Clinical Presentation of The Idiopathic Inflammatory Myopathies Clinics Clinics 2002
Diunggah oleh
Alexis May UcDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Clinical Presentation of The Idiopathic Inflammatory Myopathies Clinics Clinics 2002
Diunggah oleh
Alexis May UcHak Cipta:
Format Tersedia
Rheum Dis Clin N Am 28 (2002) 823 832
Clinical presentation of the idiopathic inflammatory myopathies
Yusuf Yazici, MDa,b,*, Lawrence J. Kagen, MDa,c
a
Hospital for Special Surgery, Weill Medical College of Cornell University, New York, NY, USA b Long Island College University, Brooklyn, NY, USA c New York Presbyterian Hospital, New York, NY, USA
The idiopathic inflammatory myopathies (IIM) are a group of disorders that are characterized by proximal muscle weakness and nonsuppurative inflammation of skeletal muscle, often accompanied by extramuscular manifestations. Subgroups of IIM with different presentations include dermatomyositis (DM), polymyositis (PM), inclusion body myositis (IBM), cancer-associated myositis, overlap syndromes, amyopathic DM, and the antisynthetase syndrome. The frequencies of signs and symptoms among clinical groups show some statistically significant differences and are particularly important in inclusion body myositis, which can exhibit more atrophy, distal, and peripheral muscle weakness [1]. Patients with IIM may present in acute, subacute, or insidious ways, and with a variety of nonspecific symptoms including fatigue, malaise, weight loss, myalgias, or arthralgias that may mimic other disorders and result in unfortunate delays in diagnosis.
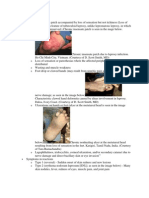
Organ systems involved Cutaneous involvement Rash The cutaneous manifestations of DM may be pathognomonic for, characteristic of, or simply compatible with, DM (Box 1) [2]. The most common rash, Gottrons papules, is a symmetric, palpable, heaped up appearing, erythematous eruption of the skin overlying the extensor surfaces of the metacarpophalangeal and interphalangeal joints of the fingers (Fig. 1). Slight scaling and,
* Corresponding author. Hospital for Special Surgery, Weill Medical College of Cornell University, 535 East 70th Street, New York, NY 10021. E-mail address: yaziciy@hss.edu (Y. Yazici). 0889-857X/02/$ see front matter D 2002, Elsevier Science (USA). All rights reserved. PII: S 0 8 8 9 - 8 5 7 X ( 0 2 ) 0 0 0 2 3 - 6
824
Y. Yazici, L.J. Kagen / Rheum Dis Clin N Am 28 (2002) 823832
Box 1. Signs and symptoms of inflammatory myopathies 1. Dermatologic (DM) A. Rash 1) Periorbital swelling 2) Heliotrope suffusion 3) Gottrons papules 4) V-sign 5) Shawl-sign B. Periungual telangiectasias C. Calcinosis 1) Usually superficial, rarely deep 2) May be complicated by infection 2. Muscle (DM, PM, inclusion body myositis [IBM]) A. Acute/subacute proximal muscle weakness B. Usually symmetric but may be asymmetric in IBM C. Myalgia early in disease course 3. Pulmonary (DM, PM) A. Fibrosing alveolitis B. Aspiration C. Weakness of muscles of respiration D. Dyspnea 4. Gastrointestinal ([GI], DM) A. Esophageal dysfunction B. Gastroparesis C. Intestinal dysfunction and inflammation 5. Cardiac (PM, DM) A. Subclinical electrocardiographic findings B. Rhythm disturbances C. Congestive heart failure 6. Joints (DM, PM, antisynthetase syndrome) A. Arthralgia B. Small joint arthritis 1) Symmetric 2) Nondeforming
Y. Yazici, L.J. Kagen / Rheum Dis Clin N Am 28 (2002) 823832
825
Fig. 1. Gottrons papules. Rash of DM with Gottrons papules over bony prominences (proximal interphalangeal [PIP] and metacarpophalangeal [MCP] joint areas).
rarely, a thick psoriasiform scale occur and telangiectasia is commonly found within the lesions. Gottrons papules are considered pathognomonic for DM. The heliotrope rash of DM is violaceous to dusky erythematous, sometimes with edema, involving the periorbital area and often the upper eyelids, in a symmetrical fashion. This rash may be slight and appear as only a mild discoloration along the eyelid margins. Its presence is highly suggestive of DM; it is rarely seen in patients with systemic lupus erythematosus (SLE) or scleroderma. Although it is considered a characteristic skin manifestation of DM, it is not present in all cases [3]. Other skin findings include a macular erythema over the lower neck and upper chest in a V distribution anteriorly (V-sign), often accompanied by a more diffuse anterior chest rash (Fig. 2), or in a shawl-like distribution posteriorly and over the upper, outer, proximal extremities (shawl-sign) (Fig. 3). The scalp and the back of the neck below the hairline may be involved. Scalp involvement is common and presents as an erythematous to violaceous psoriasiform dermatitis [2]. Clinical distinction from psoriasis or seborrheic dermatitis may be difficult and sometimes histopathologic studies may be helpful in this regard [3]. In addition to these features, severe erythema of the palms, scaling scalp plaques, and hyperkeratosis of the palmar and lateral aspects of the fingers (mechanics hands) are commonly seen. Periungual abnormalities including telangiectasias and cuticular overgrowth, and irregular, indurated plaques over the fingers with mucin accumulation in the dermis may also occur in patients with DM. Less frequently, a papular, or even vesicular rash over flat surfaces has been observed. Subcutaneous panniculitis, manifested as induration and erythematous plaques over the torso and extremities may occur [4]. Erosive lesions and exfoliative erythroderma may also be observed.
