Elimination Reactions of Alkyl HalidesA
Diunggah oleh
RSLHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Elimination Reactions of Alkyl HalidesA
Diunggah oleh
RSLHak Cipta:
Format Tersedia
Elimination Reactions of Alkyl Halides; Competition Between Substitution and Elimination Quiz 2
1.
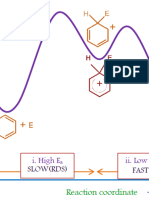
Which of the following is not a true statement? [Hint] Syn elimination is the removal of two substitutents from the same side of the molecule. Anti elimination is favored over syn elimination in an E2 reaction. Syn elimination requires the molecule to be in a staggered conformation. Syn elimination is slower than anti elimination. Anti elimination is the removal of two substitutents from opposite sides of the molecule.
2.
Which of the following describes the stereochemistry of an E2 reaction? [Hint] stereospecific regioselective and stereospecific regioselective stereoselective regioselective and stereoselective
3.
What alkene is formed as the major product of an E2 reaction between 2-bromohexane and hydroxide ion? [Hint] (E)-3-hexene (Z)-2-hexene
(Z)-3-hexene (E)-2-hexene 1-hexene
4.
What product is formed when HBr is eliminated from (2S,3S)-2-bromo-3-phenylbutane in an E2 reaction? [Hint] (Z)-3-methyl-3-phenyl-1-butene (E)-3-methyl-3-phenyl-1-butene (Z)-2-phenyl-2-butene and (E)-2-phenyl-2-butene (Z)-2-phenyl-2-butene (E)-2-phenyl-2-butene
5.
Which of the following statements is not true? [Hint] The product of the E2 reaction of 1-chloro-2,3-dimethylcyclohexane is 1,2dimethylcyclohexene. The product of the E2 reaction of cis-1-chloro-2-methylcyclohexane is 3methylcyclohexene. The product of the E2 reaction of trans-1-chloro-2-methylcyclohexane is 3methylcyclohexene. The product of the E1 reaction of 1-chloro-2,3-dimethylcyclohexane is 1,2dimethylcyclohexene. The product of the E1 reaction of 1-chloro-1,2-dimethylcyclohexane is 1,2dimethylcyclohexene.
6.
Why does cis-1-bromo-4-tert-butylcyclohexane react faster in an E2 reaction than does trans1-bromo-4-tert-butylcyclohexane? [Hint]
When Br and H are in the required equatorial positions, the large tert-butyl group is in the more stable axial position. When Br and H are in the required equatorial positions, the large tert-butyl group is in the more stable equatorial position. The required syn elimination can occur with all the substituents in the most stable equatorial position. Since the Br is in an equatorial position, syn elimination can occur. When Br and H are in the required axial positions, the large tert-butyl group is in the more stable equatorial position.
7.
Which of the following equations defines the "deuterium kinetic isotope effect"? [Hint] kH/kD kD - kH (kH - kD)/kD kD / kH kH - kD
8.
What size of ring is formed from the intramolecular reaction of Br-CH2-(CH2)3-CH2-O-? [Hint] a 3-membered ring a 4-membered ring a 5-membered ring a 6-membered ring a 7-membered ring
9.
Which of the following alkyl halides would form the greatest yield of 3-heptene as a result of reaction with methoxide ion? [Hint] 2-bromoheptane 5-bromoheptane 4-bromoheptane 3-bromoheptane 1-bromoheptane
10 .
