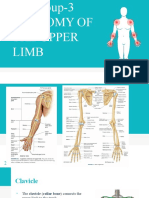
Water Lose
Diunggah oleh
JAKLIN EMPOLDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Water Lose
Diunggah oleh
JAKLIN EMPOLHak Cipta:
Format Tersedia
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
The Body's Water Fluid Compartments Measuring Volumes Ionic Composition Units of Measure Calculating Osmolarity The Anion Gap Clinical Examples Fluid Movements Transport Mechanisms Membrane Transport Osmosis ICF-ECF Exchange Calculating Exchange Examples ISF-Plasma Exchange Capillary Pressures Study Questions Answers
Water and its dissolved constituents make up the bulk of your body, and determine the nature of nearly every physiological process. In most individuals, approximately 60% of the total weight is water. This percentage varies between 50% and 70%, with the exact value primarily dependent on a person's fat content. Since fat has very low water, individuals with more fat will have a lower overall percentage of body weight as water. In the first sections of this document, we'll examine how the body's water is distributed into several functional compartments, with significant differences in the ionic composition of each.
1999, Joe Patlak Department of Molecular Physiology and Biophysics University of Vermont Page 1 of 29 (joe.patlak@uvm.edu) 06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Fluid Compartments in the Body
The body's water is effectively compartmentalized into several major divisions. Intracellular Fluid (ICF) comprises 2/3 of the body's water. If your body has 60% water, ICF is about 40% of your weight. The ICF is primarily a solution of potassium and organic anions, proteins etc. (Cellular Soup!). The cell membranes and cellular metabolism control the constituents of this ICF. ICF is not homogeneous in your body. It represents a conglomeration of fluids from all the different cells. Extracellular Fluid (ECF) is the remaining 1/3 of your body's water. ECF is about 20% of your weight. The ECF is primarily a NaCl and NaHCO3 solution. The ECF is further subdivided into three subcompartments: Interstitial Fluid (ISF) surrounds the cells, but does not circulate. comprises about 3/4 of the ECF. It
Plasma circulates as the extracellular component of blood. It makes up about 1/4 of the ECF. Transcellular fluid is a set of fluids that are outside of the normal compartments. These 1-2 liters of fluid make up the CSF, Digestive Juices, Mucus, etc.
40% x 70 kg = 28 L water
ISF, 10 L
Plasma, 4L
T r a n s , 1 L
The 60-40-20 Rule: 60 % of body weight is water 40% of body weight is intracellular fluids 20% of body weight is extracellular fluid
Intracellular Water =40%
Extracellular=20%
Total Body Water = 60% of weight
Page 2 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Special Notes:
All the body's fluid compartments are in osmotic equilibrium (except for transient changes). The ions and small solutes that constitute the ECF are in equilibrium with similar concentrations in each subcompartment. The ECF volume is proportional to the total Na content.
Measuring the Volumes of the Body's Compartments
It is sometimes necessary to know more precisely how water is distributed between the various compartments in a particular individual. The volumes of some of the compartments can be measured by the dilution method. For the dilution method, one adds an extrinsic, measurable, compound that distributes fully within the compartment of interest. This method relies on the formula: Concentration = Amount / Volume or:
Volume = Amount Added-Amount Lost / Measured Concentration
Directly Measurable volumes:
Total Body Water: Use D20 or radioactive water (tritiated). Distributes throughout all aqueous solutions. ECF Volume: Use Inulin (a starch) or Sucrose. These distribute throughout body, but are excluded from cells. Plasma Volume: Use radioactive albumin or dye (Evans Blue) that stay in plasma only.
Indirectly Measurable Volumes:
There is no practical way to measure only the intracellular or the interstitial volumes. Rather, these are calculated by combining the measured volumes given above. Page 3 of 29 06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Interstitial Volume: Equal to the Extracellular volume minus the plasma volume. Intracellular Volume: Equal to the Total Water minus the Extracellular Volume.
Ionic Composition of Body Fluids:
The following table gives representative values for the primary ionic constituents of the major fluid compartments. Also, follow the links at left for additional important information.
Electrolyte Cations: Sodium Potassium Calcium Magnesium Total Cations:
Plasma Water Plasma, (mEq/L) Interstitial Fluid Intracellular Fluid (mEq/L) [molarity] (mEq/L) (mEq/L) [molality]
142 4 5 2 153
153 4.3 5.4 2.2 165
145 4 5 2 156
10 160 2 26 198
Anions: Chloride Bicarbonate Phosphate Sulphate Organic Acid Protein Total Anions: 101 27 2 1 6 16 153 108.5 29 2.2 1 6.5 17 165 114 31 2 1 7 1 156 65 198 3 10 100 20
Page 4 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Important Notes:
The units of measure given in the table are in equivalents, referred to either liter volume or liter water (plasma). If you are not familiar with the terms molarity, molality, osmolarity, osmolality, equivalents, and tonicity, please review Units of Measure. Plasma has approximately 7% (by volume) proteins and lipids. However, the ionic activity is limited to the aqueous portion of the solution. Labs report concentrations as mEq/Liter Plasma. You must correct for the 7% non-aqueous portion to obtain the actual concentrations (if needed). This is why the numbers in the second plasma column are higher than the first. Not all of the reported concentrations are FREE. Some ions are bound to proteins or other ions. Proteins have many negative charges per molecule. The equivalents given above are therefore much higher than the molarity of those same proteins.