826
Y. Yazici, L.J. Kagen / Rheum Dis Clin N Am 28 (2002) 823832
Fig. 2. V-sign and anterior chest rash. Rash of DM evident over the anterior thorax as well as over the upper outer arms.
Although the rash is not usually a severe medical problem, dehiscence of the rash can occur and may be severe. Open lesions of this type on the trunk usually connote a poor prognostic sign. Histologically, skin biopsies have demonstrated hyperkeratosis, focal epidermal atrophy, liquefaction of the basal level, mucin deposition, dermal edema, and a mononuclear cell infiltrate composed of CD4+ T cells and macrophages proximal to small vessels in the dermis. At the dermo epidermal junction and in
Fig. 3. Shawl-sign. Rash of DM over the posterior thorax.
Y. Yazici, L.J. Kagen / Rheum Dis Clin N Am 28 (2002) 823832
827
dermal microvessels, IgG and complement components are found. Direct immunofluorescence reveals a band of immunoglobulin and complement at the basement membrane zone [5]. These histologic findings are similar to those of subacute cutaneous lupus erythematosus (SCLE). The skin lesions of DM are photoaggravated in some patients [6]. Other aspects of IIM, however, including muscle disease, may also flare after sunlight exposure. The rash of DM often appears before signs of myopathy become evident. Dermatologic involvement may be confused with that of SLE or of other papulosquamous skin disorders and histologically cannot be differentiated from SLE skin manifestations [3]. Calcinosis Skin calcinosis is a vexing and often disabling complication, most commonly seen in children or young adults, but also encountered in adult patients, as well. Hard, yellow-white nodules can arise over bony prominences and often in areas of repeated trauma. These deposits of calcium may erupt onto the skin surface and be the site of secondary infections. This may be extremely troublesome, particularly at the olecranon bursa area. The infections are often caused by Staphylococcus aureus. The deposits, generally several millimeters in diameter, can also be extremely painful, particularly at the fingertips. Other patterns of calcification that have been noted, include: (1) deep deposits with linear masses, as well as investments of the fascia of the musculature; and (2) reticular subcutaneous deposits [11]. Muscle involvement Most patients present with acute or subacute onset of proximal muscle weakness that is usually symmetric. Initially, myalgia, which is experienced as soreness or a Charley horse sensation in the proximal musculature of the upper and lower extremities, may be present. Myalgia is generally not a limiting feature of these disorders and often disappears as the illness becomes more chronic [7]. Muscular weakness represents the major source of disability faced by patients with inflammatory myopathies. There is difficulty in raising the head when supine, lifting, carrying, climbing steps or street curbs, and dressing. Many patients find they need to use the handrail of stairways as a way of pulling themselves up, as well as to holding on to other supports to prevent a fall when going down. Although strength of dorsiflexion of the ankle may be preserved, loss of hip flexion power may result in tripping over curbs or stumbling on uneven terrain. Proximal muscles are generally affected to a greater degree; however, with chronicity, increasing severity, and in the case of patients with inclusion body myositis, distal musculature is also involved. Not all skeletal muscles are equally involved; facial musculature, innervated by the cranial nerves, is generally spared. Neck extensors, although involved, are less affected than flexors. Flexors of the knee are less involved than extensors. Weakness also involves the musculature of the torso, making arising from the supine position difficult. In order to arise from
828
Y. Yazici, L.J. Kagen / Rheum Dis Clin N Am 28 (2002) 823832
bed the patient may need to roll to the side, and dangle the legs over the edge as a counterweight while pushing with the arms. Weakness of proximal musculature of the lower extremities, impairing the ability to arise from a seat, severely limits independent function. Histologically, muscle biopsies in patients with DM demonstrate a mononuclear cell infiltrate, emanating from blood vessels and extending into the myofibers. Myofiber necrosis and regeneration are seen, and in chronic cases, replacement of parts of the fascicle with adipose tissue and collagen occurs. The inflammatory infiltrate is composed mainly of B cells and CD4+ lymphocytes in perivascular areas. CD8+ lymphocytes may also be present in the endomysium. Macrophages are encountered in areas of myofiber necrosis, along with polymorphonuclear leukocytes. Reduction in the number of capillaries and microvessels, and perifascicular atrophy are evidence of the vasculopathy seen in this disorder. As a part of the inflammatory process, MHC determinants of Class I and Class II can be demonstrated on myofibers. Adhesion molecules, ICAM-1 and VCAM-1 are upregulated, as are cytokines TNF a and IL-1 along with other cytokines and monocyte attractant proteins that serve to attract leukocytes to the inflammation site [8]. PM is characterized by the same pattern of muscle weakness as DM but without the rash. Histologic features of muscle are similar with the exception of a preponderance of CD8+ lymphocytes in the endomysium on immunostaining. In patients with PM the risk of associated interstitial pulmonary disease seems to be higher, but the risk of malignancy is much lower than in patients with DM. Pulmonary involvement Respiratory complaints can be prominent and a serious feature in patients with inflammatory muscle disease. Interstitial lung disease secondary to fibrosing alveolitis is seen in patients