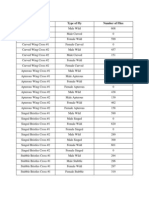
Biology - Diffusion Through Membranes Lab Analysis Questions
Diunggah oleh
lanichungHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Biology - Diffusion Through Membranes Lab Analysis Questions
Diunggah oleh
lanichungHak Cipta:
Format Tersedia
Chung 1
Lani Chung Mr. Nakamura Biology HL Period 2B 4 February 2012 Diffusion through Membranes Lab Analysis Questions 1. What conclusion can you draw from the data in Table 2? When the concentration of salt increased from 1% to 5% and then to 10% concentration, the rate of diffusion consistently increased by about 2 mg/L/s at each step. This might be attributed to the way diffusion occurs faster when there is a higher concentration inside or outside of a membrane because a greater amount of molecules are required to flow across the membrane for equilibrium to be reached. Thus there is a direct correlation between salt concentration and the rate of diffusion across a membrane: As salt concentration increases, the rate of diffusion increases. 2. How did your conclusion compare to your prediction for Part I? Can you account for any differences? My prediction proved to be correct as I predicted that as salt concentration increases, the rate of diffusion will increase because greater concentrations of salt will need to flow into the membrane. Just as I had predicted, the results showed that as the salt concentration was gradually increased from 5-10%, the rate of diffusion had also increased steadily. 3. If the rates in any of the three experiments varied in Part I, calculate how much faster each rate was compared to that for the 1% salt solution. For instance, if the rate of the 1% solution was 1 s/s, then the rate of diffusion for the 10% solution would be (5/1) five times the rate of the 1% salt solution.
Chung 2
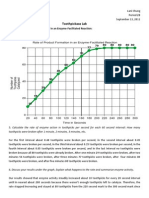
In the 1% salt concentration test the rate of diffusion was about 1 mg/L/s, in the 5% it was about 3 mg/L/s, and in the 10% it was about 5 mg/L/s. With these rounded estimates, you can say that the rate of the 5% salt concentration test was about three times that of the 1% salt concentration test and the rate of the10% salt concentration test was about five times that of the 1% salt concentration test. 4. Compare the ionic concentration of pure water with a sugar water solution. How do you account for this? The ionic concentration of water is 572 mg/L while the ionic concentration of sugar solution is 506 mg/L. This can be due to the fact that the water that was tested was not purified or distilled. Since tap water normally has some dissolved molecules, it is quite possible that the conductivity probe detected the ions found in the water. However, if the water had been distilled like it was supposed to, there is a great likelihood that the sugar solution would have a higher ionic concentration than that of the water. This is because sugar ions would be present in the sugar solution while no ions would be present in the distilled water. 5. What conclusion can you draw from the data in Tables 3 and 4? Through the data in tables 3 and 4, I can conclude that sugar can be an inhibitor as it prevented the 5% salt concentration from diffusing through the membrane since the rate of diffusion had seen a decrease from 3.1946 mg/L/s to 2.5076 mg/L/s. I can also infer that the ionic concentration of a solution can have an impact on the rate of diffusion of a substance through a membrane.
