PSet 8 FL13
Diunggah oleh
cacer009Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
PSet 8 FL13
Diunggah oleh
cacer009Hak Cipta:
Format Tersedia
MatS 2001 - Fall 2013 Problem Set:8 Due 11/15/13
8-1 Using the copper-silver phase diagram (p 298) answer the following questions: a. what is the maximum solubility of Ag in Cu? Cu in Ag? 70 g Cu is mixed with 30 g of Ag and heated to 1000C. Then the mixture is slowly cooled to room temperature. b. Identify the phases present at 780C, the composition, and amount of each phase. Sketch the microstructure. c. Identify the phases present at 778C, the composition, and amount of each phase. Sketch the microstructure. d. Identify the phases present at room temperature, the composition of each phase, and the amount. Assume equilibrium compositions. (Of course the microstructure will not be at equilibrium). 8-2 Using the magnesium-lead phase diagram (p 313) a. what is the maximum solubility of Pb in Mg? of Mg in Pb(be careful on this one)? b. An alloy is prepared by heating a mixture of 50 kg lead and 50 kg magnesium to 700C, then cooling to just above the eutectic temperature, 465C. What is the composition and mass of each phase? c. The alloy is then cooled to 464C. Sketch the microstructure. What is the composition and mass of each phase? d. Finally the alloy is cooled to room temperature. What are the mass of each microstructure and the mass of each phase in the different microstructures? Note, because the phase boundary separating the +Mg2Pb (two-phase) and regions is rather temperature dependent you will need to keep track of the changes between the eutectic temperature and room temperature. 8-3 Using the copper-zinc phase diagram (p312) a. what is the maximum solubility of Zn in Cu? Why does zinc not have unlimited solubility in cooper? (hint: consider the Hume-Rothery rules) b. A common commercial alloy is cartridge brass which has 30 wt% zinc. Find the yield stress and tensile stress for this brass in Chapter 6 and compare it to pure copper. Give some reasons for the difference. What assumptions need to be made?
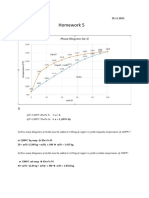
8-4 The attached figure shows the pressuretemperature phase diagram for H2O. Apply the Gibbs phase rule at points A, B, and C; that is, specify the number of degrees of freedom at each of the points. (Degrees of freedom are the number of externally controllable variables that need be specified to completely define the system.) 8-5 Consider the phase diagrams for two binary mixtures, Mo-W and Mo-Si, which are attached at the end of the problem set.
a. Label all phase fields in both diagrams. There are two extremely tiny fields on the Mo-Si diagram that you can leave unlabeled. Note: Stoichiometric compounds Mo3Si, Mo5Si3 and MoSi2 exist at the compositions denoted by the vertical arrows. You need to label the arrows with the right compound. What is the melting point of MoSi2? b. The phase behaviors of Mo-W mixtures and Mo-Si mixtures are markedly different. Give a detailed explanation for why this is. 8-6 Suppose you have a Mo-W alloy that is 70 wt% W. Describe a process that would allow you to isolate an enriched mixture that is 80 wt% W. What would your yield be for a two-step process? Show your calculations and indicate the process on the Mo-W phase diagram. 8-7 10 kg of graphite (C) is added to 990 kg of Fe and the mixture is heated to 1600 C. Slowly cool as follows. a. Cool to 1400C. Sketch the morphology. Calculate the mass of each microstructure and the mass and composition of each phase within each microstructure. b. Cool to 1000C. Sketch the morphology. Calculate the mass of each microstructure and the mass and composition of each phase within each microstructure. c. Cool to 728C. Sketch the morphology. Calculate the mass of each microstructure and the mass and composition of each phase within each microstructure. d. Cool to 726C. Sketch the morphology. Calculate the mass of each microstructure and the mass and composition of each phase within each microstructure. e. Is this a hypo- or hypereutectoid alloy? Why? 8-8 Phase Transformations. Consider an iron-carbon alloy of eutectoid composition. If the sample is held at 760C and then subjected to following timetemperature treatments, specify the nature of the final microstructure (microconstituents present and approximate percentages). a. Rapidly cool to 350C, hold for 10,000 s, then quench to room temperature. b. Rapidly cool to 250C, hold for 100 s, then quench to room temperature. c. Rapidly cool to 650C, hold for 20s, rapidly cool to 400C, hold for 1000s, then quench to room temperature.