with DM or PM and is associated with esophageal involvement as well as with the presence of antibodies to aminoacyl-transfer RNA synthetase [9]. In some patients, chest radiographs or pulmonary function testing can indicate the presence of intrinsic lung disease in the absence of clinically apparent signs or symptoms. In others, exertional dyspnea is an indolently progressive problem. Finally, some patients can present acutely with aggressive diffuse lung disease, with nonproductive cough, fever, and severe, rapidly progressive dyspnea. Exertional dyspnea simulating lung disease can also result from weakness of the diaphragm and intercostal muscles. Additionally, pharyngeal and esophageal dysfunction may lead to chronic aspiration with concomitant pulmonary involvement. Pulmonary hypertension is a rare but very worrisome finding in some patients. It is usually associated with pulmonary fibrosis, and predicts a poor prognosis. GI involvement The most common GI symptom is dysphagia, caused by the weakness of the tongue, pharynx, or esophagus, and disordered esophageal motility [10]. Esoph-
Y. Yazici, L.J. Kagen / Rheum Dis Clin N Am 28 (2002) 823832
829
ageal dysfunction occurs in 15% to 50% of patients and can be associated with pulmonary involvement as the result of eructation of solids and liquids with concomitant aspiration. Symptoms of reflux esophagitis, abdominal pain, and constipation alternating with diarrhea, may result from GI tract inflammation. Delay of gastric emptying has been documented in apparently asymptomatic patients and severe gastroparesis, although rare, may result in postprandial nausea, vomiting, and malaise and impair adequate nutrition. Vasculitis of the GI system is a rare event that may be complicated by intestinal bleeding. Cardiac involvement Cardiac involvement requiring therapy is uncommon because most involvement is subclinical. When cardiomyopathy from myocarditis causes symptoms, however, the outcome is very poor. Rhythm disturbances, conduction defects, less commonly congestive heart failure, and rarely, pericarditis, have been observed. The outcome of these patients is much worse when cardiac manifestations occur in conjunction with pulmonary disease [3]. Joint involvement DM and PM are systemic disorders and arthralgia can be present in one fourth of patients. It is generally noted early in the disease and responds to therapy of the underlying muscle disease. It commonly involves hands, wrists, feet, and ankles symmetrically and is nondeforming. Morning stiffness is common.
Clinical manifestations in selected subgroups Cancer-associated myositis The exact nature of the relationship between malignancy and myositis has created continued controversy; however, most authorities agree that there is an increased incidence of malignancy in myositis patients [12]. The association is much stronger for DM than PM, especially in the first 5 years after diagnosis of myositis. The malignancies seen in patients with DM and PM reflect those found in an age-matched population, except for a possible preponderance of ovarian cancer. Most experts recommend that patients with DM have an age-and riskspecific examination for occult malignancy with further evaluation of any suggestive symptoms or abnormal findings. Older age at onset, and male gender may be independent predictive factors; interstitial lung disease may be protective for malignancy in myositis patients [15]. Amyopathic DM Some patients with characteristic cutaneous findings of DM have no overt clinical evidence of muscle disease. This syndrome, also known as DM sine
830
Y. Yazici, L.J. Kagen / Rheum Dis Clin N Am 28 (2002) 823832
myositis, makes up about 10% of all patients with rashes consistent with DM. Overt muscle weakness may not be demonstrated; however, fatigue may be a complaint and extensive testing can reveal subtle, subclinical myopathic abnormalities [14]. Many of these patients go on to develop obvious myopathy after a delay of several months, and rarely, longer. Antisynthetase syndrome This entity occurs most often among women in their middle years and is characterized by inflammatory myopathy associated with low grade fevers, interstitial pulmonary infiltration, Raynauds syndrome, mechanics hands, and arthritis of the small joints of the hands. The hallmark of this disorder is the presence of antibodies to aminoacyl-tRNA synthetases in the serum of affected individuals. In some patients, the associated findings of Raynauds syndrome and pulmonary disease may precede or even overshadow the concurrent presence of myopathy. IBM IBM is an illness much like chronic PM. It usually occurs in older patients, often men, and patients may have more distal weakness, particularly of the muscle of the forearm and hand. The disease onset is frequently insidious and a considerable delay of months to years may elapse before recognition of the disease occurs. In many patients, weakness, initially ascribed to the effects of age, may delay diagnosis leading to irreparable myofiber loss, with atrophy and disability prior to presentation. Most patients wirth IBM patients do not have extramuscular manifestations, but dysphagia is not uncommon. Histologically, the inflammatory infiltrate is similar to that of PM with the presence of cytotoxic CD8+ lymphocytes invading the endomysium. Additionally, rimmed or beaded vacuoles, containing eosinophilic inclusions, are present in the cytoplasm. Eosinophilic inclusions can also be found in the nucleus. Immunohistology revealed the presence of b amyloid, ubiquitin, phosphorylated t protein, B crystalline, and transglutaminases as well as other components within inclusions [13]. The role of these materials in the pathogenesis of inclusion body myositis remains to be fully elucidated.
