First Pass at Cancer Genome
Diunggah oleh
Mariano PerezDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
First Pass at Cancer Genome
Diunggah oleh
Mariano PerezHak Cipta:
Format Tersedia
NEWS>>
THIS WEEK
A boost for Germany
No end to earmarks
1373
CANCER
1374
ants, and changes that didnt alter a protein. They ultimately found that the average breast or colon tumor has 93 mutated genes, and at least 11 are thought to be cancer-promoting. This yielded a total of 189 candidate cancer genes. Although some are familiarthe tumor-suppressor gene p53, for example most had never been found mutated in cancer before. And the abundance of certain types of genes, such as those involved in cell adhesion and transcription, suggested that these processes play a huge role in cancer. The results, says Ronald DePinho of the Dana-Farber Cancer Institute in Boston, are a treasure trove. Verifying that each candidate gene is important to cancer wont be simple. Not only did the cancer genes differ between colon and breast cancers, but each tumor had a different pattern of mutations. The number of genes suggests that there may be more steps to cancer than thought. Its a much more complex picture than we had anticipated, Vogelstein says. At least two other pilot cancer-genome projectsone funded by NHGRI and one led by Michael Stratton and P. Andrew Futreal of the Sanger Institute in Hinxton, U.K.are yielding similar results. The Sanger effort is looking at 500 genes in a larger number of tumor samples and cancer types and, according to an e-mail from Stratton and Futreal, has also found a tremendous diversity of mutation number and pattern between cancers. DePinho says the mutation differences from tumor to tumor could help explain why 90% of drugs fail in patients. Elledge, for his part, says the relatively small number of new genes common to the tumors reinforces his concerns about The Cancer Genome Atlas. He suggests that some of the governments money would be better spent on more direct studies, such as screens for lethal genes in cancer cells. The cost of the Hopkins study aloneVogelstein says it took about $5 million, mostly from private funding sourcescould fund five National Institutes of Health (NIH) grants on such topics, Elledge notes. Despite such doubts, the atlas project gets under way next week. NIH will announce the three cancers to be studied in the pilot phase and a set of repositories that will supply tissue samples for sequencing. Centers that will characterize the genes will be announced in early October. The project is on an extremely aggressive timeline, says DePinho, who co-chairs its advisory committee. JOCELYN KAISER
First Pass at Cancer Genome Reveals Complex Landscape
Scientists have long known that the sparks that kindle cancer are mutations in a cells genes. But most cancer-causing mutations have been discovered by looking in obvious places, such as in the genes that control cell division. Now it seems these efforts have barely glimpsed the big picture. As reported online this week in Science (www.sciencemag.org/cgi/content/abstract/ 1133427), researchers have shined a searchlight across the genomes of breast and colorectal cancer cells, looking for mutations in more than half of all known human genes. And what theyve uncovered is a much larger