Anda mungkin juga menyukai
- Che 3330 - Spring 2012 HW 5Dokumen5 halamanChe 3330 - Spring 2012 HW 5Brett CasserlyBelum ada peringkat
- Supercapacitors Based on Carbon or Pseudocapacitive MaterialsDari EverandSupercapacitors Based on Carbon or Pseudocapacitive MaterialsBelum ada peringkat
- Assignment PhaseDiaDokumen5 halamanAssignment PhaseDiaAnshu Kumar GuptaBelum ada peringkat
- Engineering Materials Phase DiagramsDokumen6 halamanEngineering Materials Phase DiagramsOmar AssalBelum ada peringkat
- Advanced Battery MaterialsDari EverandAdvanced Battery MaterialsChunwen SunBelum ada peringkat
- Tutorial Problems 5Dokumen8 halamanTutorial Problems 5Majak MarialBelum ada peringkat
- Tutorial 7 - Phase DiagramsDokumen4 halamanTutorial 7 - Phase DiagramsSYAFIQAH ISMAILBelum ada peringkat
- ASSIGNMENT # 3 W KEYDokumen16 halamanASSIGNMENT # 3 W KEYRashedul IslamBelum ada peringkat
- Phase Diagram Exercises - Worked Answers - CorrectedDokumen14 halamanPhase Diagram Exercises - Worked Answers - CorrectedHayden Reeves75% (4)
- Phase Diagram AssignmentDokumen10 halamanPhase Diagram AssignmentSyafiqah RusdiBelum ada peringkat
- Eng Mat Chapter 4Dokumen126 halamanEng Mat Chapter 4VC Chua Yee LeongBelum ada peringkat
- Phase Diagrams and Microstructures in AlloysDokumen4 halamanPhase Diagrams and Microstructures in AlloysTia AgustinBelum ada peringkat
- Assignment 4 Phase DiagramDokumen4 halamanAssignment 4 Phase DiagramAhmedAhmedBelum ada peringkat
- HW 8 TrustedDokumen20 halamanHW 8 TrustedEldeniz AliyevBelum ada peringkat
- Recitation 2 QuestionsDokumen14 halamanRecitation 2 QuestionsfzfwsbyxrhBelum ada peringkat
- Tutorial 2 HelperDokumen3 halamanTutorial 2 HelperDedy SaputraBelum ada peringkat
- TYPD ExercisesDokumen10 halamanTYPD ExercisesConstance Lynn'da GBelum ada peringkat
- Chapter 4-Phase DiagramDokumen16 halamanChapter 4-Phase Diagramtky96Belum ada peringkat
- Chapter N9 Proplems HW - Ch9Dokumen1 halamanChapter N9 Proplems HW - Ch9xuantoni1112003Belum ada peringkat
- Assignment 23Dokumen5 halamanAssignment 23Jenny GoBelum ada peringkat
- MME 9602 Term Test SolutionsDokumen8 halamanMME 9602 Term Test Solutionszubair ahmedBelum ada peringkat
- MSE 260 Phase Transformations AssignmentDokumen2 halamanMSE 260 Phase Transformations Assignmentmichael ananaBelum ada peringkat
- CH3 Questions: Fig. 10-20 Unary Phase Diagram For Carbon. (Region For Diamond Formation Is Shown With A Dotted Line)Dokumen4 halamanCH3 Questions: Fig. 10-20 Unary Phase Diagram For Carbon. (Region For Diamond Formation Is Shown With A Dotted Line)Mohammed IbrahimBelum ada peringkat
- r05220802 Chemical Engineering Thermodynamics IDokumen5 halamanr05220802 Chemical Engineering Thermodynamics ISrinivasa Rao GBelum ada peringkat
- HW5-Phase Diagram-2023Dokumen5 halamanHW5-Phase Diagram-2023nanniedefiBelum ada peringkat
- Chapt 08Dokumen21 halamanChapt 08Jesse McClure100% (5)
- 2019 20-ExOrd19dic2019 V19dic SS Ing VfinalDokumen8 halaman2019 20-ExOrd19dic2019 V19dic SS Ing VfinalIñigoBelum ada peringkat
- Week 11Dokumen12 halamanWeek 11lduran_63Belum ada peringkat