Summary The hallmark of the inflammatory myopathies is muscle weakness. Although this feature can lead to significant disability and impairment of activities of daily living, its initial presentation may not be recognized early. Older individuals, in particular, may feel that the changes caused by myositis reflect the effects of aging rather than those of a disease process, and diagnosis, therefore, may be delayed. This factor has negative impact on the response to therapy. Inclusion
Y. Yazici, L.J. Kagen / Rheum Dis Clin N Am 28 (2002) 823832
831
body myositis, with its insidious onset in older people, and laboratory findings which may not be markedly abnormal, presents a diagnostic challenge. DM, with its characteristic symptomatic rash, is generally brought to medical attention more quickly. Another area of diagnostic concern occurs when associated organ involvement precedes myopathy. This has been observed, for example, with interstitial lung disease, and again represents a challenge to physicians. In this connection, the antisynthetase syndrome presenting with fevers, Raynauds features, arthritis, or pulmonary involvement may not initially be recognized as a manifestation of inflammatory muscle disease. Each subgroup of IIM may present with a variety of extramuscular features that can complicate diagnosis and alter therapy and prognosis. This is particularly true for the pulmonary, GI, and cardiac manifestations and when cancer is associated with myositis. For these reasons, such features of IIM should be carefully evaluated, treated, and monitored over the course of the illness; in some cases these may play a greater role in determining the outcome of patients with IIM than the muscle involvement itself. It is hoped that in the future increased familiarity with the manifestations of the inflammatory myopathies, together with a better understanding of the underlying pathogenesis, will lead to more rapid diagnosis and more effective treatments.
References
[1] Love LA, Leff RL, Fraser DD, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991;70:360 74. [2] Spiera R, Kagen L. Extramuscular manifestations in idiopathic inflammatory myopathies. Curr Opin Rheumatol 1998;10:556 61. [3] Callen JP. Dermatomyositis. Lancet 2000;335:53 7. [4] Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. B J Dermatol 1993; 128:451 3. [5] Vaughan Jones SA, Black MM. The value of direct immunofluorescence as a diagnostic aid in dermatomyositis a study of 35 cases. Clin Exp Dermatol 1997;22:77 81. [6] Cheong WK, Hughes GRV, Norris PG, et al. Cutanoeus photosensitivity in dermatomyositis. Br J Dermatol 1994;131:205 8. [7] Kagen LJ. History, physical examination and laboratory tests in the evaluation of myopathy. In: Diseases of skeletal muscle. Wortmann RL, editor. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 255 66. [8] Hausmann G, Mascaro Jr. JM, Herrero C, et al. Cell adhesion molecule expression in cutaneous lesions of dermatomyositis. Acta Derm Nenereol 1996;76:222 5. [9] Hirakata M, Nagai S. Interstitial lung disease in polymyositis and dermatomyositis. Curr Opin Rheumatol 2000;12:501 8. [10] Eshraghi N, Farahmand M, Maerz LL, et al. Adult-onset dermatomyositis with severe gastrointestinal manifestations: case report and review of the literature. Surgery 1998;123:356 8. [11] Blane CE, White SJ, Braunstein EM, et al. Patterns of calcification in childhood dermatomyositis. Am J Roentgenol 1984;142:397 400. [12] Yazici Y, Kagen LJ. The association of malignancy with myositis. Curr Opin Rheumatol 2000; 12:498 500.
832
Y. Yazici, L.J. Kagen / Rheum Dis Clin N Am 28 (2002) 823832
[13] Dalakas MC. The molecular and cellular pathology of inflammatory muscle diseases. Curr Opin Pharmacol 2001;1:300 6. [14] Olsen NJ, Wortmann RL. Inflammatory and metabolic diseases of muscle. In: Klippel JH, editor. Primer on the rheumatic disease. 2nd edition. Atlanta: Arthritis Foundation; 1997. p. 276 82. [15] Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case control study. Br J Dermatol 2001;144:825 31.