and richer set of cancer genes than expected. The findings, hailed as a tour de force by other cancer scientists, should speed the race for new drugs, diagnostics, and a better understanding of tumor development. It will take a long time to unravel all of this, but this is what cancer is , says Bert Vogelstein of Johns Hopkins Kimmel Cancer Center in Baltimore, Maryland, a co-leader of the sequencing effort. The results also appear to bolster The Cancer Genome Atlas, an ambitious $1.5 billion federal project to systematically search for genes mutated in dozens of cancer types (Science, 29 July 2005, p. 693). I see this as a big shot in the arm for the argument that this strategy is going to work, says Francis Collins, director of the National Human Genome Research Institute in Bethesda, Maryland, which together with the National Cancer Institute (NHGRI) will s o o n a n n o u n c e d e t a i l s o f a $100 million, 3-year pilot effort for the atlas. Adds Eric Lander, director of the Broad Institute in Cambridge, Massachusetts, who first proposed sequencing the cancer genome, This is a beautiful demonstration that if you turn over every rock, there is a lot more to be found. Yet even supporters of the atlas say this first, quick pass at describing all cancer mutations reveals daunting complexity. And not everyone has been convinced of the larger projects value. Geneticist Stephen Elledge of Harvard Medical School in Boston, while predicting that the new study will become a classic paper, says that a costly sequencing project will give short shrift to functional genomics studies and take money away from investigators working on equally important cancer efforts. I still believe we need a more balanced approach, says Elledge, who first expressed those concerns last year (Science, 21 October 2005, p. 439). To conduct this minicancer-genome project, a 29-person team, headed by Vogelstein and Hopkins colleagues Kenneth Kinzler and Victor Velculescu, began with a database of 13,023 genes that are considered the beststudied and annotated of the 21,000 known genes in the human genome. Led by postdoc Tobias Sjblom, the team resequenced the protein-coding regions of the genes in 11 breast cancer samples and 11 colon cancer samples, yielding 800,000-plus possible mutations. The team then winnowed out more than 99% of the mutations by removing errors, normal vari-
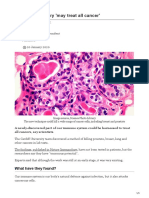
Genetic bounty. Breast (top) and colorectal (bottom) cancer cells contain many mutated genes.
1370
8 SEPTEMBER 2006
VOL 313
SCIENCE
www.sciencemag.org
Published by AAAS
CREDITS (TOP TO BOTTOM): DAVID BECKER/GETTY IMAGES; EYE OF SCIENCE/PHOTO RESEARCHERS INC.
Anda mungkin juga menyukai
- Villa La Paws Operations Manual-Detail 03-26-2014Dokumen167 halamanVilla La Paws Operations Manual-Detail 03-26-2014Anonymous JKqvy986U100% (1)
- Safety Legislation, Regulation and PolicyDokumen110 halamanSafety Legislation, Regulation and PolicyNichoBelum ada peringkat
- Mental HealthDokumen80 halamanMental HealthSingYen QuickBelum ada peringkat
- Automatic Stop Orders (ASO)Dokumen3 halamanAutomatic Stop Orders (ASO)Bae BeeBelum ada peringkat
- (SAMPLE THESIS) Research and Analysis Project (Obu)Dokumen66 halaman(SAMPLE THESIS) Research and Analysis Project (Obu)Murtaza Mansoor77% (35)