- Jntuworld: R07 Set No. 2Dokumen6 halamanJntuworld: R07 Set No. 2Dolly PriyaBelum ada peringkat
- rr220802 Chemical Engineering Thermodynamics IDokumen8 halamanrr220802 Chemical Engineering Thermodynamics ISRINIVASA RAO GANTABelum ada peringkat
- Closed-Book Practice-Ch 10 (2017!08!08)Dokumen12 halamanClosed-Book Practice-Ch 10 (2017!08!08)Juan0% (1)
- r05220802 Chemical Engineering Thermodynamics IDokumen6 halamanr05220802 Chemical Engineering Thermodynamics ISRINIVASA RAO GANTABelum ada peringkat
- Excercise 5-Chapter 8Dokumen10 halamanExcercise 5-Chapter 8Rachel So Jia XinBelum ada peringkat
- Problems 2Dokumen13 halamanProblems 2yesildalertugrulBelum ada peringkat
- 07 - BEME (CE) Section-A& BDokumen2 halaman07 - BEME (CE) Section-A& BDarya MemonBelum ada peringkat
- PROBLEMSDokumen14 halamanPROBLEMSChristian John DumoBelum ada peringkat
- MM 209 Assignment 2 2016Dokumen4 halamanMM 209 Assignment 2 2016SiddharthaSheringBelum ada peringkat
- ChE101 PSDokumen2 halamanChE101 PSKevin JangBelum ada peringkat
- MTDKDokumen9 halamanMTDKraviteja1840Belum ada peringkat
- rr222102 Engineering ThermodynamicsDokumen8 halamanrr222102 Engineering ThermodynamicsSRINIVASA RAO GANTABelum ada peringkat
- (NagpurStudents - Org) Chemical Reactor DesignDokumen4 halaman(NagpurStudents - Org) Chemical Reactor Designsiddharth sharmaBelum ada peringkat
- St. Joseph'S College (Autonomous) Tiruchirappalli - 620002 - APRIL 2021Dokumen3 halamanSt. Joseph'S College (Autonomous) Tiruchirappalli - 620002 - APRIL 2021dharaniBelum ada peringkat
- Homework 5Dokumen4 halamanHomework 5Ferhat PeynirciBelum ada peringkat
- ME604 Thermal Engineering Sem VI exam questionsDokumen2 halamanME604 Thermal Engineering Sem VI exam questionsYash BeleBelum ada peringkat
- Effect of Austempering Time On Microstructure and Properties of A Low-Carbon Bainite SteelDokumen7 halamanEffect of Austempering Time On Microstructure and Properties of A Low-Carbon Bainite Steelمسعود بوزويرBelum ada peringkat
- Atomic Structure and Bonding in MaterialsDokumen16 halamanAtomic Structure and Bonding in MaterialsRasyidi AhmadBelum ada peringkat
- Mat101 w12 Hw6 SolutionsDokumen8 halamanMat101 w12 Hw6 SolutionsKonark PatelBelum ada peringkat
- Computer Exercises A B CDokumen16 halamanComputer Exercises A B CAlex JhonBelum ada peringkat
- Phase Diagram Analysis of the Al-Cu Binary SystemDokumen6 halamanPhase Diagram Analysis of the Al-Cu Binary Systemnilanga123Belum ada peringkat
- WWW - Manaresults.Co - In: II B. Tech I Semester Regular/Supplementary Examinations, October/November - 2018 ThermodynamicsDokumen8 halamanWWW - Manaresults.Co - In: II B. Tech I Semester Regular/Supplementary Examinations, October/November - 2018 Thermodynamicsashoku24007Belum ada peringkat
- Engineering Materials and MetallurgyDokumen14 halamanEngineering Materials and Metallurgyashok pradhanBelum ada peringkat
- ME6403-Engineering Materials and MetallurgyDokumen12 halamanME6403-Engineering Materials and Metallurgysanthanam102Belum ada peringkat
- 2Dokumen2 halaman2faizrummanBelum ada peringkat