Anda mungkin juga menyukai
- Clinical Manifestations of Dermatomyositis and Polymyositis in AdultsDokumen18 halamanClinical Manifestations of Dermatomyositis and Polymyositis in AdultsSyl NMBelum ada peringkat
- Polymyositis and Dermatomyositis: SeminarDokumen12 halamanPolymyositis and Dermatomyositis: SeminarMisaeldpdBelum ada peringkat
- PolymyositisDokumen5 halamanPolymyositisdeea03Belum ada peringkat
- Transverse Myelitis, A Simple Guide To The Condition, Treatment And Related DiseasesDari EverandTransverse Myelitis, A Simple Guide To The Condition, Treatment And Related DiseasesPenilaian: 5 dari 5 bintang5/5 (1)
- Scleroderma PDFDokumen9 halamanScleroderma PDFAustine OsaweBelum ada peringkat
- Clinical Manifestations of Dermatomyositis and Polymyositis in Adults - UpToDate PDFDokumen35 halamanClinical Manifestations of Dermatomyositis and Polymyositis in Adults - UpToDate PDFGiiszs AlvarezBelum ada peringkat
- DermatomyositisDokumen4 halamanDermatomyositisLakshya J BasumataryBelum ada peringkat
- Dermatomyositis: By: Mariam Qais Hatef - 5 Stage Supervised By: DR - Sabeeh AlmashhadaniDokumen6 halamanDermatomyositis: By: Mariam Qais Hatef - 5 Stage Supervised By: DR - Sabeeh AlmashhadaniMariam QaisBelum ada peringkat
- 11 194scleroderma A Case ReportDokumen3 halaman11 194scleroderma A Case ReportAfiatul MukarromahBelum ada peringkat
- Dermatomyositis: Constantin Haber Pgyi - SurgeryDokumen32 halamanDermatomyositis: Constantin Haber Pgyi - SurgeryconstantinhaberBelum ada peringkat
- Transverse Myelitis: Differential DiagnosisDokumen14 halamanTransverse Myelitis: Differential DiagnosissekiannBelum ada peringkat
- Dermatomyositis: Zhang Nian NianDokumen41 halamanDermatomyositis: Zhang Nian NianMohsin Tanmoy100% (1)
- Pathology QuizDokumen4 halamanPathology QuizGamal ShohdyBelum ada peringkat
- Differential Diagnosis of Transverse MielitisDokumen14 halamanDifferential Diagnosis of Transverse MielitisLuminița VelceanBelum ada peringkat
- An Overview of DermatomyositisDokumen6 halamanAn Overview of DermatomyositisInternational Journal of Innovative Science and Research TechnologyBelum ada peringkat
- Lamb 2017Dokumen6 halamanLamb 2017Carolina González RiveraBelum ada peringkat
- Eritema Elevatum DiutinunDokumen8 halamanEritema Elevatum DiutinunVar AndaBelum ada peringkat
- 07 BMDokumen9 halaman07 BMMajid KhanBelum ada peringkat
- Localized Scleroderma: Clinical Spectrum and Therapeutic UpdateDokumen12 halamanLocalized Scleroderma: Clinical Spectrum and Therapeutic UpdateMati AjaBelum ada peringkat
- Scleroderma 1Dokumen2 halamanScleroderma 1Alaa AhmedBelum ada peringkat
- Infections of The Bones, Joints, and Soft Tissues Musculoskeletal KeyDokumen1 halamanInfections of The Bones, Joints, and Soft Tissues Musculoskeletal KeyDiana Alejandra González SánchezBelum ada peringkat
- Referensi NeurologiDokumen15 halamanReferensi NeurologiFathma AisyahBelum ada peringkat
- Septic ArthritisDokumen38 halamanSeptic ArthritisayeshasadiqamukhtarBelum ada peringkat
- SclerodermaDokumen13 halamanSclerodermanevelle4667Belum ada peringkat
- Eosinophilic Fasciitis - UpToDateDokumen15 halamanEosinophilic Fasciitis - UpToDateFelicia MustateaBelum ada peringkat
- Paper CTSDokumen27 halamanPaper CTSnawabBelum ada peringkat
- CellulitisDokumen8 halamanCellulitisTricia Llorin100% (1)
- MainDokumen11 halamanMainMuhammad Nur Ardhi LahabuBelum ada peringkat
- Selulitis DGN Ulkus VarikosumDokumen1 halamanSelulitis DGN Ulkus VarikosumdeaBelum ada peringkat
- Week 6 SpondyloarthropathiesDokumen124 halamanWeek 6 SpondyloarthropathiesJoeBelum ada peringkat
- DR G Avinash Rao Fellow in Hand and Microsurgery: ArthrogryposisDokumen74 halamanDR G Avinash Rao Fellow in Hand and Microsurgery: Arthrogryposisavinashrao39100% (1)
- Seminar On Spread of Oral Infections: Presented By:-Dr. Ipsha DhaliDokumen97 halamanSeminar On Spread of Oral Infections: Presented By:-Dr. Ipsha DhaliDua'a Ma'anBelum ada peringkat
- Myositis Disorders: Polymyositis, Inclusion Body Myositis, and DermatomyositisDokumen3 halamanMyositis Disorders: Polymyositis, Inclusion Body Myositis, and DermatomyositisLaura ChandraBelum ada peringkat
- Special Studies of Discoid Lupus ErythematosusDokumen12 halamanSpecial Studies of Discoid Lupus ErythematosusDuong Ngoc Phuong UyenBelum ada peringkat