What is the most likely reaction for a primary halide to undergo in the presence of relatively small base/nucleophile? [Hint] SN1 E1 E2 E1 and SN1 SN2
Anda mungkin juga menyukai
- NH4BH4Dokumen1 halamanNH4BH4RSLBelum ada peringkat
- Coordination Isomers ListDokumen1 halamanCoordination Isomers ListRSLBelum ada peringkat
- Carbon Monoxide or Carbonyl: MO DescriptionDokumen3 halamanCarbon Monoxide or Carbonyl: MO DescriptionRSLBelum ada peringkat
- Mechanism of Organic ReactionDokumen4 halamanMechanism of Organic ReactionRSLBelum ada peringkat
- S-Block Elments: Inorganic ChemistryDokumen8 halamanS-Block Elments: Inorganic ChemistryRSLBelum ada peringkat
- DebereinerDokumen4 halamanDebereinerRSLBelum ada peringkat
- Basics of ElectrochemistryDokumen22 halamanBasics of ElectrochemistryRSLBelum ada peringkat
- Colloids: Thomas Graham (1861) Studied The Ability of Dissolved Substances ToDokumen28 halamanColloids: Thomas Graham (1861) Studied The Ability of Dissolved Substances ToRSLBelum ada peringkat
- Turkevich1985 Article ColloidalGoldPartII PDFDokumen7 halamanTurkevich1985 Article ColloidalGoldPartII PDFRSLBelum ada peringkat
- Epoxides Ring-Opening - Chemistry LibreTextsDokumen3 halamanEpoxides Ring-Opening - Chemistry LibreTextsRSLBelum ada peringkat
- Colloidal Gold. Part I: Historical and Preparative Aspects, Morphology and StructureDokumen6 halamanColloidal Gold. Part I: Historical and Preparative Aspects, Morphology and StructureRSL100% (1)
- © 1934 Nature Publishing GroupDokumen2 halaman© 1934 Nature Publishing GroupRSLBelum ada peringkat
- Piezoelectric Materials: Crystal Orientation and Poling Direction - COMSOL Blog PDFDokumen4 halamanPiezoelectric Materials: Crystal Orientation and Poling Direction - COMSOL Blog PDFRSLBelum ada peringkat
- Ionic Equilibrium - Salt Hydrolysis Practice Questions (Level-1)Dokumen3 halamanIonic Equilibrium - Salt Hydrolysis Practice Questions (Level-1)RSLBelum ada peringkat
- Colligative Properties: Cryoscopy & EbulliosDokumen30 halamanColligative Properties: Cryoscopy & EbulliosRSL100% (1)
- 2019dec-03 - Ionic Equilibrium - PracticeSheetDokumen2 halaman2019dec-03 - Ionic Equilibrium - PracticeSheetRSLBelum ada peringkat
- Analysis Report of Criminal and Financial Background Details of Winners in Lok Sabha 2019 ElectionsDokumen397 halamanAnalysis Report of Criminal and Financial Background Details of Winners in Lok Sabha 2019 ElectionsRSLBelum ada peringkat
- Koopmans TheoremDokumen14 halamanKoopmans TheoremRSLBelum ada peringkat
- Priority List IUPACDokumen1 halamanPriority List IUPACRSLBelum ada peringkat
- List of Straight-Chain AlkanesDokumen6 halamanList of Straight-Chain AlkanesRSLBelum ada peringkat
- Redox Reactions and Balancing Using Oxidation Number & NfactorDokumen1 halamanRedox Reactions and Balancing Using Oxidation Number & NfactorRSLBelum ada peringkat
- Factors Affecting Relative Strengths of Acids and BasesDokumen1 halamanFactors Affecting Relative Strengths of Acids and BasesRSL80% (5)
- Chemistry Organic GOC Reaction Mechanism Elimination E1 E2 E1cBDokumen4 halamanChemistry Organic GOC Reaction Mechanism Elimination E1 E2 E1cBRSL100% (1)
- EAS Worksheet 1 SubjDokumen1 halamanEAS Worksheet 1 SubjRSLBelum ada peringkat
- Crossword Coordination CompoundsDokumen2 halamanCrossword Coordination CompoundsRSLBelum ada peringkat
- Isoelectric Point, PiDokumen2 halamanIsoelectric Point, PiRSLBelum ada peringkat
- The World of Chemistry Episode 5 - A Matter of StateDokumen2 halamanThe World of Chemistry Episode 5 - A Matter of StateRSL0% (1)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5795)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (345)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1091)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Question Bank 2019Dokumen1 halamanQuestion Bank 2019Priyank KhirsariyaBelum ada peringkat