- THERMODYNAMICS Oct-Nov 2019Dokumen8 halamanTHERMODYNAMICS Oct-Nov 2019Karthik CruiseBelum ada peringkat
- Tutorial Questions For Part 1Dokumen5 halamanTutorial Questions For Part 1Ng Yan XiongBelum ada peringkat
- PP Set 1 & 2Dokumen3 halamanPP Set 1 & 2Raveendra kumar KosireddiBelum ada peringkat
- University of LondonDokumen6 halamanUniversity of LondonShootingStarPhotonsBelum ada peringkat
- 9A03301 Materials Science and EngineeringDokumen4 halaman9A03301 Materials Science and EngineeringsivabharathamurthyBelum ada peringkat
- Science Chapter 1 Class 10Dokumen5 halamanScience Chapter 1 Class 10joshi_rljBelum ada peringkat
- 0620 Sow Unit 11 Redox Electrochemistry Group VIIDokumen7 halaman0620 Sow Unit 11 Redox Electrochemistry Group VIIPakardan TeaBelum ada peringkat
- ICP Determination of Metal in Waste WaterDokumen18 halamanICP Determination of Metal in Waste WaterNorshafiza Mohd Rosli100% (5)
- Standarization of Volumetric SolutionsDokumen9 halamanStandarization of Volumetric SolutionsZahid IqbalBelum ada peringkat
- Graphite in The English River Metasedimentary Rocks - 2014Dokumen43 halamanGraphite in The English River Metasedimentary Rocks - 2014Fernando GuedesBelum ada peringkat
- Chemistryproject On Alloy Extraction Class 12Dokumen17 halamanChemistryproject On Alloy Extraction Class 12Shobhit VarshneyBelum ada peringkat
- Examen de Evaluación API 571Dokumen18 halamanExamen de Evaluación API 571berray2007100% (2)
- Ss LectureDokumen18 halamanSs LectureFasil ParuvanathBelum ada peringkat
- Lecture 5. Chemical Reaction (Part 2)Dokumen38 halamanLecture 5. Chemical Reaction (Part 2)Dione Gale NavalBelum ada peringkat
- (Enppi) Corrosion & Cathodic Protection in Petroleum Industry Course (Yasser Tawfik)Dokumen545 halaman(Enppi) Corrosion & Cathodic Protection in Petroleum Industry Course (Yasser Tawfik)nachitosis100% (4)
- Inventory Management NalcoDokumen73 halamanInventory Management NalcoAnonymous rhw2oi80% (1)
- Carbides, Fullerenes & Fluorocarbons (Inorg)Dokumen21 halamanCarbides, Fullerenes & Fluorocarbons (Inorg)SamanBelum ada peringkat
- Material Science & MetallurgyDokumen2 halamanMaterial Science & MetallurgyBhavesh Pipaliya0% (1)
- Wire Rope Technical Manual: Choose QualityDokumen62 halamanWire Rope Technical Manual: Choose QualityMelchorRdzBelum ada peringkat
- Gold RefiningDokumen6 halamanGold Refiningelizaldesf50% (2)
- Technical Specs PDFDokumen12 halamanTechnical Specs PDFcsolanki6586100% (1)
- Unistrut: Application, Installation, Specification GuideDokumen20 halamanUnistrut: Application, Installation, Specification GuideCAR6Belum ada peringkat
- BHMA Builders Hardware Finishes Reference GuideDokumen5 halamanBHMA Builders Hardware Finishes Reference GuideRey Eduard Q. UmelBelum ada peringkat
- High Performance Steel MaterialsDokumen38 halamanHigh Performance Steel MaterialsDavid CrownBelum ada peringkat
- Ferrous Metals Guide - Classification, Properties and Uses of Iron, Cast Iron, Wrought Iron and SteelDokumen18 halamanFerrous Metals Guide - Classification, Properties and Uses of Iron, Cast Iron, Wrought Iron and SteelJohnBelum ada peringkat