Units of Measure in Solutions:
Concentrations are often given in terms of weight/volume. For example, mg/L, or mg/100 mL (common clinical units), are used. These units do not depend on knowledge of the molecular structure of the measured substance. For a substance with a known molecular structure, one can define a "Mole" of that substance. 1 Mole is the weight of 6.23X10^23 molecules, and is commonly calculated as the sum of the atomic weights of each atom in a molecule. A Molar Solution is an aqueous solution consisting of one mole of a substance plus enough water to make one Liter of solution. A Molal Solution is an aqueous solution consisting of one mole of a substance plus 1 kg of water (usually very close to 1 L water). The total volume may thus be more than 1 L. The difference between molar and molal is important when a solution contains a large amount of non-aqueous substance. For example, cream has 20% fat (homogenized in very small droplets). There would be a 20% difference between the molarity of salt and its molality. A lab might report a Na concentration of 20 mMolar (moles/L cream), but really all the Na would be in the 80% that was water. It's "real" concentration (molality) would be 24 mMolal (moles/L water). Page 5 of 29 06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Concentrations of ions are often given in Equivalents (or milliequivalents, mEq) per Liter. The equivalents of an ion is equal to the molarity times the number of charges per molecule. Thus Equivalents is the measure of CHARGE concentration. Osmoles refers to the number of impermeable particles dissolved in a solution, regardless of charge. This will be important for determining the diffusional movement of water. For substances that maintain their molecular structure when they dissolve (e.g. glucose), the osmolarity and the molarity are essentially the same. For substances that dissociate when they dissolve, the osmolarity is the number of free particles times the molarity. Thus for a pure NaCl solution, a 1 Molar solution would be 2 Osmolar (1 for Na, and 1 for Cl). When measured as osmoles per liter, one obtains the osmolarity. kg water, one obtains osmolality. For osmoles per
Calculating Osmolarity in Complex Solutions:
As described under Units of Measure, the osmolarity of a simple solution is equal to the molarity times the number of particles per molecule.
Glucose has 1 particle NaCl has two MgCl2 has three.
Real solutions can be much more complex:
Proteins with many equivalents/L may only contribute a small amount to the osmolarity, since they consist of a few very large "particles". Not all the solution volume is aqueous. For example, plasma has 7% dissolved proteins and lipids. Not all ions are free in a solution. Cations may be bound to other anions or to proteins. For complete accuracy, all constituents should be included in the calculation. Page 6 of 29 06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Shortcut:
The difficulties given above leave many uncertainties when calculating the osmolarity of a solution like plasma. Plasma osmolarity can be estimated easily, however: Take the reported Na concentration (mEq/Liter Plasma) and double it. This obviously erroneous calculation (given all of the above) comes very close, since the errors tend to cancel each other! In some clinical settings, one must also account for the effects of elevated plasma glucose or urea.
Osmolarity vs. Tonicity:
Osmolarity measures the effective gradient for water assuming that all the osmotic solute is completely impermeant. It is simply a count of the number of dissolved particles. Therefore a 300 millimolar solution of glucose, a 300 millimolar solution of urea, and a 150 millimolar solution of NaCl each have the same osmolarity. A cell, placed in each of these solutions, would behave very differently, however. In a 150 mM NaCl solution, there would be equal osmotic strengths on both sides (NaCl is impermeant), and the cell would maintain the same volume. Urea is very permeable through most cell membranes. It exerts little osmotic force against a real cell and its membrane. A cell placed in 300 mM urea would rapidly swell as both urea and water entered the cell down their activity gradients. Tonicity is a functional term that describes the tendency of a solution to resist expansion of the intracellular volume. Two solutions are isosmotic when they have the same number of dissolved particles, regardless of how much water would flow across a given membrane barrier. In contrast, two solutions are isotonic when they would cause no water movement across a membrane barrier, regardless of how many particles are dissolved. In the example given above, a 150 mM NaCl solution would be isosmotic to the inside of a cell, and it would also be isotonic--the cell would not swell or shrink when placed in this solution. On the other hand, a 300 mM urea solution, while still isosmotic would cause the cell to swell and burst (due to its permeability). This isosmotic ureas solution is not isotonic. Instead it has a lower tonicity (called hypotonic).
Page 7 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
The Anion Gap:
The equivalents of cations in a solution always balances the equivalents of anions. However, by tradition, not every ionic constituent is reported by the lab. (Do you really want to know that much detail in any case?!). The simplest reports may only give the [Na+], [Cl-], and the [HCO3-]. The "real" balance is given by the equation: [Na] + [other cations] = [Cl] + [HCO3] + [other anions] rearranging: [Na] - ([Cl] + [HCO3]) = [other anions] - [other cations] = "Anion Gap" Normal values for the Anion Gap are 8-16 mEq/L plasma The Anion Gap is a useful shorthand measure, particularly in the differential diagnosis of acid/base disorders.
Page 8 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Summary of common body fluid distrubances.
Most clinical body fluid disturbances fall into one of a small number of classifications. These are summarized in the table below, along with the changes in ECF and ICF volume and osmolarity. Note that the nomenclature gives you important clues: It is based on the condition of the ECF following the alteration. For example, hypo-osmotic expansion means the osmolality is reduced and the ECF volume has increased. Therefore you can immediately predict the changes in the EC fluid based on the words used to describe the clinical condition. Also note that shifts in osmolarity are always in the same direction. This is because the EC and IC compartments are always in osmotic equilibrium. Focusing on the name of the disturbance thus gives you three of the four changes listed in the table. The fourth column is also easy to derive: Since the number of disolved particles in the intracellular compartment is fixed (they don't cross the cell membrane), the only way for IC osmolarity to change transiently is by the addition or subtraction of water. If the osmolarity decreases, then the volume must go up. Note that in the chart below, the IC volume arrows are always opposite those for the IC osmolarity.
Condition
Example
EC Fluid Osmolality Volume
IC Fluid Osmolality Volume
Hyposmotic expansion Hyposmotic contraction Isosmotic expansion Isosmotic contraction Hyperosmotic expansion Hyperosmotic contraction
excessive water intake salt wasting (Loss by kidneys) IV infusion, edema hemorrhage, burns drink conc. saline severe sweating
In the table, denotes a decrease, an increase, and no change. Page 9 of 29 06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Fluid Movements
This section reviews the basic forces of diffusion, membrane transport of solutes, and the osmosis of water across membranes. Since the section probably repeats information from your undergraduate studies, you may safely skip it if you feel completely comfortable with its definitions and demonstrations. Fluid movement is caused primarily by the application of pressure. In a macroscopic sense, the application of hydrostatic pressure causes bulk flow, e.g. blood flowing through your artieries. Pressure also moves fluid at a microscopic level. Through pores within cell membranes, and between the cells, pressure causes water and its dissolved particles to move. The amount of movement is equal to the pressure gradient, the area, and the leakiness of the barriers. The random movement of particles in the presence of a concentration gradient is called diffusion, which also acts as a microscopic pressure that causes movement within solutions and across membranes. The mechanism by which water and other solutes move across cell membranes is reviewed here in the section on membrane transport. Diffusion of water is called osmosis, which is another critical microscopic pressure controlling fluid movement. These forces control the exchange of water and solutes between the intracellular and extracellular fluids as well as between the interstitial and the plasma compartments of the ECF. Follow these sections to examine these forces in more detail.