- Workbook Answer KeyDokumen27 halamanWorkbook Answer Keysalcedo4479% (56)
- Info - Iec 62305-2 2010Dokumen10 halamanInfo - Iec 62305-2 2010Anonymous HMhAx1BoWBelum ada peringkat
- Infection Control LectureDokumen21 halamanInfection Control LectureJessica Medina100% (1)
- Zheng Hong 郑 红 Department of Medical Genetics & Cell BiologyDokumen63 halamanZheng Hong 郑 红 Department of Medical Genetics & Cell BiologyinakiBelum ada peringkat
- BBB Month 10 Workout LogDokumen8 halamanBBB Month 10 Workout LogLi SaBelum ada peringkat
- Chapter6 - Feeds and Feeding The FishDokumen23 halamanChapter6 - Feeds and Feeding The Fishፋሲል ታደሰ100% (3)
- Fu Zhen and TurtleDokumen7 halamanFu Zhen and Turtledrakpo100% (2)
- Human Genome Discoveries Reach The BedsideDokumen12 halamanHuman Genome Discoveries Reach The BedsideireneBelum ada peringkat
- Lists of Cancer Mutations Awash With False Positives - Nature News & CommentDokumen3 halamanLists of Cancer Mutations Awash With False Positives - Nature News & CommentMazikeen MorningstarBelum ada peringkat
- 2010-08-09 - NY Times - DNA Test May Speed Colon Cancer DiagnosticsDokumen3 halaman2010-08-09 - NY Times - DNA Test May Speed Colon Cancer DiagnosticsnamkoongBelum ada peringkat
- UpdatesDokumen2 halamanUpdatesCLBelum ada peringkat
- Science Magazine 5696 2004-10-22Dokumen120 halamanScience Magazine 5696 2004-10-22WillimSmithBelum ada peringkat
- Hereditary Link: Oncology Issues in FocusDokumen2 halamanHereditary Link: Oncology Issues in FocusMuhammad MotaweaBelum ada peringkat
- Nucleotides Haploid Reference GenomeDokumen2 halamanNucleotides Haploid Reference GenomeDaniH46Belum ada peringkat
- 44 Ter PersonDokumen4 halaman44 Ter PersonFlor Paredes VelasquezBelum ada peringkat
- Research Paper On Cancer GeneticsDokumen7 halamanResearch Paper On Cancer Geneticsrtggklrif100% (1)
- A Tipping Point in Cancer Research - InfographicDokumen2 halamanA Tipping Point in Cancer Research - InfographicdamonrunyonBelum ada peringkat
- Gene CloningDokumen17 halamanGene CloningOmaraye JoshuaBelum ada peringkat
- Human Genome Project EssayDokumen2 halamanHuman Genome Project EssayJanice CamposBelum ada peringkat
- Genetics of Cancer and Its Recent ProgressDokumen8 halamanGenetics of Cancer and Its Recent ProgressAaron OsorioBelum ada peringkat
- 1-HLA Gene May Predict If Cancer Immunotherapy Will Work - National Cancer InstituteDokumen7 halaman1-HLA Gene May Predict If Cancer Immunotherapy Will Work - National Cancer InstituteAhmed Abd El AzizBelum ada peringkat
- Genome Project Article 2Dokumen2 halamanGenome Project Article 2api-243328977Belum ada peringkat
- Top Ten Tips For Success at UniversityDokumen2 halamanTop Ten Tips For Success at UniversityFhai EscioBelum ada peringkat
- 655506v1 Full PDFDokumen36 halaman655506v1 Full PDFgarrobosBelum ada peringkat
- Annotated BibliographyDokumen6 halamanAnnotated Bibliographyapi-339995167Belum ada peringkat
- 02.16.2009 Push Is On To Tailor Cancer Care To Tumor GenesDokumen2 halaman02.16.2009 Push Is On To Tailor Cancer Care To Tumor GenesCTBHIBelum ada peringkat
- A Blood Test For CancerDokumen2 halamanA Blood Test For CancerHazel Joy Bragado TungpalanBelum ada peringkat
- 02 Fal The Importance of Human Based Models in Gene and Drug DiscoveryDokumen12 halaman02 Fal The Importance of Human Based Models in Gene and Drug DiscoveryluyawinBelum ada peringkat