- Plain X Rays of BonesDokumen17 halamanPlain X Rays of BonesEmmanuella NoelBelum ada peringkat
- Autoimmune Disorders Part I:: Rheumatoid Arthritis, Osteoarthritis, & Gouty ArthritisDokumen220 halamanAutoimmune Disorders Part I:: Rheumatoid Arthritis, Osteoarthritis, & Gouty ArthritisMaya SwariBelum ada peringkat
- CellulitisDokumen3 halamanCellulitisAllen MallariBelum ada peringkat
- Iseasesof ONE: Section I Pseudo-DiseasesDokumen16 halamanIseasesof ONE: Section I Pseudo-Diseasesseunnuga93Belum ada peringkat
- Sclerosis Vs FibrosisDokumen4 halamanSclerosis Vs FibrosisAndu1991100% (1)
- 1908 Juvenile Dermatomyositis - Advances in Clinical Presentation, Myositis Specifc Antibodies and TreatmentDokumen13 halaman1908 Juvenile Dermatomyositis - Advances in Clinical Presentation, Myositis Specifc Antibodies and TreatmentFlorian LamblinBelum ada peringkat
- Skin Manifestations of Common DiseasesDokumen5 halamanSkin Manifestations of Common DiseasesAya AmerBelum ada peringkat
- Up To Date. Closed Spinal DysraphismDokumen30 halamanUp To Date. Closed Spinal DysraphismGuardito PequeñoBelum ada peringkat
- Therapeutics in Practice: Disorders of The Sclera and EpiscleraDokumen7 halamanTherapeutics in Practice: Disorders of The Sclera and EpiscleraAchmad Deddy FatoniBelum ada peringkat
- Inflammatory Myopathies - An Update For NeurologistsDokumen11 halamanInflammatory Myopathies - An Update For Neurologistsrafael rocha novaesBelum ada peringkat
- Infectious Diseases - 05Dokumen27 halamanInfectious Diseases - 05Arthur YanezBelum ada peringkat
- Dekubitus MateriDokumen11 halamanDekubitus MateriAwalia riska nur - DIII GIZIBelum ada peringkat
- Scleroderma - Oral Manifestations and Treatment ChallengesDokumen5 halamanScleroderma - Oral Manifestations and Treatment ChallengesLujainBelum ada peringkat
- A Stroke or Cerebrovascular Accident and CellulitisDokumen3 halamanA Stroke or Cerebrovascular Accident and CellulitisCjhay Polintan ParasoBelum ada peringkat
- Pressure Ulcers - Surgery I March 2019Dokumen63 halamanPressure Ulcers - Surgery I March 2019daniyfondoBelum ada peringkat
- Polymyositis DermatomyositisDokumen2 halamanPolymyositis DermatomyositisTay Woo ChiaoBelum ada peringkat
- Red Flags of SclerodermaDokumen4 halamanRed Flags of SclerodermanonniBelum ada peringkat
- Multiple Dermatofibromas in An Adult FemaleDokumen3 halamanMultiple Dermatofibromas in An Adult FemaleDeba P SarmaBelum ada peringkat
- LeprosyDokumen6 halamanLeprosyMaricel Y. LizadaBelum ada peringkat
- Wolff 2001Dokumen10 halamanWolff 2001MARIELLA AMANDA RAMOS VALDIVIEZOBelum ada peringkat
- Miopatías Inflamatorias Dr. José HuamánDokumen20 halamanMiopatías Inflamatorias Dr. José HuamánEstrella CutiBelum ada peringkat
- Ioana Rosca - SclerodermaDokumen6 halamanIoana Rosca - SclerodermaMinnossBelum ada peringkat
- Intergrowth Ga Calculator 1.1Dokumen5 halamanIntergrowth Ga Calculator 1.1Alexis May UcBelum ada peringkat
- Management of The Critically Ill Patient With Severe Acute Pancreatitis SCCM ATS 2004Dokumen13 halamanManagement of The Critically Ill Patient With Severe Acute Pancreatitis SCCM ATS 2004Alexis May UcBelum ada peringkat
- Nutricion Parenteral en PediatriaDokumen6 halamanNutricion Parenteral en PediatriaAlexis May UcBelum ada peringkat
- Thiazolidinediones For The Treatment of Type 2 Diabetes EJIM 2007Dokumen8 halamanThiazolidinediones For The Treatment of Type 2 Diabetes EJIM 2007Alexis May UcBelum ada peringkat
- Infective Endocarditis Lancet SeminarDokumen11 halamanInfective Endocarditis Lancet SeminarAlexis May UcBelum ada peringkat
- Eponym Guillain Barre Lancet 2004Dokumen3 halamanEponym Guillain Barre Lancet 2004Alexis May UcBelum ada peringkat
- Novel Therapies For Chronic Leukaemia Blood Rev 2004Dokumen12 halamanNovel Therapies For Chronic Leukaemia Blood Rev 2004Alexis May UcBelum ada peringkat
- Acute Lymphoblastic Leukaemia Lancet 2008Dokumen14 halamanAcute Lymphoblastic Leukaemia Lancet 2008Alexis May UcBelum ada peringkat
- Acute Myocardial Infarction Lancet 2003Dokumen12 halamanAcute Myocardial Infarction Lancet 2003Alexis May UcBelum ada peringkat
- Does Dopamine Administration in Shock Influences OutcomeDokumen9 halamanDoes Dopamine Administration in Shock Influences OutcomeAlexis May UcBelum ada peringkat