- Lesson 3Dokumen6 halamanLesson 3Kyl Luna Louise MontefalcoBelum ada peringkat
- ME525SP2023HW4Dokumen1 halamanME525SP2023HW4Dylan HsiehBelum ada peringkat
- A Chart Comparing The SN1 Vs SN2 Reactions: That Can Prevent This Reaction From Occurring?Dokumen3 halamanA Chart Comparing The SN1 Vs SN2 Reactions: That Can Prevent This Reaction From Occurring?মেঘলা আকাশBelum ada peringkat
- Technip Separations PDFDokumen50 halamanTechnip Separations PDFProcess Engineer100% (1)
- 06 SKKK1113 201415 - 2 Chap 4.4 4.6Dokumen13 halaman06 SKKK1113 201415 - 2 Chap 4.4 4.6Eunice AnneBelum ada peringkat
- CatalogueDokumen50 halamanCatalogueDiegoBelum ada peringkat
- To Study The Kinetics of Persulphate-Iodide Ion Reaction by Initial Rate Method (Iodine Clock Reaction)Dokumen12 halamanTo Study The Kinetics of Persulphate-Iodide Ion Reaction by Initial Rate Method (Iodine Clock Reaction)Nishika GeraBelum ada peringkat
- IGCSE Chem ch1 Questions PDFDokumen2 halamanIGCSE Chem ch1 Questions PDFRajesh100% (1)
- Kittiwake Mak19642pa F&L Cabinet DatasheetDokumen4 halamanKittiwake Mak19642pa F&L Cabinet DatasheetCorina StanculescuBelum ada peringkat
- ON Delayed Coker Unit: Prepared byDokumen42 halamanON Delayed Coker Unit: Prepared bymujeebtalibBelum ada peringkat
- 10.chemical Thermodynamics Objective by RajputDokumen3 halaman10.chemical Thermodynamics Objective by RajputMuruganantham MajesticBelum ada peringkat
- SEM Picture of The Multi-Layer Structure of A 0.05 Micron MembraneDokumen12 halamanSEM Picture of The Multi-Layer Structure of A 0.05 Micron MembraneRuben SerraBelum ada peringkat
- Chapter 9 QuizDokumen6 halamanChapter 9 QuizDuong Nguyen100% (1)
- A Comprehensive Kinetic Theory To Model Thermolysis, Aquathermolysis, Gasification, Combustion, and Oxidation of Athabasca BitumenDokumen31 halamanA Comprehensive Kinetic Theory To Model Thermolysis, Aquathermolysis, Gasification, Combustion, and Oxidation of Athabasca BitumenMejbahul SarkerBelum ada peringkat
- Organic Chemistry 4th Edition Gorzynski Test BankDokumen17 halamanOrganic Chemistry 4th Edition Gorzynski Test Bankdigonousconcrewh2zxi100% (31)
- 28-3-62-Methane Dry Reforming Over Ni-Co-Al2O3Dokumen10 halaman28-3-62-Methane Dry Reforming Over Ni-Co-Al2O3Wassachol SumarasinghaBelum ada peringkat
- Chem 223 SI - Chapter 19 Part 2Dokumen5 halamanChem 223 SI - Chapter 19 Part 2Yvonne ChuehBelum ada peringkat
- Nautilus Box Boom Crane Reference ListDokumen6 halamanNautilus Box Boom Crane Reference Listvipin_nair01100% (2)
- Ethylene and Acetylene Plant PDFDokumen405 halamanEthylene and Acetylene Plant PDFاحمد الدلالBelum ada peringkat
- Experiment 2: Haloalkanes: Reaction of HaloalkanesDokumen6 halamanExperiment 2: Haloalkanes: Reaction of HaloalkanesEssay NationBelum ada peringkat
- 2009 Margarita Seminar - 10 Successful Applications of Casale Technology To Grass-Roots PlantsDokumen32 halaman2009 Margarita Seminar - 10 Successful Applications of Casale Technology To Grass-Roots PlantsIvonneBelum ada peringkat
- Te MegDokumen5 halamanTe MegTaylor Santiago Salinas Del PezoBelum ada peringkat
- Combustion - Related Fuel PropertiesDokumen57 halamanCombustion - Related Fuel PropertiesNUREEN DAYANA BINTI MOHD IZMANIZAN A21ET0194Belum ada peringkat
- Process - Mosad Mosad DawoodDokumen4 halamanProcess - Mosad Mosad DawoodHatem HusseinBelum ada peringkat
- Lec 1-Vapor Liquid Equilibrium-Part 1Dokumen30 halamanLec 1-Vapor Liquid Equilibrium-Part 1DianaBelum ada peringkat
- Assignment 1B (Part 1) - ConDokumen2 halamanAssignment 1B (Part 1) - ConRUTH MALABABelum ada peringkat
- Oxygen MSDSDokumen4 halamanOxygen MSDSsalcabesBelum ada peringkat
- APS Company Profile Presentation To KSO PTM EP Telaga Said March 11 2014Dokumen26 halamanAPS Company Profile Presentation To KSO PTM EP Telaga Said March 11 2014Nik AbduhBelum ada peringkat
- TLC SimulatorDokumen1 halamanTLC SimulatorKrys tallBelum ada peringkat