- 9701 Chemistry Data Booklet 2016Dokumen20 halaman9701 Chemistry Data Booklet 2016Hamza JunaidBelum ada peringkat
- Tds - Fischer ProductlineDokumen24 halamanTds - Fischer Productlinesrpati_55555Belum ada peringkat
- Stud Bolt Specification 2Dokumen7 halamanStud Bolt Specification 2santoshblonkarBelum ada peringkat
- Aluminum Analysis ReportGlient: Here is a concise, SEO-optimized title for the document:TITLE IGAS Research Reports Aluminum Alloy Purity and PropertiesDokumen3 halamanAluminum Analysis ReportGlient: Here is a concise, SEO-optimized title for the document:TITLE IGAS Research Reports Aluminum Alloy Purity and PropertiessalmanBelum ada peringkat
- CLASS-10TH - CHAPTER - 3 Metals and Non-MetalsDokumen3 halamanCLASS-10TH - CHAPTER - 3 Metals and Non-MetalsTanmay LahaBelum ada peringkat
- PRE4122 Exercise No. 4 Imperfections in SolidsDokumen9 halamanPRE4122 Exercise No. 4 Imperfections in SolidsعبداللهأحمدBelum ada peringkat
- Coordination Compound and Its ChemistryDokumen38 halamanCoordination Compound and Its ChemistryAvinash RaiBelum ada peringkat
- Grab Sampling For Underground Gold Mine Grade Control PDFDokumen11 halamanGrab Sampling For Underground Gold Mine Grade Control PDFmzulfikarmuslimBelum ada peringkat
- Honda Pump BrochureDokumen18 halamanHonda Pump Brochurefser04Belum ada peringkat
- Electroplating of Cu-Sn Alloys andDokumen81 halamanElectroplating of Cu-Sn Alloys andcicerojoiasBelum ada peringkat
- Guidelines for Implementing Process Safety ManagementDari EverandGuidelines for Implementing Process Safety ManagementBelum ada peringkat
- Safety Critical Systems Handbook: A Straight forward Guide to Functional Safety, IEC 61508 (2010 EDITION) and Related Standards, Including Process IEC 61511 and Machinery IEC 62061 and ISO 13849Dari EverandSafety Critical Systems Handbook: A Straight forward Guide to Functional Safety, IEC 61508 (2010 EDITION) and Related Standards, Including Process IEC 61511 and Machinery IEC 62061 and ISO 13849Penilaian: 4 dari 5 bintang4/5 (5)
- Introduction to Petroleum Process SafetyDari EverandIntroduction to Petroleum Process SafetyPenilaian: 3 dari 5 bintang3/5 (2)
- A Poison Like No Other: How Microplastics Corrupted Our Planet and Our BodiesDari EverandA Poison Like No Other: How Microplastics Corrupted Our Planet and Our BodiesPenilaian: 5 dari 5 bintang5/5 (1)
- Fire in the Night: The Piper Alpha DisasterDari EverandFire in the Night: The Piper Alpha DisasterPenilaian: 4.5 dari 5 bintang4.5/5 (5)
- Practical Industrial Safety, Risk Assessment and Shutdown SystemsDari EverandPractical Industrial Safety, Risk Assessment and Shutdown SystemsPenilaian: 4 dari 5 bintang4/5 (11)
- Chemical Process Safety: Learning from Case HistoriesDari EverandChemical Process Safety: Learning from Case HistoriesPenilaian: 4 dari 5 bintang4/5 (14)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsDari EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsBelum ada peringkat
- Incidents That Define Process SafetyDari EverandIncidents That Define Process SafetyBelum ada peringkat