- Coccidioidomycosis GuidelinesDokumen7 halamanCoccidioidomycosis GuidelinesAndreas IoannouBelum ada peringkat
- Tuberous Sclerosis Lancet 2008Dokumen12 halamanTuberous Sclerosis Lancet 2008Alexis May UcBelum ada peringkat
- Whipples Disease Lancet 2003Dokumen8 halamanWhipples Disease Lancet 2003Alexis May UcBelum ada peringkat
- Advances in Burn Critical CareDokumen6 halamanAdvances in Burn Critical CareaccedeperuBelum ada peringkat
- Abdominal Compartment Syndrome PMJ 2008Dokumen7 halamanAbdominal Compartment Syndrome PMJ 2008Alexis May UcBelum ada peringkat
- A Medical Center Is Not A Hospital Editorial CCJM 2008Dokumen2 halamanA Medical Center Is Not A Hospital Editorial CCJM 2008Alexis May UcBelum ada peringkat
- Accuracy of The Physical Examination in Evaluating Pleural EffusionDokumen7 halamanAccuracy of The Physical Examination in Evaluating Pleural EffusionTeresa MontesBelum ada peringkat
- Ankylosing Spondylitis NEJM 2008Dokumen1 halamanAnkylosing Spondylitis NEJM 2008Alexis May UcBelum ada peringkat
- Abdominal Aortic Aneurysm Lancet 2005Dokumen13 halamanAbdominal Aortic Aneurysm Lancet 2005Alexis May UcBelum ada peringkat
- 1-8 Physiologic Consequences of Acute Renal 2005Dokumen10 halaman1-8 Physiologic Consequences of Acute Renal 2005Alexis May UcBelum ada peringkat
- Ovario PDokumen6 halamanOvario PAlexis May UcBelum ada peringkat
- Inventory Management NestleDokumen40 halamanInventory Management Nestlesrinivas2help883675% (4)
- Contractor Safety Management ProcessDokumen14 halamanContractor Safety Management Processsrkam100% (2)
- P CLS14 Powertec Compact Leg Sled ManualDokumen15 halamanP CLS14 Powertec Compact Leg Sled ManualElizabeth GuzmanBelum ada peringkat
- Evaluation Tool (Competency Based) - NonescostDokumen35 halamanEvaluation Tool (Competency Based) - NonescostFroilan GuilleranBelum ada peringkat
- EMResource - NEDOCS Definitions and Tracking LogDokumen2 halamanEMResource - NEDOCS Definitions and Tracking LogkingdonBelum ada peringkat
- ASSIGNMENT AND ACTIVITIES in Contemp FinalDokumen14 halamanASSIGNMENT AND ACTIVITIES in Contemp FinalalmahairaBelum ada peringkat
- AntiConvulsants Drugs in Brief PDFDokumen28 halamanAntiConvulsants Drugs in Brief PDFSunilBelum ada peringkat
- Diverifikasi DPJP AgustusDokumen6 halamanDiverifikasi DPJP AgustusKadek candraBelum ada peringkat
- SMK Sinar Bintang, Segambut Kuala Lumpur Yearly Plan Science Form 3Dokumen16 halamanSMK Sinar Bintang, Segambut Kuala Lumpur Yearly Plan Science Form 3Azie HarunBelum ada peringkat
- Cancer - 2011 - Hajdu - A Note From History Landmarks in History of Cancer Part 2Dokumen10 halamanCancer - 2011 - Hajdu - A Note From History Landmarks in History of Cancer Part 2Pilar AufrastoBelum ada peringkat
- Barangay Anti-Drug Abuse Council (Badac) Training: Activity DesignDokumen3 halamanBarangay Anti-Drug Abuse Council (Badac) Training: Activity DesignMaria Fiona Duran Merquita100% (2)
- ProposalDokumen41 halamanProposalMark B. BarrogaBelum ada peringkat
- How To Build Your Creative Confidence PDFDokumen2 halamanHow To Build Your Creative Confidence PDFSteph GonzagaBelum ada peringkat
- Treinamento Sensório MotorDokumen8 halamanTreinamento Sensório MotorDébora SouzaBelum ada peringkat
- Air PollutionDokumen3 halamanAir PollutionGaurav Singh JantwalBelum ada peringkat
- Pathologist Job SpecificationDokumen16 halamanPathologist Job SpecificationLalaluluBelum ada peringkat
- ST Regis OriginalDokumen224 halamanST Regis OriginalNeeraj AgarwalBelum ada peringkat
- Statistic - Id873655 - Almond Consumption Per Capita in India 2012 2021Dokumen8 halamanStatistic - Id873655 - Almond Consumption Per Capita in India 2012 2021Rohith kumarBelum ada peringkat
- Road Safety EAL 338-1Dokumen53 halamanRoad Safety EAL 338-1NasrulBelum ada peringkat
- VACCP Template Checklist - SafetyCultureDokumen7 halamanVACCP Template Checklist - SafetyCulturepattysaborio520Belum ada peringkat
- Max Bupa Claim Form NewDokumen9 halamanMax Bupa Claim Form NewViral ShuklaBelum ada peringkat
- Headache Management Guideline For Adults Version 10Dokumen6 halamanHeadache Management Guideline For Adults Version 10TomiBelum ada peringkat
- Reflective Journal 1 2 and 3Dokumen11 halamanReflective Journal 1 2 and 3api-321980896Belum ada peringkat
- BTPB RepertoryDokumen24 halamanBTPB Repertoryanamika mishra100% (1)
