes: Driving Comfort & DurabilityDokumen8 halamanProducts For Polyurethanes: Driving Comfort & DurabilityA MahmoodBelum ada peringkat
- Section 1.4: Isotopes, Radioisotopes, and Atomic MassDokumen4 halamanSection 1.4: Isotopes, Radioisotopes, and Atomic MassgzboyzoneBelum ada peringkat
- Gas Flotation Package (Asbea-A-2703) 32294 Ponticelli - Al Shaheen PWTDokumen54 halamanGas Flotation Package (Asbea-A-2703) 32294 Ponticelli - Al Shaheen PWTTĩnh Hồ TrungBelum ada peringkat
- T1 1 E Automotive 072Dokumen15 halamanT1 1 E Automotive 072Marian OstrowskiBelum ada peringkat
- Milk, An Excerpt From American Farmstead CheeseDokumen14 halamanMilk, An Excerpt From American Farmstead CheeseChelsea Green PublishingBelum ada peringkat
- Laboratory Report Sheet 02 Properties of SolidsDokumen10 halamanLaboratory Report Sheet 02 Properties of SolidsCzarina RelleveBelum ada peringkat
- Intern J Hydrogen Energy - Hydrogen Embrittlement in Low-Density TWIP Steel 2014Dokumen13 halamanIntern J Hydrogen Energy - Hydrogen Embrittlement in Low-Density TWIP Steel 2014cesar barandaBelum ada peringkat
- 2019FA-Organic CHEM-2423-61310Dokumen10 halaman2019FA-Organic CHEM-2423-61310Lissette RegisBelum ada peringkat
- Physicochemical Processes: Dr. Sana Hanif Assistant ProfessorDokumen12 halamanPhysicochemical Processes: Dr. Sana Hanif Assistant ProfessorMohammad ZohaibBelum ada peringkat
- Mil-Std-3004d W-CH1 PDFDokumen266 halamanMil-Std-3004d W-CH1 PDFPaul BarnardBelum ada peringkat
- The Iodometric Estimation of MercaptansDokumen2 halamanThe Iodometric Estimation of MercaptansSteven Alvarez AguilarBelum ada peringkat
- Effect of Chemical FertilizerDokumen4 halamanEffect of Chemical FertilizerMrithulaVaidyanathanBelum ada peringkat
- Heap Leaching With OxygenDokumen6 halamanHeap Leaching With OxygenArief RHBelum ada peringkat
- Good Practice Guide Operation Drinking Water Research PRJ 1074Dokumen52 halamanGood Practice Guide Operation Drinking Water Research PRJ 1074Michael FredericoBelum ada peringkat
- Carboxymethyl Cellulose 1KGDokumen6 halamanCarboxymethyl Cellulose 1KGJawir JenorBelum ada peringkat
- Applied Thermodynamics For Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, KharagpurDokumen19 halamanApplied Thermodynamics For Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, KharagpurTommyVercettiBelum ada peringkat
- Bolts, threaded parts and rivets allowable loadsDokumen2 halamanBolts, threaded parts and rivets allowable loadsMary MarasiganBelum ada peringkat
- Experimental Study of Humidification AndDehumidifiDokumen19 halamanExperimental Study of Humidification AndDehumidifiHanz GianBelum ada peringkat
- Reference BS 449 Part 2: Design of Moment Connection Flush End Plate Moment ConnectionDokumen2 halamanReference BS 449 Part 2: Design of Moment Connection Flush End Plate Moment ConnectionMazenMowafyBelum ada peringkat
- Protein Synthesis EssayDokumen5 halamanProtein Synthesis Essaymarybrownarlington100% (2)
- Mark Scheme (Results) Summer 2015: GCE Chemistry (6CH05/01)Dokumen24 halamanMark Scheme (Results) Summer 2015: GCE Chemistry (6CH05/01)Arpan SahaBelum ada peringkat
- Human Digestive SystemDokumen13 halamanHuman Digestive SystemEvansBelum ada peringkat
- High-Performance Concrete Characteristics and PropertiesDokumen16 halamanHigh-Performance Concrete Characteristics and PropertiesChukwuma OgbonnaBelum ada peringkat
- Lecture BIOCHEMISTRY of CYTOSOL AlfiDokumen56 halamanLecture BIOCHEMISTRY of CYTOSOL Alfisennaavia12Belum ada peringkat
- Sample Paper-01 Chemistry (Theory) Class – XIDokumen4 halamanSample Paper-01 Chemistry (Theory) Class – XISarthakBelum ada peringkat