Anda mungkin juga menyukai
- Act 3 Gollon, Hanz Chua, ValiantDokumen5 halamanAct 3 Gollon, Hanz Chua, Valiants9036282Belum ada peringkat
- Lab Report 2Dokumen5 halamanLab Report 2Jessica TysonBelum ada peringkat
- Discussion: Replace With StudyDokumen1 halamanDiscussion: Replace With StudyjoaBelum ada peringkat
- Salting In, Salting Out, and Dialysis of ProteinsDokumen5 halamanSalting In, Salting Out, and Dialysis of ProteinsspeknatsBelum ada peringkat
- Act 3Dokumen4 halamanAct 3Rose CostaBelum ada peringkat
- Osmosis Lab ReportDokumen4 halamanOsmosis Lab ReportDean DoneenBelum ada peringkat
- The Effects of Concentration of Glucose and Temperature On The Rate of OsmosisDokumen4 halamanThe Effects of Concentration of Glucose and Temperature On The Rate of OsmosisAisha Nicole Saito50% (2)
- Summative Photosynthesis LabDokumen4 halamanSummative Photosynthesis Labapi-201421417Belum ada peringkat
- Acid-Base Lab ReportDokumen6 halamanAcid-Base Lab Reportapi-279614944Belum ada peringkat
- Diffusion LabDokumen7 halamanDiffusion LabtinkerloveBelum ada peringkat
- Lab Introduction Title:: Magna?Dokumen3 halamanLab Introduction Title:: Magna?Alex LeeBelum ada peringkat
- Laboratory 8: Effect of Temperature and PH On Amylase ActivityDokumen4 halamanLaboratory 8: Effect of Temperature and PH On Amylase ActivityPedro Pepe Perez Munoz LopezBelum ada peringkat
- Movement in and Out of The Cells (Diffusion, Osmosis)Dokumen12 halamanMovement in and Out of The Cells (Diffusion, Osmosis)Mentari Ulfah100% (1)
- Enzyme Liver Catalase Hydrogen PeroxideDokumen9 halamanEnzyme Liver Catalase Hydrogen PeroxideCarmen Muir100% (2)
- Biochemistry Experiment On OsmosisDokumen5 halamanBiochemistry Experiment On OsmosisEna FarillasBelum ada peringkat
- Rates of Reaction Experiment v.1.02Dokumen6 halamanRates of Reaction Experiment v.1.02Muhammad Bilal AnwarBelum ada peringkat
- Tintin's 1ST Lab Act BioDokumen2 halamanTintin's 1ST Lab Act BioChristine Jade TanBelum ada peringkat
- Osmosis Coursework ConclusionDokumen5 halamanOsmosis Coursework Conclusionafaydoter100% (2)
- Diffusion and Osmosis LabDokumen2 halamanDiffusion and Osmosis Labkcinvincibleman83% (6)
- Factors Influenc, Ing The Reduction of Alkaline Copper Reagents by GlucoseDokumen18 halamanFactors Influenc, Ing The Reduction of Alkaline Copper Reagents by GlucoseNurul Aulia HusainBelum ada peringkat
- Physiological Practical 1Dokumen4 halamanPhysiological Practical 1Khezia ChalweBelum ada peringkat
- Labreport 2Dokumen9 halamanLabreport 2Anne MasangkayBelum ada peringkat
- Save NemoDokumen8 halamanSave Nemoapi-358984084Belum ada peringkat
- Diffusion Grade 9Dokumen9 halamanDiffusion Grade 9Mario ButlerBelum ada peringkat
- Department of Other Natural Science Lab Activity 3 Course Code Biol 1012Dokumen12 halamanDepartment of Other Natural Science Lab Activity 3 Course Code Biol 1012Aysheshim NegaBelum ada peringkat
- Rishika Individual InvetigationDokumen9 halamanRishika Individual InvetigationAviman Pratap SinghBelum ada peringkat
- Lab 7 - Diffusion and OsmosisDokumen2 halamanLab 7 - Diffusion and Osmosisallicockn50% (2)
- Deborah Chen December 17, 2012 AP Biology C BlockDokumen4 halamanDeborah Chen December 17, 2012 AP Biology C BlockdebochenBelum ada peringkat
- Bokashi Balls Lab RepDokumen5 halamanBokashi Balls Lab RepIris BercesBelum ada peringkat