- Ijc Heart & Vasculature: SciencedirectDokumen8 halamanIjc Heart & Vasculature: SciencedirectFerdi YuanBelum ada peringkat
- Outpatient Dental & Clinical - Claim FormDokumen1 halamanOutpatient Dental & Clinical - Claim FormYiki TanBelum ada peringkat
- Application Form For Registration of Assessment AgenciesDokumen2 halamanApplication Form For Registration of Assessment AgenciesAnkurBelum ada peringkat
- Medihuanna Brand GuidelinesDokumen71 halamanMedihuanna Brand GuidelinespuskickBelum ada peringkat
- MiesDokumen40 halamanMiessanthiyasandyBelum ada peringkat
- Hun Yuan Qigong - All NotesDokumen19 halamanHun Yuan Qigong - All Notespilillo50% (2)
- The Obesity Code: Unlocking the Secrets of Weight LossDari EverandThe Obesity Code: Unlocking the Secrets of Weight LossPenilaian: 4 dari 5 bintang4/5 (6)
- The Age of Magical Overthinking: Notes on Modern IrrationalityDari EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityPenilaian: 4 dari 5 bintang4/5 (28)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsDari EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsBelum ada peringkat
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDari EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BePenilaian: 2 dari 5 bintang2/5 (1)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDDari EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDPenilaian: 5 dari 5 bintang5/5 (1)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionDari EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionPenilaian: 4 dari 5 bintang4/5 (404)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsDari EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsPenilaian: 5 dari 5 bintang5/5 (1)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisDari EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisPenilaian: 4.5 dari 5 bintang4.5/5 (42)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedDari EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedPenilaian: 5 dari 5 bintang5/5 (81)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsDari EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsPenilaian: 3.5 dari 5 bintang3.5/5 (3)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisDari EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisPenilaian: 4 dari 5 bintang4/5 (1)
- Why We Die: The New Science of Aging and the Quest for ImmortalityDari EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityPenilaian: 4 dari 5 bintang4/5 (3)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaDari EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- The Comfort of Crows: A Backyard YearDari EverandThe Comfort of Crows: A Backyard YearPenilaian: 4.5 dari 5 bintang4.5/5 (23)
- Sleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningDari EverandSleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningPenilaian: 4 dari 5 bintang4/5 (3)
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Dari EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Belum ada peringkat
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisDari EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisPenilaian: 3.5 dari 5 bintang3.5/5 (2)
- Gut: the new and revised Sunday Times bestsellerDari EverandGut: the new and revised Sunday Times bestsellerPenilaian: 4 dari 5 bintang4/5 (392)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryDari EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryPenilaian: 4 dari 5 bintang4/5 (44)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Dari EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Penilaian: 4.5 dari 5 bintang4.5/5 (110)
- The Marshmallow Test: Mastering Self-ControlDari EverandThe Marshmallow Test: Mastering Self-ControlPenilaian: 4.5 dari 5 bintang4.5/5 (58)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessDari EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessPenilaian: 4.5 dari 5 bintang4.5/5 (328)
- Dark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingDari EverandDark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingPenilaian: 4 dari 5 bintang4/5 (1138)
- Troubled: A Memoir of Foster Care, Family, and Social ClassDari EverandTroubled: A Memoir of Foster Care, Family, and Social ClassPenilaian: 4.5 dari 5 bintang4.5/5 (26)