- LNG Risk Based Safety: Modeling and Consequence AnalysisDari EverandLNG Risk Based Safety: Modeling and Consequence AnalysisBelum ada peringkat
- Safety Fundamentals and Best Practices in Construction IndustryDari EverandSafety Fundamentals and Best Practices in Construction IndustryBelum ada peringkat
- Trevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationDari EverandTrevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationBelum ada peringkat
- Inherently Safer Chemical Processes: A Life Cycle ApproachDari EverandInherently Safer Chemical Processes: A Life Cycle ApproachPenilaian: 5 dari 5 bintang5/5 (1)
- Rules of Thumb for Maintenance and Reliability EngineersDari EverandRules of Thumb for Maintenance and Reliability EngineersPenilaian: 4.5 dari 5 bintang4.5/5 (12)
- A Complete Guide to Safety Officer Interview Questions and AnswersDari EverandA Complete Guide to Safety Officer Interview Questions and AnswersPenilaian: 4 dari 5 bintang4/5 (1)
- The Invisible Rainbow: A History of Electricity and LifeDari EverandThe Invisible Rainbow: A History of Electricity and LifePenilaian: 4.5 dari 5 bintang4.5/5 (21)
- One Health: Integrated Approach to 21st Century Challenges to HealthDari EverandOne Health: Integrated Approach to 21st Century Challenges to HealthJoana C. PrataBelum ada peringkat
- Radium Girls: Women and Industrial Health Reform, 1910-1935Dari EverandRadium Girls: Women and Industrial Health Reform, 1910-1935Penilaian: 4.5 dari 5 bintang4.5/5 (4)
- Fire Fighting Pumping Systems at Industrial FacilitiesDari EverandFire Fighting Pumping Systems at Industrial FacilitiesPenilaian: 4.5 dari 5 bintang4.5/5 (3)
- The Single Cure: Human Life Extension to 300+ YearsDari EverandThe Single Cure: Human Life Extension to 300+ YearsBelum ada peringkat
- Handbook of Fire and Explosion Protection Engineering Principles: for Oil, Gas, Chemical and Related FacilitiesDari EverandHandbook of Fire and Explosion Protection Engineering Principles: for Oil, Gas, Chemical and Related FacilitiesPenilaian: 4.5 dari 5 bintang4.5/5 (2)
- Electrical Safety Code Manual: A Plain Language Guide to National Electrical Code, OSHA and NFPA 70EDari EverandElectrical Safety Code Manual: A Plain Language Guide to National Electrical Code, OSHA and NFPA 70EPenilaian: 3 dari 5 bintang3/5 (6)
- The Safety Critical Systems Handbook: A Straightforward Guide to Functional Safety: IEC 61508 (2010 Edition), IEC 61511 (2015 Edition) and Related GuidanceDari EverandThe Safety Critical Systems Handbook: A Straightforward Guide to Functional Safety: IEC 61508 (2010 Edition), IEC 61511 (2015 Edition) and Related GuidancePenilaian: 5 dari 5 bintang5/5 (1)
- Guidelines for Process Safety in Bioprocess Manufacturing FacilitiesDari EverandGuidelines for Process Safety in Bioprocess Manufacturing FacilitiesBelum ada peringkat
- Establishing an occupational health & safety management system based on ISO 45001Dari EverandEstablishing an occupational health & safety management system based on ISO 45001Penilaian: 4 dari 5 bintang4/5 (5)