- Ethical Issues of The Human Genome ProjectDokumen7 halamanEthical Issues of The Human Genome Projectsam_max_bladerunnerBelum ada peringkat
- Research Essay Final - Aidan HargettDokumen10 halamanResearch Essay Final - Aidan Hargettapi-559292279Belum ada peringkat
- Immune Discovery May Treat All Cancer - 2020jan20Dokumen5 halamanImmune Discovery May Treat All Cancer - 2020jan20ryan aniceteBelum ada peringkat
- Prentice Testimony HCBAR GO Fetal Tissue 12.13.18Dokumen10 halamanPrentice Testimony HCBAR GO Fetal Tissue 12.13.18Umang PrajapatiBelum ada peringkat
- Genomes Project Gives New Map of Genetic DiversityDokumen2 halamanGenomes Project Gives New Map of Genetic DiversityScheila Avila E SilvaBelum ada peringkat
- The Hallmarks of Cancer Review: 2000 by Cell PressDokumen14 halamanThe Hallmarks of Cancer Review: 2000 by Cell PressAashiq HussainBelum ada peringkat
- Note Jan 30 2014Dokumen8 halamanNote Jan 30 2014api-243334077Belum ada peringkat
- Hallmark of CancerDokumen14 halamanHallmark of CanceriinsabatiniBelum ada peringkat
- 'New Era' of Personalised Cancer Drugs, Say Doctors: DNA CluesDokumen2 halaman'New Era' of Personalised Cancer Drugs, Say Doctors: DNA CluesDhira OktaviantyBelum ada peringkat
- Dilemma 1Dokumen6 halamanDilemma 1api-296594594Belum ada peringkat
- Art Culo 1 Genetic Code Unlocking Future of MedicineDokumen3 halamanArt Culo 1 Genetic Code Unlocking Future of MedicineAnonymous 4XKNqMBelum ada peringkat
- Reclassify Cancers To Improve TreatmentDokumen3 halamanReclassify Cancers To Improve TreatmentJonathan EnglerBelum ada peringkat
- Dissertation On Stem CellsDokumen8 halamanDissertation On Stem CellsCollegePaperWritingHelpCanada100% (1)
- Stem Cell Transplant in Spinal CordDokumen3 halamanStem Cell Transplant in Spinal Cordberry12343Belum ada peringkat
- The Prognosis of Breast Cancer in MalesDokumen8 halamanThe Prognosis of Breast Cancer in Malesd17oBelum ada peringkat
- A Practical Guide to Human Cancer GeneticsDari EverandA Practical Guide to Human Cancer GeneticsPenilaian: 5 dari 5 bintang5/5 (1)
- Notocias Sobre OncogenesDokumen1 halamanNotocias Sobre Oncogenesalbertohz1Belum ada peringkat
- 2012-9..4-Whole-Exome Sequencing Combined With Functional Genomics Reveals Novel Candidate Driver Cancer Genes in Endometrial CancerDokumen10 halaman2012-9..4-Whole-Exome Sequencing Combined With Functional Genomics Reveals Novel Candidate Driver Cancer Genes in Endometrial Cancershidis1028Belum ada peringkat
- Functional Genomics in HCC 2005Dokumen6 halamanFunctional Genomics in HCC 2005johnyap11Belum ada peringkat
- Aiden Psczulkoski - Literature Review 1Dokumen11 halamanAiden Psczulkoski - Literature Review 1api-608831675Belum ada peringkat
- Final DraftDokumen12 halamanFinal Draftapi-609560720Belum ada peringkat
- Diagnostic and Prognostic Markers For Gastrointestinal Stromal Tumors in NorwayDokumen8 halamanDiagnostic and Prognostic Markers For Gastrointestinal Stromal Tumors in NorwayNengLukmanBelum ada peringkat
- Cea PPFDokumen37 halamanCea PPFmarcolius11Belum ada peringkat
- ACFrOgBmyygIVNBtJXCqo - lJdM9 r4K7 - XepillAovHk1ddWQSmfucV3m pyMEK92I2OM1IFoMS9dc1pz3CsvfNlpwKQW1OumyTeZb82T2t9IwNmvCfjWc933LCF HWDokumen3 halamanACFrOgBmyygIVNBtJXCqo - lJdM9 r4K7 - XepillAovHk1ddWQSmfucV3m pyMEK92I2OM1IFoMS9dc1pz3CsvfNlpwKQW1OumyTeZb82T2t9IwNmvCfjWc933LCF HWArrush AhujaBelum ada peringkat