Page 10 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Membrane Transport Mechanisms
There are four primary means that water and other small molecules cross into or out of cells. They are reviewed here since they play such an important role in body fluids and many other physiological functions.
Diffusion
Anions and cations shown moving at random, as via diffusion in a solution. Put the cursor over the Net movement is from regions of high box above to start the simulation. concentration to low. Click within the box to reset all Does not saturate as the concentration the particles to a single point. or gradient changes For a more extensive review of Powered by random movement of molecules in a solution Diffusion of different substances do not Diffusion, Osmosis, and Nernst Potentials, see my separate interfere with each other (no chapter on that subject. competition). Net flux (amount of movement) is proportional to the concentration difference and the permeability of any barrier like a membrane. Substances can cross membranes by diffusion if they can dissolve in the oily interior of the membrane (hydrophobic) Diffusion can occur through tight junctions or within bulk solutions. Diffusion of water down its concentration gradient is called osmosis.
Page 11 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Facilitated Diffusion
Proteins act as carriers or pores permit flux of substances that cannot diffuse directly through the membrane. Movement is still passive (like diffusion), from high concentration to low. Occurs across cell membranes only. Saturates when substance reaches high concentrations due to lack of available protein. Related substances can compete for the same carrier or pore. Maximum rate of transport (fully saturated) is called Tm, the transport maximum.
Primary Active Transport
Proteins in the membrane can also act as pumps. Move ions or small molecules from low concentration to high concentration (i.e. up their gradients). Require cellular energy, usually as ATP Saturates when substance reaches high concentrations due to lack of available protein. Example: Na-K ATPase Present in nearly every cell in the body
Page 12 of 29
06/18/99 ;
Body Fluids Pumps 3 Na ions out in exchange for 2 K ions pumped in (cost=1 ATP)
http://cats.med.uvm.edu/physiology/bodyfluids
Other pumps include the CaATPase, and the H-ATPase.
Secondary Active Transport
Uses proteins similar to those for facilitated diffusion.
Couples the movement of several different molecules in each cycle.
Saturates when substance reaches high concentrations due to lack of available protein. Cotransport moves 2 or more molecules in the same direction across the membrane. Counter transport moves molecules in opposite directions. The gradient for one molecule can cause the other to move against its own diffusion gradient. Normal active transport (Na-K ATPase) makes a strong Na gradient, which in turn powers many secondary active transport mechanisms. Example: Na-Glucose cotransport.
Page 13 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Osmosis:
Osmosis is the diffusion of water down its concentration gradient. Normally one thinks of water as the solvent, and focuses on the concentration of the solutes, but water itself has a concentration in any solution. Pure water has a molecular weight of 18 grams/mole, so its concentration is approximately 55 Molar! Solutes take up space that would otherwise have been occupied by water in a solution, and they also associate with a number of the water molecules, further lowering its activity (effective concentration). The following demonstration simulates the process of dissolving a solute in water. Watch what happens to the concentration of free water as you use the chooser menu to increase the number of solute particles. Simulation of Water and Solute in an Aqueous Solution
This simulation shows the behavior of water and solute in an idealized, simple solution. Initially only 150 water molecules are present, and they are exhibiting Brownian motion. Use the pulldown menu at the lower right to increase (or decrease) the number of solute molecules in solution. Clicking on the large compartment itself resets the simulation to its starting condtion. Each additional solute associates with several water molecules: in this simulation, 3. When no solute is present, 150 water molecules are free. When 10 solute atoms are added, and the initial volume maintained at 150 particles, only 140 water molecules are present and 110 of these are free. What do you think would happen to the solution, if the solute amount were increased to 30? Try it and see! For a more extensive review of Diffusion, Osmosis, and Nernst Potentials, see my separate chapter on that subject.
The other critical component for osmosis is a barrier that permits water to cross, but holds back some or all of the solutes. Under these conditions, a gradient in solute concentration means that there is also a gradient in the free water concentration (the other way!). The following demo illustrates this process.
Page 14 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids Simulation of Osmotic Flow Between Two Flexible Compartments Fluctuating volumes and effective Molar concentrations for each compartment are depicted numerically across the top of the simulation. Use the mouse and the pull-down bar at the right to change the solute number in the right-hand compartment from 10 to 5 particles and describe what happens. (This experiment may take as much as 30 minutes to reach equilibrium!) Does the membrane ever return to its original location? How about a change from 10 to 20? And then from 20 to 5? Repeat these experiments several times to obtain a statistical sample.
The demonstration above shows the membrane moving (and the volume changing) as water moves. This is just what would occur between two flexible compartments that always had equal hydrostatic pressure. Cells behave this way, as will be discussed below As we have also seen, hydrostatic pressure can also cause water to move. When pressure is applied against the direction of osmotic movement, then the osmotic flow will be slowed or even reversed. When the pressure is just enough to stop the osmotic flow an equilibrium is reached. This pressure, by definition, is called the "Osmotic Pressure" of a solution.
Page 15 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Exchange between Intracellular and Extracellular Compartments:
The ICF and the ECF are separated by the membranes of the body's cells. The protein components of these membranes give them substantial permeability to water while carefully controlling the permeability of selected ions. Cell membranes are flexible. If water flows into (out of) the cell, it expands (contracts). Hydrostatic pressure therefore does not play a significant role, and osmosis results in flows rather than pressure. As discussed above, osmosis occurs when there is a gradient of impermeable solute across a water-permeable membrane. In cells, osmotic flow would occur if there were an osmotic gradient between the intracellular and extracellular fluids. In the body, these two compartments are always in osmotic equilibrium, even though the composition of the fluids in them is very different. The addition or subtraction of water or solutes from one or more of the body's fluid compartments will result in water exchange between the ICF and the ECF if there is an alteration in the resulting osmolarity. These relationships were discussed more extensively in the clinical examples given above, but here you will view more detail regarding how to calculate exactly how much water moves where during distrubances.
Calculating Extracellular fluid - intracellular fluid exchange
In the simplest of examples, it is possible to understand intuitively how the body fluids move between the intracellular and extracellular compartments. However, for more complex cases, it is often necessary to calculate the exact shifts based on the total osmolar content of the two compartments. This section discusses methodologies for making such calculations.
