Living Kidney Donation
Diunggah oleh
atul_desai_3Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Living Kidney Donation
Diunggah oleh
atul_desai_3Hak Cipta:
Format Tersedia
Editorial Living Kidney Donation: Biochemical Changes and Patient Outcomes
Related Article, p. 577
very year more than 27,000 individuals worldwide choose to undergo living kidney donation to help someone in need.1 The question has been raised of whether the immediate decrement in glomerular ltration rate (GFR) after kidney donation portends the same biochemical changes and patient outcomes as chronic kidney disease (CKD).2 When certain biochemical features and clinical outcomes are observed in patients with CKD, it is difcult to know whether they are attributable entirely to the decrease in GFR. This is because patients with CKD are older and frequently have comorbid conditions that may inuence observed associations. In this issue of AJKD, Kasiske et al3 report the 6-month (interim) results of an important prospective cohort study of approximately 200 living kidney donors and 200 nondonor controls (the ALTOLD [Assessing Long Term Outcomes Of Living Donation] Study; funded by the US National Institutes of Health). Kasiske et al3 offer unique insights into the biochemical effects of mild isolated decrements in GFR after nephrectomy with less confounding than studies of patients with decreased GFR from kidney disease. This study provides more reliable estimates of effects previously described in cross-sectional and retrospective studies of living kidney donors.4,5 In brief, in this study, donors and nondonor controls were prospectively enrolled from 8 transplantation centers in the United States between 2006 and 2011. In order to investigate outcomes associated with kidney donation, it is vital to choose the best type of nondonor controls to compare with donors. Potential donors undergo a thorough selection process, which means that individuals who go on to donate are already inherently healthier than the general population. In the study by Kasiske et al,3 nondonor controls were healthy individuals who theoretically could have been donors at each enrolling site. Controls were screened with a medical history, physical examination, and basic blood and urine laboratory tests for kidney disease, but did not undergo other screening tests, such as renal imaging, that were performed in
Address correspondence to Amit X. Garg, MD, PhD, London Kidney Clinical Research Unit, Room ELL-121, Westminster, London Health Sciences Centre, 800 Commissioners Road East, London, Ontario N6A 4G5, Canada. E-mail: amit.garg@lhsc.on.ca 2013 by the National Kidney Foundation, Inc. 0272-6386/$36.00 http://dx.doi.org/10.1053/j.ajkd.2013.06.006
448
donors. Assays were done in a single study laboratory. Of the participants, 32% were men, 95% were of white ethnicity, 23% were obese (body mass index 30 kg/m2), and 12% were current smokers; average blood pressure was 117/70 mm Hg. Fifteen percent of nondonor controls and 31% of donors had a family history of a rst-degree relative with kidney failure. One strength of the study is that participants GFRs were measured (iohexol) at baseline and follow-up. Approximately 10% of participants were lost to 6-month follow-up. Kidney donation resulted in a 28% decline in iohexol-measured GFR (average, 97 mL/min/1.73 m2 before donation and 68 mL/min/1.73 m2 6 months after donation). The average decrement observed in measured GFR was comparable to the average decrement observed in estimated GFR, which is reassuring for clinical practice, in which estimated GFR is typically used to follow up donors. Six months after donation, the authors found no substantive difference between donors and nondonor controls in blood pressure, body weight, or body mass index and no difference in lipid, glycemic, or inammatory parameters. Donors had differences in some parameters of mineral metabolism (higher parathyroid hormone and lower serum phosphate levels), slightly lower hemoglobin levels, and higher serum uric acid and homocysteine levels versus