- Brain Tumor Epidemiology: Consensus From The Brain Tumor Epidemiology ConsortiumDokumen16 halamanBrain Tumor Epidemiology: Consensus From The Brain Tumor Epidemiology ConsortiumscrugnlorBelum ada peringkat
- Let Aratu ReDokumen18 halamanLet Aratu ReRayon AutoriusBelum ada peringkat
- JNCI J Natl Cancer Inst 2010 Garber 448 50Dokumen3 halamanJNCI J Natl Cancer Inst 2010 Garber 448 50Brian SalvatoreBelum ada peringkat
- 9-Test Finds Residual Medulloblastoma in Kids After Treatment - National Cancer InstituteDokumen7 halaman9-Test Finds Residual Medulloblastoma in Kids After Treatment - National Cancer InstituteAhmed Abd El AzizBelum ada peringkat
- Why Most Gene Expression Signatures of Tumors Have Not Been Useful in The ClinicDokumen3 halamanWhy Most Gene Expression Signatures of Tumors Have Not Been Useful in The ClinicSELBelum ada peringkat
- Cancerization: Evidence and Clinical Implications A Genetic Explanation of Slaughter's Concept of FieldDokumen5 halamanCancerization: Evidence and Clinical Implications A Genetic Explanation of Slaughter's Concept of FieldEliana MuisBelum ada peringkat
- CNCR 21619Dokumen7 halamanCNCR 21619Syed Shah MuhammadBelum ada peringkat
- Story Source:: Fred Hutchinson Cancer Research CenterDokumen5 halamanStory Source:: Fred Hutchinson Cancer Research CenterCheska RamosBelum ada peringkat
- Epigenetic Plasticity Cooperates With Cell-Cell Interactions ToDokumen37 halamanEpigenetic Plasticity Cooperates With Cell-Cell Interactions ToIvan PerezBelum ada peringkat
- Cummings Et Al-2014-The Journal of PathologyDokumen9 halamanCummings Et Al-2014-The Journal of Pathologyalicia1990Belum ada peringkat
- Scientific Project Manager Liaison Writer in Detroit MI Resume Nandita BarnabasDokumen6 halamanScientific Project Manager Liaison Writer in Detroit MI Resume Nandita BarnabasNanditaBarnabasBelum ada peringkat
- Texto Sobre TumoresDokumen5 halamanTexto Sobre TumoresDaianaTolozaArceBelum ada peringkat
- TC Ga BrochureDokumen4 halamanTC Ga BrochureroelsmajorBelum ada peringkat
- Stem Cell Shifts: Current Biology Vol 19 No 6 R224Dokumen1 halamanStem Cell Shifts: Current Biology Vol 19 No 6 R224Candra BumiBelum ada peringkat
- Peroxiredoxin Systems Structures and FunctionsDokumen409 halamanPeroxiredoxin Systems Structures and FunctionsMariano PerezBelum ada peringkat
- Protein Dna RecognitionDokumen31 halamanProtein Dna RecognitionMariano PerezBelum ada peringkat
- Disulfide Bond Formation and Cysteine Exclusion inDokumen10 halamanDisulfide Bond Formation and Cysteine Exclusion inMariano PerezBelum ada peringkat
- A Pathway For Disulfide Bond Formation in VivoDokumen5 halamanA Pathway For Disulfide Bond Formation in VivoMariano PerezBelum ada peringkat
- CHOP Induces Death by Promoting Protein Synthesis and Oxidation in The Stressed Endoplasmic ReticulumDokumen13 halamanCHOP Induces Death by Promoting Protein Synthesis and Oxidation in The Stressed Endoplasmic ReticulumMariano PerezBelum ada peringkat
- The Ubiquitin-Proteasome Proteolytic System From Classical Biochemistry To Human DiseasesDokumen242 halamanThe Ubiquitin-Proteasome Proteolytic System From Classical Biochemistry To Human DiseasesMariano PerezBelum ada peringkat