Page 16 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Analytic principles and assumptions
1. Water moves freely between intracellular and extracellular compartments, driven by osmotic pressure differences. Ion movement is variable, but for simplicity of analysis it is reasonable to assume that there are no transient shifts of ions between ECF and ICF . 2. Exchanges of water and solutes with the environment, for example intravenous infusion or water intake, take place via the ECF. Change of the ICF occurs only by way of fluid shifts, which are seen only if the ECF osmolality is perturbed. 3. The ECF and ICF are in osmotic equilibrium (although the equilibrium may be disrupted by brief transients that take place over periods of seconds or minutes). Thus one assumes equality of plasma, interstitial fluid, and intracellular fluid osmolality. 4. When one considers the effect of adding or removing water or solutes from the body, mass must be conserved, i.e. neither water nor solutes are created or destroyed as the compartments come to equilibrium. See the following for specific examples of such fluid shift calculations. knowledge on the study questions at the end. Also, try out your
Example of calculations
Assume the standard 70 kg male, in whom the original osmolality of the ECF is 290 mOsm/kg water. Consider addition of 2 liters of distilled water to the ECF. Describe in words the shifts that take place, and calculate the effect of this addition on the volumes and osmolality of the ECF and ICF. Addition of water to the ECF increases its volume and reduces its osmolality. We assume there are no significant movements of solutes across the cell membranes. The cell membranes are permeable to water, and the increased water concentration in the ECF causes water to enter cells, increasing the volume of the ICF and reducing the ECF volume (but not returning it to its initial volume). The shift of water results in re-establishment of osmotic equilibrium between ECF and ICF, with both having increased volumes and decreased osmolalities.
Page 17 of 29
06/18/99 ;
Body Fluids Initial conditions: Initial total body water Initial ICF volume Initial ECF volume
http://cats.med.uvm.edu/physiology/bodyfluids
0.6 x 70 kg = 42 liters 0.4 x 70 kg = 28 liters 0.2 x 70 kg = 14 liters TBW volume x osmolality
Initial total body osmoles
42 liters x 290 mOsm/liter 12180 mOsm ICF volume x osmolality
Initial ICF osmoles
28 liters x 290 mOsm/liter 8120 mOsm ECF volume x osmolality
Initial ECF osmoles
14 liters x 290 mOsm/liter 4060 mOsm
Final conditions: Final osmolality (Total body osmoles)/(new TBW) 12180 mOsm/(42+2) kg water 277 mOsm/kg water Final ICF volume (ICF osmoles)/(new osmolality) 8120 mOsm/(277 mOsm/kg water) 29.3 kg water = 29.3 liters Final ECF volume (ECF osmoles)/(new osmolality) 4060 mOsm/(277 mOsm/kg water) 14.65 kg water = 14.65 liters It will be apparent that one might get these results in other ways (for example, note that the added two liters distributes between the two compartments in proportion to their original size (2/3 to ICF, 1/3 to ECF). However, the systematic approach has advantages because it can predict the correct result even when the body fluid changes include alterations to both water and solutes simultaneously. Page 18 of 29 06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Plasma-Interstitial Fluid Exchange
The aqueous solutions that compose the plasma and the interstitial fluid exchange readily through the thin walls of most of your body's capillaries. The primary forces that govern this exchange are hydrostatic pressure (the blood pressure within the capillaries), and osmosis.
Hydrostatic Pressure at the Capillary:
The capillary wall acts as a filtration "barrier". Most of the fluid within the capillaries is retained, but some filters through pores between the cells, pushed by the pressure difference between the capillary blood and the ISF. Water and small solutes can pass freely through these pores. The net effect of the hydrostatic pressure alone is a net loss of water and solute from plasma to the ISF. The capillary wall (both cells and pores) are, however, impermeable to the plasma proteins and lipids. Under normal conditions, these stay within the plasma. Note that following injury, the capillaries can also leak protein. The hydrostatic pressure in the capillaries is lower than that of the arteries, and decreases along the length of the capillary as blood flows through. At the arteriolar end of the capillary, the pressure is usually about 35 mm Hg (due to the pressure drop caused by the resistance arterioles). On the venule end of the capillary, the pressure is in the range of 15 mm Hg. The mid-capillary pressure profile can be assumed to be linear (see figures below).
Osmotic forces in the capillaries:
Because the capillary wall is permeable to water, but essentially impermeant to the plasma proteins, these molecules generate an osmotic pressure. Furthermore, since these proteins are negatively charged, they tend to hold additional cations in the plasma (the Gibbs-Donnan effect), further enhancing an osmotic gradient between the plasma and the interstitial fluid. The combined effect (osmotic and Gibbs-Donnan) results in a pressure that draws water out of the interstitium and into the plasma. This pressure is known as the Colloid Oncotic Pressure (often shortened to the Oncotic Pressure). This pressure is proportional to the difference in protein concentration between the Page 19 of 29 06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
plasma and the ISF. Compared to pure saline, the plasma exerts about 28 mm Hg Oncotic pressure, whereas the ISF has only about 3 mm Hg. The net Oncotic Pressure is thus about 25 mm Hg. This value remains roughly constant over the length of most capillary beds.
Starling's Relationship
The British physiologist Starling first identified the interrelationship between the hydrostatic pressure and the oncotic forces within the capillary. Hydrostatic pressure tends to cause fluid to leave the plasma, and oncotic pressure pulls it back. These two forces tend to balance each other. The hydrostatic forces, however, are gradually decreasing over the length of the capillary, while the oncotic pressure remains constant. If these pressures were graphed, they would look approximately like the following figure:
On the arteriole end, the hydrostatic pressure is higher than the oncotic, so there is fluid movement from plasma to interstitium. The magnitude of this water flow is indicated by the light blue area on the left (downward arrows). On the venule end, the hydrostatic pressure has dropped below the oncotic. Fluid moves back from the interstitium to the plasma. The magnitude of this reverse flow is indicated by the green area on the right (upward arrows). In a normal capillary bed, fluid gain and loss from the plasma are closely balanced, so there is little or no net change in plasma and ISF volumes. Note that this can be seen graphically, since the blue and green (right and left) regions have equal areas. Note that a small excess in fluid loss from plasma to ISF is normally drained back to the circulation via the lymphatics.