controls. The authors conclude that kidney donors have some but not all of the biochemical abnormalities seen with mild CKD, an assertion fully supported by their data. This new knowledge of biochemical changes after donor nephrectomy is welcome. A prospective cohort design, as used in this study, is well suited to describe biochemical, GFR, and blood pressure changes after donor nephrectomy. It also is well suited to understanding the psychosocial outcomes of donation, such as evaluating the adequacy of informed consent, any changes in quality of life or anxiety, and the nancial expenses incurred by donors. However, one of the challenges with a prospective study design is that participants will need to be followed up for decades to characterize many clinically relevant outcomes that matter to donors, recipients, and their health professionals. The threat of lost follow-up is a major concern in long-term prospective studies, and thousands of participants need to be followed up to provide adequate statistical power for reliable estimates of risk. In this regard, the information for clinical and surrogate outcomes from prospective studies can prompt efforts to obtain safety information for patient outcomes in a timely way using other types of study
Am J Kidney Dis. 2013;62(3):448-449
Editorial
designs. For example, studies by Kasiske et al3 and others4,5 now describe changes in levels of markers of bone mineral metabolism in kidney donors; higher levels of serum broblast growth factor 23 (FGF-23), plasma parathyroid hormone, and fractional excretion of urinary inorganic phosphate and lower levels of serum calcitriol and phosphate. To ensure that donors are not experiencing a higher rate of osteoporotic fractures with these biochemical changes, we recently used a retrospective cohort design using large health care databases.6 We were reassured that living kidney donors were not manifesting a higher risk of osteoporotic fractures compared with nondonor controls when followed up for almost 2 decades. In conclusion, different types of study designs will continue to be used to better understand biochemical changes and clinically important long-term outcomes in living kidney donors. This information will inform the decision-making process for potential kidney donors and recipients, strengthen the publics trust in the transplantation system, allow the nephrology community to expand to new types of living donors safely, and guide the follow-up care we provide to donors to maintain optimal long-term health.7 Amit X. Garg, MD, PhD Western University London, Canada
ACKNOWLEDGEMENTS
Support: Dr Garg and colleagues in the Donor Nephrectomy Outcomes Research (DONOR) Network have received investigatorinitiated grants from Astellas and Roche to support a Canadian Institutes of Health Researchfunded prospective study on living kidney donation. Financial Disclosure: The author declares that he has no relevant nancial interests.
REFERENCES
1. Horvat LD, Shariff SZ, Garg AX. Global trends in the rates of living kidney donation. Kidney Int. 2009;75(10):1088-1098. 2. Levey AS, Danovitch G, Hou S. Living donor kidney transplantation in the United Stateslooking back, looking forward. Am J Kidney Dis. 2011;58(3):343-348. 3. Kasiske BL, Anderson-Haag T, Ibrahim HN, et al. A prospective controlled study of kidney donors: baseline and 6-month follow-up. Am J Kidney Dis. 2013;62(3):577-586. 4. Young A, Nevis IF, Geddes C, et al. Do biochemical measures change in living kidney donors? A systematic review. Nephron Clin Pract. 2007;107(3):c82-c89. 5. Young A, Hodsman AB, Boudville N, et al. Bone and mineral metabolism and broblast growth factor 23 levels after kidney donation. Am J Kidney Dis. 2012;59(6):761-769. 6. Garg AX, Pouget J, Young A, et al. Fracture risk in living kidney donors: a matched cohort study. Am J Kidney Dis. 2012; 59(6):770-776. 7. Leichtman A, Abecassis M, Barr M, et al. Living kidney donor follow-up: state-of-the-art and future directions, conference summary and recommendations. Am J Transplant. 2011;11(12): 2561-2568.