- Gyrase Inhibitors Induce An Oxidative Damage Cellular Death Pathway in Escherichia ColiDokumen15 halamanGyrase Inhibitors Induce An Oxidative Damage Cellular Death Pathway in Escherichia ColiMariano PerezBelum ada peringkat
- Redox Sensing by Escherichia Coli Effects of DithiothreitolDokumen4 halamanRedox Sensing by Escherichia Coli Effects of DithiothreitolMariano PerezBelum ada peringkat
- Contribution of Calcium Influx in Mediating Glucose-Stimulated Oxygen Consumption in Pancreatic IsletsDokumen11 halamanContribution of Calcium Influx in Mediating Glucose-Stimulated Oxygen Consumption in Pancreatic IsletsMariano PerezBelum ada peringkat
- Generation of Rho0 Cells Utilizing A Mitochondrially Targeted Restriction Endonuclease and Comparative AnalysesDokumen10 halamanGeneration of Rho0 Cells Utilizing A Mitochondrially Targeted Restriction Endonuclease and Comparative AnalysesMariano PerezBelum ada peringkat
- Microplate-Based Kinetic Method For Assay of Mitochondrial NADH - and Succinate - Cytochrome C Reductase ActivitiesDokumen3 halamanMicroplate-Based Kinetic Method For Assay of Mitochondrial NADH - and Succinate - Cytochrome C Reductase ActivitiesMariano PerezBelum ada peringkat
- Targeting Metabolism To Diagnose and TreatDokumen4 halamanTargeting Metabolism To Diagnose and TreatMariano PerezBelum ada peringkat
- A Molecular Mechanism of Pyruvate Protection Against Cytotoxicity of Reactive Oxygen SpeciesDokumen11 halamanA Molecular Mechanism of Pyruvate Protection Against Cytotoxicity of Reactive Oxygen SpeciesMariano PerezBelum ada peringkat
- Mitochondria/Cytosol Fractionation Kit: Sufficient For Analysis of 50 SamplesDokumen8 halamanMitochondria/Cytosol Fractionation Kit: Sufficient For Analysis of 50 SamplesMariano PerezBelum ada peringkat
- Reactive Oxygen Species in Cancer Cells Live by The Sword Die by The Sword 2006 Cancer CellDokumen2 halamanReactive Oxygen Species in Cancer Cells Live by The Sword Die by The Sword 2006 Cancer CellMariano PerezBelum ada peringkat
- Selective Removal of Mitochondria Via MitophagyDokumen7 halamanSelective Removal of Mitochondria Via MitophagyMariano PerezBelum ada peringkat
- Phase II Trial of Temozolomide Plus O6-Benzylguanine in Adults With Recurrent, Temozolomide-Resistant Malignant GliomaDokumen6 halamanPhase II Trial of Temozolomide Plus O6-Benzylguanine in Adults With Recurrent, Temozolomide-Resistant Malignant GliomaMariano PerezBelum ada peringkat
- Mitochondria/Cytosol Fractionation Kit: Sufficient For Analysis of 50 SamplesDokumen8 halamanMitochondria/Cytosol Fractionation Kit: Sufficient For Analysis of 50 SamplesMariano PerezBelum ada peringkat
- The Rate of Oxygen Utilization by CellsDokumen32 halamanThe Rate of Oxygen Utilization by CellsMariano PerezBelum ada peringkat
- NAD+ and NADH in Cellular Functions and Cell DeathDokumen3 halamanNAD+ and NADH in Cellular Functions and Cell DeathMariano PerezBelum ada peringkat
- Selective Removal of Mitochondria Via MitophagyDokumen7 halamanSelective Removal of Mitochondria Via MitophagyMariano PerezBelum ada peringkat
- Protein Folding in The Bacterial PeriplasmDokumen7 halamanProtein Folding in The Bacterial PeriplasmMariano PerezBelum ada peringkat
- Oxidative Protein Folding Is DrivenDokumen11 halamanOxidative Protein Folding Is DrivenMariano PerezBelum ada peringkat