Page 20 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Starling forces in disease:
The balance described above can be altered by changes to the capillary pressure near the arterioles or near the venules, as well as alterations in the Oncotic Pressure.
Understanding how changing resistance changes capillary pressures:
The vascular system is a highly branched set of tubes that carry the blood to all parts of the body. Blood pressure results from the pumping of the heart, and affects the movement of fluids throughout the vascular system. This subject will be studied in more detail in April, but many other systems depend how the blood pressure is "felt" in the capillaries as a function of arterial resistance. Fluids move through blood vessels (as with any kind of pipe) when there is a pressure difference between the start and end of the pipe. Just as water moves continuously downhill as a river flows, the pressure drops continuously from the start to the end of a vessel. Since the capillaries are (in a sense) midway between arteries and veins, their pressure will be lower than the central arterial pressure and higher than the central venous pressure. Also, within the capillaries themselves, the pressure will gradually drop from their arteriolar side to their venule side. When a much bigger resistance to flow is introduced in the middle of this pipe (like a damn built on a river, or a "squeeze" on a tube), the pressure upstream from that resistance will be closer to that near the beginning of the pipe, and the pressure downstream will be closer to that near the end. Arterioles squeeze the blood vessels, giving a relatively large resistance to flow. Upstream, the pressure is much closer to central arteriolar pressure, while downstream at the start of the capillaries the pressure is about 35 mm Hg. When the arterioles dilate (and make less resistance), the pressure in the capillaries rises (closer to arterial pressure). When the arterioles constrict (more resistance), then the pressure upstream will rise even more, while it will fall at the capillaries. These changes in capillary pressure are critical for understanding: 1. 2. 3. Starling Forces of the capillary. Production of saliva and other excretory filtrates. Glomerular filtration in the kidney.
Page 21 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
The following examples illustrate the range of imbalances that can occur:
Increased Blood Pressure at the capillaries: Vasodilation
Vasodilation reduces the pressure drop across the arterioles, bringing the capillaries closer to the arterial pressure. The venous pressure may not be altered. In this case, there is a greater region where fluid leaves the plasma, and a reduced regions where it returns. This imbalance results in a net loss of fluid from the plasma. The result is an expansion of the interstitial fluid in this tissue. If this expansion continued, it would result in the clinical symptom known as edema.
Decreased Blood Pressure at the Capillaries: Shock
Page 22 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
When the central blood pressure declines, the pressure at the capillaries usually also decreases. In addition, most vascular beds will participate in reflex attempts to maintain the central blood pressure via arteriolar vasoconstriction. This further reduces the pressure at the start of the capillary. The decrease in hydrostatic pressure results in a diminution in the region where fluid is lost from the plasma, and an expansion in the region where fluid returns back to the plasma. There is a net gain of fluid to the plasma. This net gain, taken over most of the body's vascular beds leads to an "autotransfusion" that helps to compensate for plasma loss during hemorrhagic shock.
Increased Venous Pressure: Congestive Heart Failure
When the heart's function is compromised, it cannot pump blood as effectively, so venous pressure rises. This elevation in venous pressure is also felt at the capillaries, as illustrated above. This rise in venous pressure diminishes the region where fluid is reabsorbed into the plasma. As with the case (above) of vasodilation, this condition results in a net loss of fluid from plasma to ISF. The resulting edema can be seen in the swollen ankles (and other tissues) that are symptomatic of congestive heart failure.
Page 23 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Decreased Oncotic Pressure: Protein deficiency and tissue damage
When the plasma does not contain sufficient protein, or the interstitial fluids contain too much, then the difference in oncotic forces diminishes. As shown above, this results in a net increase in the region where hydrostatic pressure exceeds oncotic. Edema results. This imbalance can be seen in a number of important conditions. The swollen bellies of children with severe protein malnourishment are due to such edema within the peritoneum. Also, when capillaries are damaged, as in severe burns, they permit proteins to leak into the interstitium. Added to the proteins released from lysed cells, the net result is a diminution of the oncotic pressure difference, and edema.
Page 24 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
BODY FLUIDS - STUDY QUESTIONS
1. What is the concentration of water in pure water? 2. What is the water concentration of a 1 M NaCl solution? 3. Distinguish between "tonicity" and "osmolality." Illustrate the lack of identity between "isotonic" and "isosmotic" by an example in which the two clearly are different. 4. The edema of congestive heart failure involves many factors. How might the finding of increased central venous pressure contribute to this fluid accumulation? 5. A 70 kg male has a body fluid osmolality of 290 mOsm/kg water. Assume 290 millimoles of NaCl are added to the ECF. Describe in words what fluid and solute shifts, if any, take place. Calculate the final osmolality of the body fluids, and the final ECF and ICF volumes. 6. A 70 kg male has a body fluid osmolality of 290 mOsm/kg water. Assume 2 liters of isotonic saline are added to the ECF. Describe in words what fluid and solute shifts, if any, take place. Calculate the final osmolality of the body fluids and the final ECF and ICF volumes. 7. A patient on the renal ward weighed 100 kg, had a plasma osmolality of 290 mOsm/kg water, and the following volume distribution: TBW 60% body weight, ICF volume 40% body weight, and ECF volume 20% body weight. After drinking 5 liters of water rapidly, and following complete absorption but prior to any excretion, what would be the new ICF and ECF volumes (in liters)? 8. In the patient in 7, what would be the new plasma osmolality in mOsm/kg water?
Page 25 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
ANSWERS
1. What is the concentration of water in pure water? 1 L water weighs 1000 g. Gram molecular wt water = 18 grams (1000g/L)/(18grams/mole) = 55.5 moles/L or 55.5 molar 2. What is the water concentration of a 1 M NaCl solution? 1 M NaCl --> 2 osmoles. Each osmole displaces approximately 1 mole of water. Therefore the water concentration is (55.5-2)moles/L, or 53.5 molar. 3. Distinguish between... Tonicity of a particular solution is defined by whether steady-state cell volumes change when they are placed in the solution. Osmolality is determined by the number of particles, i.e. ions or molecules, in solution. An "isotonic" solution is one in which cells neither swell nor shrink in the steady state. An " isosmotic" solution (with respect to plasma) is one that has the same osmolality as plasma. The tonicity of a solution depends only on the osmolality of impermeant solutes, whereas the osmolality depends on the concentrations of all solutes. A solution containing 300 mOsm/kg water of urea is isosmotic with plasma, because both have the same osmolality, but it is not isotonic because urea permeates cell membranes easily. Cells placed in a 300 mOsm/kg urea solution will swell as urea and water enter. 4. The edema of congestive heart failure... The central venous pressure is the pressure in the right atrium and thoracic venae cavae. Heart failure can be defined as any abnormality in the pumping action of the heart that reduces its ability to perform external work. Central venous pressure rises as cardiac output declines. The mean arterial blood pressure may stay normal because of compensatory reflexes (homeostatic control), and only decline in the late stages of heart failure. Applying the Starling concept of capillary filtration and reabsorption of fluid, normal arterial pressure and increased venous pressure will decrease reabsorption of fluid at the venous ends of capillaries and hence cause edema.