Am J Kidney Dis. 2013;62(3):448-449
449
Anda mungkin juga menyukai
- Annotated Chess GameDokumen3 halamanAnnotated Chess Gameatul_desai_3100% (1)
- Nrneph 2013 232Dokumen11 halamanNrneph 2013 232atul_desai_3Belum ada peringkat
- Alekhine 1Dokumen1 halamanAlekhine 1atul_desai_3Belum ada peringkat
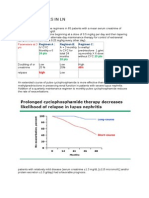
- Clinical Trials in LNDokumen19 halamanClinical Trials in LNatul_desai_3Belum ada peringkat
- Costimulatory BlockadeDokumen11 halamanCostimulatory Blockadeatul_desai_3Belum ada peringkat
- Kasparov Vs IvanchukDokumen1 halamanKasparov Vs Ivanchukatul_desai_3Belum ada peringkat
- Therapy of LN Lessons LearntDokumen8 halamanTherapy of LN Lessons Learntatul_desai_3Belum ada peringkat
- 3 Hepatmon 12 11 7359Dokumen9 halaman3 Hepatmon 12 11 7359atul_desai_3Belum ada peringkat
- Anca VasculitisDokumen12 halamanAnca Vasculitisatul_desai_3100% (2)
- 2028 Full PDFDokumen8 halaman2028 Full PDFdhineyBelum ada peringkat
- Hou YifanDokumen1 halamanHou Yifanatul_desai_3Belum ada peringkat
- Game 2Dokumen2 halamanGame 2atul_desai_3Belum ada peringkat
- Chess GameDokumen3 halamanChess Gameatul_desai_3Belum ada peringkat
- Power of Passed PawnDokumen2 halamanPower of Passed Pawnatul_desai_3Belum ada peringkat
- Clinical Trials in Lupus NephritisDokumen19 halamanClinical Trials in Lupus Nephritisatul_desai_3Belum ada peringkat
- Diag.1 Diag.2 Diag.3: Find The Move For Black, and Calculate The Response of White To It Blacks Response?Dokumen2 halamanDiag.1 Diag.2 Diag.3: Find The Move For Black, and Calculate The Response of White To It Blacks Response?atul_desai_3Belum ada peringkat
- Calcium MetabolismDokumen51 halamanCalcium Metabolismatul_desai_3Belum ada peringkat
- Laszlo Vs Geller Find Moves For BlackDokumen2 halamanLaszlo Vs Geller Find Moves For Blackatul_desai_3Belum ada peringkat
- Approach Hypophosphatemic RickeDokumen1 halamanApproach Hypophosphatemic Rickeatul_desai_3Belum ada peringkat
- DIET in Chronic Kidney DiseaseDokumen3 halamanDIET in Chronic Kidney Diseaseatul_desai_3Belum ada peringkat
- Renal Artery Stenosis-Predicting Responses To RevascularizaDokumen1 halamanRenal Artery Stenosis-Predicting Responses To Revascularizaatul_desai_3Belum ada peringkat
- Anca VasculititsDokumen6 halamanAnca Vasculititsatul_desai_3Belum ada peringkat
- Association of Dialysate Bicarbonate Concentration With Mortality in The Dialysis Outcomes and Practice Patterns Study (DOPPS)Dokumen9 halamanAssociation of Dialysate Bicarbonate Concentration With Mortality in The Dialysis Outcomes and Practice Patterns Study (DOPPS)atul_desai_3Belum ada peringkat
- Permaeability Factor in FSGSDokumen4 halamanPermaeability Factor in FSGSatul_desai_3Belum ada peringkat
- Update On Cystatin CDokumen9 halamanUpdate On Cystatin Catul_desai_3Belum ada peringkat
- Atypical Haemolytic Uraemic Syndrome With UnderlyingDokumen14 halamanAtypical Haemolytic Uraemic Syndrome With Underlyingatul_desai_3Belum ada peringkat
- Complicated Urinary Tract InfectionsDokumen39 halamanComplicated Urinary Tract InfectionsAndreas IoannouBelum ada peringkat
- Obesity-Related Cardiorenal DiseaseDokumen13 halamanObesity-Related Cardiorenal Diseaseatul_desai_3Belum ada peringkat
- Renal Cancer in Von Hippel-Lindau DiseaseDokumen10 halamanRenal Cancer in Von Hippel-Lindau Diseaseatul_desai_3Belum ada peringkat
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Maximizing Growth Factors for Height IncreaseDokumen29 halamanMaximizing Growth Factors for Height IncreaseMegaV100% (2)
- NURSING CARE PLAN The Child Undergoing Surgery For ScoliosisDokumen3 halamanNURSING CARE PLAN The Child Undergoing Surgery For ScoliosisscrewdriverBelum ada peringkat