- Oxidative Protein Folding Is DrivenDokumen11 halamanOxidative Protein Folding Is DrivenMariano PerezBelum ada peringkat
- An Assessment of Prediction Algorithms For Nucleosome PositioningDokumen1 halamanAn Assessment of Prediction Algorithms For Nucleosome PositioningMariano PerezBelum ada peringkat
- An Assessment of Prediction Algorithms For Nucleosome PositioningDokumen1 halamanAn Assessment of Prediction Algorithms For Nucleosome PositioningMariano PerezBelum ada peringkat
- Ratiometric Calcium IndicatorsDokumen1 halamanRatiometric Calcium IndicatorsMariano PerezBelum ada peringkat
- Fluorescent Dyes Introduction Ratiometric AssaysDokumen2 halamanFluorescent Dyes Introduction Ratiometric AssaysMariano PerezBelum ada peringkat
- Ratiometric Calcium IndicatorsDokumen1 halamanRatiometric Calcium IndicatorsMariano PerezBelum ada peringkat
- Antibiotic Efficacy Is Linked To Bacterial Cellular Respiration.Dokumen8 halamanAntibiotic Efficacy Is Linked To Bacterial Cellular Respiration.Mariano PerezBelum ada peringkat
- American Architecture Began With Frank Lloyd WrightDokumen8 halamanAmerican Architecture Began With Frank Lloyd WrightAdheith South NgalamBelum ada peringkat
- MSDS-Irganox 1010Dokumen10 halamanMSDS-Irganox 1010Seshagiri KalyanasundaramBelum ada peringkat
- Tdetails: Examination Question PaperDokumen14 halamanTdetails: Examination Question PaperPriyanshu GehlotBelum ada peringkat
- Buy Affordable Residential Flats in Zirakpur at Escon ArenaDokumen26 halamanBuy Affordable Residential Flats in Zirakpur at Escon ArenaEscon ArenaBelum ada peringkat
- Steam Inhalation Definition:-: A. by Jug MethodDokumen4 halamanSteam Inhalation Definition:-: A. by Jug Methodss100% (1)
- NHP Original 1985 PDFDokumen4 halamanNHP Original 1985 PDFAndrei IordanBelum ada peringkat
- List of Shelters in The Lower Mainland, June 2007Dokumen2 halamanList of Shelters in The Lower Mainland, June 2007Union Gospel Mission100% (1)
- Radiation PhysicsDokumen307 halamanRadiation PhysicsHarley Alejo MBelum ada peringkat
- Nove Farmakološke Strategije U Lečenju Nesitnoćelijskog Karcinoma PlućaDokumen8 halamanNove Farmakološke Strategije U Lečenju Nesitnoćelijskog Karcinoma PlućaMomcilo Moca DjurovicBelum ada peringkat
- GHP557 - Fundamentals of Global Health - Syllabus - 28aug2021 - FinalDokumen32 halamanGHP557 - Fundamentals of Global Health - Syllabus - 28aug2021 - Finalps.harsha2004Belum ada peringkat
- IP Environmental Chem PDFDokumen15 halamanIP Environmental Chem PDFAshish Kumar JhaBelum ada peringkat
- INGREDIENTSDokumen17 halamanINGREDIENTSPaulo BacayBelum ada peringkat
- MCN Questionnaire DraftDokumen8 halamanMCN Questionnaire DraftJenny AjocBelum ada peringkat
- Intjmi v8n2p84 FaDokumen7 halamanIntjmi v8n2p84 FaRein KarnasiBelum ada peringkat
- FAQ Mid Day MealsDokumen7 halamanFAQ Mid Day Mealsvikramhegde87Belum ada peringkat
- DizzinessDokumen65 halamanDizzinessעידית בנימיןBelum ada peringkat
- Nov Art IsDokumen6 halamanNov Art IsSaran KuttyBelum ada peringkat
- Impact of Digital Media On Sleep Pattern Disturbance in Medical and Nursing StudentsDokumen10 halamanImpact of Digital Media On Sleep Pattern Disturbance in Medical and Nursing StudentsValarmathiBelum ada peringkat
- Takaful MaybankDokumen4 halamanTakaful MaybankSHABelum ada peringkat