Page 26 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
5. A 70 kg male has a body fluid osmolality... This question was drawn from Berne and Levy, 3rd Ed., pp 756ff. Addition of 290 mMoles of NaCl to the ECF adds 580 mOsm to the ECF. We assume NaCl is restricted to the ECF--i.e. does not cross cell membranes. The osmolality of the ECF will increase, which will cause net water flow from the ICF to the ECF. The flow of water increases osmolality of the ICF. Net water flux ceases when the ECF and ICF have the same effective osmolality.
Calculations: Initial conditions: Initial total body water (TBW): 70 kg x 0.6 = 42 kg or 42 L waterInitial ICV volume: 42 x 2/3 = 28 L Initial ECF volume: 42 x 1/3 = 14 L Initial total body osmoles: 42 kg x 290 mOsm/kg = 12180 mOsm Initial ICF osmoles: 28 kg x 290 mOsm/kg = 8120 mOsm Initial ECF osmoles: 14 kg x 290 mOsm/kg = 4060 mOsm
Final conditions:
Final osmolality = (new total body osmoles)/(TBW) (12180 mOsm + 580 mOsm)/(42kg water) = 303.8 mOsm/kg water
Final ICF volume = ICF osmoles/(new osmolality) 8120 mOsm/(303.8 mOsm/kg water) = 26.7 kg or 26.7 L
Final ECF volume = TBW - ICF = 42 - 26.7 = 15.3 L
(Could also have been calculated as follows:)
Final ECF volume = (new ECF osmoles)/(new osmolality) (4060mOsm + 580mOsm)/(303.8 mOsm/kg water) 4640/303.8 = 15.3 kg water or 15.3 L
Page 27 of 29
06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
Note that addition of NaCl to the ECF has altered the fractions of the TBW contained in the ECF and ICF. The two compartiments no longer contain 1/3 and 2/3 of the TBW.
6. A 70 kg male has a body fluid osmolality... This is also taken from examples in Berne and Levy, 3rd Ed. Addition of isotonic saline to the ECF does not change the effective osmolality of the ECF. There is, therefore, no driving force for net water movement. The two liters of saline remains in the ECF. The initial conditions are just as calculated for question 5. The final conditions are:
Osmolality of TBW, ECF, ICF unchanged.
Final ECF volume = 14 L + 2 L = 16 LFinal ICF volume = unchanged.
7. A patient on the renal ward...Initial conditions: Initial TBW = 100 kg x .6 = 60 kg or 60 L water Initial ICF volume = 100 kg x 0.4 = 40 kg or 40 L water Initial ECF volume = 100 kg x 0.2 = 20 kg or 20 L water
Initial Total body osmoles = 60 kg x 290 mOsm/kg = 17400 mOsm Initial ICF osmoles = 40 kg x 290 mOsm/kg = 11600 mOsm Initial ECF osmoles = 20 kg x 290 mOsm/kg = 5800 mOsm
Final conditions: Question indicates water absorbed but not excreted. Water will be added to ECF, then equilibration of water between ECF and ICF will take place. Assume solutes do not move between compartments.Final TBW = 60 L + 5 L = 65 LNew osmolality = 17400 mOsm/65 kg water = 267.7 mOsm/kg water in ECF and ICF ECF Volume = (ECF osmoles)/(new ECF osmolality) 11600 mOsm/(267.7 mOsm/kg water) = 43.3 kg or 43.3 L ICF Volume = (ICF osmoles)/(new ICF osmolality) Page 28 of 29 06/18/99 ;
Body Fluids
http://cats.med.uvm.edu/physiology/bodyfluids
5800 mOsm/(267.7 mOsm/kg water) = 21.7 kg or 21.7 L As a check, note that 21.7L + 43.3L = 65 L Note also, that the new water is proportioned in the same 1/3, 2/3 ratio as existed originally, since the ratio of milliosmoles is 1/3, 2/3 and does not change when pure water is added. 8. In the patient in 7... The new plasma osmolality would be the same as the osmolality of the other fluids, 267.7 mOsm/kg water.