- Meridians and Points PDFDokumen35 halamanMeridians and Points PDFHamdon Hamad100% (9)
- Circulatory System: Heart, Blood Vessels & Their FunctionsDokumen17 halamanCirculatory System: Heart, Blood Vessels & Their FunctionskangaanushkaBelum ada peringkat
- Anatomy and Diseases of The UveaDokumen102 halamanAnatomy and Diseases of The UveaVishakh IsloorBelum ada peringkat
- Population Genetics TutorialDokumen164 halamanPopulation Genetics TutorialMichelle GBelum ada peringkat
- The Relativistic Brain by Ronald Cicurel and Miguel L. Nicolelis (2015)Dokumen5 halamanThe Relativistic Brain by Ronald Cicurel and Miguel L. Nicolelis (2015)Sinem SerapBelum ada peringkat
- Bfhi-Session-5-How Breastfeeding WorksDokumen16 halamanBfhi-Session-5-How Breastfeeding Worksreema arshadBelum ada peringkat
- ECG Interpretation For ACLSDokumen27 halamanECG Interpretation For ACLSZH. omg sarBelum ada peringkat
- Lipid Membrane Composition and SignificanceDokumen4 halamanLipid Membrane Composition and SignificanceZainab AliBelum ada peringkat
- Importance of Functional Groups in Drug SelectionDokumen6 halamanImportance of Functional Groups in Drug SelectionNuruel HassanBelum ada peringkat
- Physiological Changes During PregnancyDokumen55 halamanPhysiological Changes During PregnancyKrishna PatelBelum ada peringkat
- Pengaruh Faktor-Faktor Psikososial Dan Insomnia Terhadap Depresi Pada Lansia Di Kota YogyakartaDokumen5 halamanPengaruh Faktor-Faktor Psikososial Dan Insomnia Terhadap Depresi Pada Lansia Di Kota YogyakartaKristina Puji LestariBelum ada peringkat
- Hematology Analyzers, Coagulation Systems Product GuideDokumen4 halamanHematology Analyzers, Coagulation Systems Product GuideElvan Dwi WidyadiBelum ada peringkat
- Body Systems NotesDokumen2 halamanBody Systems NotesAndrea González MercadoBelum ada peringkat
- Zoology: Zoology Previous Eamcet QuestionsDokumen8 halamanZoology: Zoology Previous Eamcet QuestionsGaganpreetSingh100% (1)
- Foot and Ankle ArthrokinematicsDokumen6 halamanFoot and Ankle ArthrokinematicsCraig StewartBelum ada peringkat
- AP PSYCHOLOGY EXAM REVIEW SHEETDokumen6 halamanAP PSYCHOLOGY EXAM REVIEW SHEETNathan HudsonBelum ada peringkat
- Pregnancy - Anatomy and PhysiologyDokumen5 halamanPregnancy - Anatomy and PhysiologyNiSCHEBelum ada peringkat
- 3.03 Understand Structures, Functions and Disorders of The Nervous SystemDokumen38 halaman3.03 Understand Structures, Functions and Disorders of The Nervous SystemLoriwinchesterBelum ada peringkat
- Thalassemia: SymptomsDokumen3 halamanThalassemia: SymptomsAndi BandotBelum ada peringkat
- Drug StudyDokumen11 halamanDrug StudyKimberly Ann MendozaBelum ada peringkat
- Muscles of Mastication Functions and Clinical SignificanceDokumen130 halamanMuscles of Mastication Functions and Clinical SignificanceDevanand GuptaBelum ada peringkat
- Tetanus Neonatorum LectureDokumen11 halamanTetanus Neonatorum LectureJackBelum ada peringkat
- Science: Quarter 1 - Module 1Dokumen10 halamanScience: Quarter 1 - Module 1RUTH PIANGBelum ada peringkat
- Modul Strategik Bab 1 Ting 5Dokumen6 halamanModul Strategik Bab 1 Ting 5SK Pos TenauBelum ada peringkat
- The Science of Laughing - Worksheet 3Dokumen3 halamanThe Science of Laughing - Worksheet 3Maria Alexandra PoloBelum ada peringkat
- Long-Term Memory Encoding and RetrievalDokumen24 halamanLong-Term Memory Encoding and RetrievalThavasi mari selvam NBelum ada peringkat
- Answer Key For Comprehensive Exam XVDokumen18 halamanAnswer Key For Comprehensive Exam XVQharts SajiranBelum ada peringkat