Page 29 of 29
06/18/99 ;
Anda mungkin juga menyukai
- Anatomy and Physiology Terms: Brief Definitions, Roots & Morphology; An Abecedary; Vol 2-Planes & DirectionsDari EverandAnatomy and Physiology Terms: Brief Definitions, Roots & Morphology; An Abecedary; Vol 2-Planes & DirectionsBelum ada peringkat
- Blood Vessels and Lymphatics in Organ SystemsDari EverandBlood Vessels and Lymphatics in Organ SystemsDavid AbramsonBelum ada peringkat
- Body Fluid Compartment and Formation of Edema 1Dokumen4 halamanBody Fluid Compartment and Formation of Edema 1JayricDepalobosBelum ada peringkat
- Anatomy VivaDokumen5 halamanAnatomy VivaAhmedBelum ada peringkat
- Histo - Pituitary GlandDokumen22 halamanHisto - Pituitary GlandRessam NazirBelum ada peringkat
- Lect 75control of Tissue Blood FlowDokumen23 halamanLect 75control of Tissue Blood FlowEl FaroukBelum ada peringkat
- Week12 13 RENAL PhysiologyDokumen41 halamanWeek12 13 RENAL Physiologyirwan kastellaBelum ada peringkat
- Cerebellum: Principally Because Electrical Excitation of The CerebellumDokumen42 halamanCerebellum: Principally Because Electrical Excitation of The Cerebellumdr_mksinhaBelum ada peringkat
- BloodDokumen38 halamanBloodchukwukerechimezirimBelum ada peringkat
- Chapter 21 Muscle Blood FlowDokumen17 halamanChapter 21 Muscle Blood Flowelmedina omeragicBelum ada peringkat
- Blood: 1. Medullary Cavities of Long Bones 2. Spongiosa of Vertebral Bodies, Ribs, Sternum 3. Flat Bones of The PelvisDokumen2 halamanBlood: 1. Medullary Cavities of Long Bones 2. Spongiosa of Vertebral Bodies, Ribs, Sternum 3. Flat Bones of The PelvisKrizza Mariz Bautista PolaronBelum ada peringkat
- Nerve Muscle PhysiologyDokumen79 halamanNerve Muscle Physiologyroychanchal562100% (2)
- Unit 3: Control and Regulation: Physiological HomeostasisDokumen16 halamanUnit 3: Control and Regulation: Physiological HomeostasisaclumutBelum ada peringkat
- Blood Physiology Part 2 Dr. OlivarDokumen5 halamanBlood Physiology Part 2 Dr. OlivarJorelyn FriasBelum ada peringkat
- NeurophysiologyDokumen364 halamanNeurophysiologyTaimoor Ul HassanBelum ada peringkat
- Lecture 2 Endocrine SystemDokumen53 halamanLecture 2 Endocrine SystemLouella ArtatesBelum ada peringkat
- Bio 122 FR 09 - Muscle ContractionDokumen10 halamanBio 122 FR 09 - Muscle ContractionLance CarandangBelum ada peringkat
- Bilaminar and Trilaminar Germ DiscDokumen40 halamanBilaminar and Trilaminar Germ DiscjabirBelum ada peringkat
- Endocrine System PhysiologyDokumen2 halamanEndocrine System PhysiologyLuisGabitoBelum ada peringkat
- The Cardiovascular System: Elaine N. MariebDokumen151 halamanThe Cardiovascular System: Elaine N. Mariebwhiskers qtBelum ada peringkat
- Anatomy of PelvisDokumen40 halamanAnatomy of PelvisGovGeet100% (1)
- Muscular TissueDokumen49 halamanMuscular TissueSteph VeeBelum ada peringkat
- Cardiac OutputDokumen31 halamanCardiac OutputanojBelum ada peringkat
- Upper Limbs NOTES - BRS Anatomy, Table of Muscles and BRS Questions With Answers ExplainedDokumen14 halamanUpper Limbs NOTES - BRS Anatomy, Table of Muscles and BRS Questions With Answers ExplainedJustyna PoznanskaBelum ada peringkat
- Bones of Upper and Lower Limbs - RevisionDokumen15 halamanBones of Upper and Lower Limbs - RevisionChess Nuts100% (1)
- The ABC's in Pediatric Assessment and Care: Cymbeline Joan L. Pooten, RN NUM - Pediatric ICUDokumen39 halamanThe ABC's in Pediatric Assessment and Care: Cymbeline Joan L. Pooten, RN NUM - Pediatric ICUggrrk7Belum ada peringkat
- Cerebraal Spinal FluidDokumen34 halamanCerebraal Spinal FluidElisa NBelum ada peringkat
- Circulatory System - Part 2 4-8-14 For BBDokumen23 halamanCirculatory System - Part 2 4-8-14 For BBroman7dbBelum ada peringkat
- Introduction To Physiology: General Physiology The Cell HomeostasisDokumen38 halamanIntroduction To Physiology: General Physiology The Cell HomeostasisAnanta Subedi100% (1)
- 16/09/2015 1 Mujtaba AshrafDokumen44 halaman16/09/2015 1 Mujtaba Ashrafதீரன் சக்திவேல்Belum ada peringkat
- Notes About BiologyDokumen81 halamanNotes About BiologyRichard Coffey100% (1)
- Nerve and Muscle PhysiologyDokumen82 halamanNerve and Muscle PhysiologychandsriBelum ada peringkat
- Functional Anatomy of SkullDokumen83 halamanFunctional Anatomy of SkullTaha Ahsan100% (1)
- Anatomy Part 1 - The Upper LimbDokumen27 halamanAnatomy Part 1 - The Upper LimbSolange AcostaBelum ada peringkat
- Physiology Essays & Shorts Topic WiseDokumen18 halamanPhysiology Essays & Shorts Topic Wisekoutharapu lasyapriyankaBelum ada peringkat
- Lecture 1 - Development of Respiratory SystemDokumen27 halamanLecture 1 - Development of Respiratory SystemRachmad MusyaffaBelum ada peringkat
- The Gut Tube and Body CavitiesDokumen31 halamanThe Gut Tube and Body CavitiesGeoffreyBelum ada peringkat
- Histology Exam IV Review Part 2Dokumen26 halamanHistology Exam IV Review Part 2ashdmb217Belum ada peringkat
- 7th Week Femoral Sheath, Femoral Triangle and Adductor CanalDokumen17 halaman7th Week Femoral Sheath, Femoral Triangle and Adductor CanalShah NawazBelum ada peringkat
- Thoracic Wall.Dokumen48 halamanThoracic Wall.Shimmering MoonBelum ada peringkat
- General Principles of GIT PhysiologyDokumen22 halamanGeneral Principles of GIT PhysiologyUsman Ali AkbarBelum ada peringkat
- 4.3 Bone TissueDokumen80 halaman4.3 Bone TissueManjunathBelum ada peringkat
- Practical Physiology (CVS) : Dr. Ramadan Mohamed Ahmed Associate. Prof. in PhysiologyDokumen11 halamanPractical Physiology (CVS) : Dr. Ramadan Mohamed Ahmed Associate. Prof. in PhysiologyRamadan Physiology100% (1)
- The Nervous System: Na ShaoDokumen79 halamanThe Nervous System: Na ShaoArvindhanBelum ada peringkat
- Chapter 29 - Endocrine FunctionDokumen6 halamanChapter 29 - Endocrine Functionhgfree41392Belum ada peringkat
- Key of SEQs Heart, Nerve and Muscle Revision TestDokumen11 halamanKey of SEQs Heart, Nerve and Muscle Revision TestMudassar Roomi100% (2)
- Upper Extremities Ortho SG3Dokumen119 halamanUpper Extremities Ortho SG3Nuhu Bankwhot100% (1)
- Anatomy & Physiology Unit 1Dokumen29 halamanAnatomy & Physiology Unit 1Priyanjali SainiBelum ada peringkat
- 1 Red Blood Cells Anemia and PolycythemiaDokumen43 halaman1 Red Blood Cells Anemia and PolycythemiaGeevee Naganag VentulaBelum ada peringkat
- Respiratory SystemDokumen41 halamanRespiratory SystemNikkiAbbaNiColeCartagenaBelum ada peringkat
- Anatomy Last Moment RevisionsDokumen45 halamanAnatomy Last Moment RevisionsManisanthosh KumarBelum ada peringkat
- 2.osteology Q ADokumen11 halaman2.osteology Q ADr P N N ReddyBelum ada peringkat
- 1 SEMESTER 18/19: Median Nerve-Carpal Tunnel Weakens Lateral 3 Digits Sensory Loss ParalysisDokumen60 halaman1 SEMESTER 18/19: Median Nerve-Carpal Tunnel Weakens Lateral 3 Digits Sensory Loss ParalysisInsaf AhamedBelum ada peringkat
- Topographic Anatomy of Basal NucleiDokumen44 halamanTopographic Anatomy of Basal NucleiRafique AhmedBelum ada peringkat
- Sheep Brain Observation LAB 2015Dokumen4 halamanSheep Brain Observation LAB 2015Leo MatsuokaBelum ada peringkat
- Chapter 18 Endocrine SystemDokumen40 halamanChapter 18 Endocrine SystemlolasparkleBelum ada peringkat
- Endo 3 Notes PDFDokumen9 halamanEndo 3 Notes PDFDilBelum ada peringkat
- Upper Extremity (Anatomy)Dokumen17 halamanUpper Extremity (Anatomy)Margareth Christine CusoBelum ada peringkat
- Reproduction and Sexuality in Marine Fishes: Patterns and ProcessesDari EverandReproduction and Sexuality in Marine Fishes: Patterns and ProcessesKathleen S. ColeBelum ada peringkat
- Water System PQDokumen47 halamanWater System PQsainzb8389% (9)
- ARANGUEZ NORTH SECONDARY SCHOO1. IIdocxDokumen8 halamanARANGUEZ NORTH SECONDARY SCHOO1. IIdocxDevi RambaranBelum ada peringkat
- Worksheet Ap01Dokumen11 halamanWorksheet Ap01Abhishek SuranaBelum ada peringkat
- CH 7 AquaporinDokumen10 halamanCH 7 AquaporincoryguntherBelum ada peringkat
- CH 01Dokumen31 halamanCH 01loverBelum ada peringkat
- Env Engg Lab ReportDokumen46 halamanEnv Engg Lab ReportMuhammad ShahbazBelum ada peringkat
- Solutions Test 18.06.23 Answer KeyDokumen9 halamanSolutions Test 18.06.23 Answer KeyGGEZBelum ada peringkat
- Igcse Biology 2008 0610 - Y08 - SyDokumen42 halamanIgcse Biology 2008 0610 - Y08 - SyHassan mahmudBelum ada peringkat
- Overcoming The Challenges That Delay Development of Your Lateral Flow AssayDokumen6 halamanOvercoming The Challenges That Delay Development of Your Lateral Flow Assaymicrobehunter007Belum ada peringkat
- Composites CommunicationsDokumen6 halamanComposites CommunicationsNicolle Paredes ReyesBelum ada peringkat
- Exercise On Movement of Substance Across The Plasma MembraneDokumen7 halamanExercise On Movement of Substance Across The Plasma MembraneNorliyana Ali83% (18)
- IB DP Biology 1.4 Membrane Transport QuestionDokumen19 halamanIB DP Biology 1.4 Membrane Transport QuestionikaBelum ada peringkat
- Lecture 6 - Cell Membranes and TransportDokumen42 halamanLecture 6 - Cell Membranes and TransportGloria LiBelum ada peringkat
- On The Coupling of Mechanics With Bioelectricity and Its Role in MorphogenesisDokumen12 halamanOn The Coupling of Mechanics With Bioelectricity and Its Role in MorphogenesisVishvendraBelum ada peringkat
- Instrumentaion II Case Study SampleDokumen14 halamanInstrumentaion II Case Study SampleBijay Uprety50% (2)
- 2-Excellent Chemistry Assignment SolutionsDokumen5 halaman2-Excellent Chemistry Assignment SolutionsSachin B SBelum ada peringkat
- 1.4 - Diffusion and Osmosis - CrosswordPuzzleDokumen1 halaman1.4 - Diffusion and Osmosis - CrosswordPuzzlemmssthBelum ada peringkat
- Estimating Osmolarity LabDokumen9 halamanEstimating Osmolarity LabveronicaBelum ada peringkat
- Xii ChemistryDokumen119 halamanXii ChemistryAftab AliBelum ada peringkat
- Lesson Plan For SS1 Physics 3RD TermDokumen21 halamanLesson Plan For SS1 Physics 3RD Termowei prosperBelum ada peringkat
- Naked Eggs ExperimentDokumen2 halamanNaked Eggs Experimentbarbieflores762Belum ada peringkat
- Bgcse Biology Notes ImportantDokumen200 halamanBgcse Biology Notes ImportantKordell leydBelum ada peringkat
- IX Class NotesDokumen60 halamanIX Class NotesNitin ThakurBelum ada peringkat
- Movement in and Out of CellDokumen19 halamanMovement in and Out of CellRand HusseinBelum ada peringkat
- NTSE Stage-I &II - Biology Sheet Final-2017-18Dokumen170 halamanNTSE Stage-I &II - Biology Sheet Final-2017-18Rishikesh Kumar mishra67% (3)
- Answer Key Lab Diffusion and OsmosisDokumen8 halamanAnswer Key Lab Diffusion and OsmosissabrinaBelum ada peringkat
- Biology Notes For o Level HTMLDokumen91 halamanBiology Notes For o Level HTML123456789Belum ada peringkat
- Concept and System Design For Rate Controlled Drug DeliveryDokumen88 halamanConcept and System Design For Rate Controlled Drug DeliverySoma Ranjith100% (1)
- Ejercicio 1 - Actividad 3 PhysioexDokumen4 halamanEjercicio 1 - Actividad 3 PhysioexMayra MarroquinBelum ada peringkat
- Puc II Chem Mcqs 2024Dokumen113 halamanPuc II Chem Mcqs 2024geethaathradyBelum ada peringkat