- Activity 2 - Biochemical Processes (Revised 6.8.20)Dokumen6 halamanActivity 2 - Biochemical Processes (Revised 6.8.20)Sherma Sheikh karimBelum ada peringkat
- Biology Coursework Potato OsmosisDokumen6 halamanBiology Coursework Potato Osmosisguv0k1jemen3100% (2)
- Enzyme Core PracticalDokumen7 halamanEnzyme Core PracticalJackHowley123Belum ada peringkat
- Laboratory Experiment 1Dokumen6 halamanLaboratory Experiment 1Rutchelle Desiree SenadonBelum ada peringkat
- Env PracticalDokumen21 halamanEnv PracticalHassan juttBelum ada peringkat
- AP Biology Diffusion and Osmosis Lab ReportDokumen7 halamanAP Biology Diffusion and Osmosis Lab ReportVictor Martin100% (1)
- Transportation Across Plasma Membrane Lab ReportDokumen13 halamanTransportation Across Plasma Membrane Lab Reportamansempoi33% (3)
- Chemistry Investigatiry 2019-20Dokumen13 halamanChemistry Investigatiry 2019-20kings KingsBelum ada peringkat
- Exercise No. 1 Movement of Substance Through Cell Membrane Experiment Demonstrating DiffusionDokumen8 halamanExercise No. 1 Movement of Substance Through Cell Membrane Experiment Demonstrating DiffusionBernadette VillacampaBelum ada peringkat
- Importance of Alkalinity ReportDokumen14 halamanImportance of Alkalinity ReportAayush YadavBelum ada peringkat
- Lab Report 5 Kita!!!Dokumen10 halamanLab Report 5 Kita!!!Zaidi Zakaria100% (1)
- Activity Lab 1Dokumen10 halamanActivity Lab 1Chamae IsagaBelum ada peringkat
- FR GREEN Notebook 041511 PhosphorousDokumen6 halamanFR GREEN Notebook 041511 PhosphorousLoh Ri JianBelum ada peringkat
- DissolvedoxygenlabDokumen7 halamanDissolvedoxygenlabapi-242403741Belum ada peringkat
- Laboratory Experiment No. 11 - Transport Across MembranesDokumen4 halamanLaboratory Experiment No. 11 - Transport Across MembranesMary Rose CatalbasBelum ada peringkat
- Watertreatmentsimulationandanalysis EllismcnicholDokumen5 halamanWatertreatmentsimulationandanalysis Ellismcnicholapi-302400368Belum ada peringkat
- Analyzing An Acid SpillDokumen10 halamanAnalyzing An Acid SpillMolly Zhang100% (1)
- Lab 1 - Diffusion and Osmosis Write Up - AP BiologyDokumen2 halamanLab 1 - Diffusion and Osmosis Write Up - AP BiologyFVCproductions90% (10)
- A&P Lab ReportDokumen2 halamanA&P Lab ReportddevilswayBelum ada peringkat
- Chemistry Lab Report (Back Titration)Dokumen8 halamanChemistry Lab Report (Back Titration)api-28215708280% (10)
- Bio Lab1Dokumen9 halamanBio Lab1David SolingerBelum ada peringkat
- Pex 01 03Dokumen4 halamanPex 01 03Jarren BautistaBelum ada peringkat
- Protein Precipitation by SaltsDokumen5 halamanProtein Precipitation by SaltsRüveyda Akçin100% (3)
- IB HL Biology Mock IADokumen9 halamanIB HL Biology Mock IAeleni.papazarifiBelum ada peringkat
- O Level Biology Practice Questions And Answers Movement of substancesDari EverandO Level Biology Practice Questions And Answers Movement of substancesBelum ada peringkat
- Grupo 1Dokumen14 halamanGrupo 1Suspirito MorochoBelum ada peringkat
- Osmosis Coursework GcseDokumen4 halamanOsmosis Coursework Gcseafazamfbk100% (2)
- Understanding pH Levels And Their Link To CancerDari EverandUnderstanding pH Levels And Their Link To CancerPenilaian: 4.5 dari 5 bintang4.5/5 (3)
- Fluids and Electrolytes: An Easy and Intuitive Way to Understand and Memorize Fluids, Electrolytes, and Acidic-Base BalanceDari EverandFluids and Electrolytes: An Easy and Intuitive Way to Understand and Memorize Fluids, Electrolytes, and Acidic-Base BalancePenilaian: 5 dari 5 bintang5/5 (2)
- Letter To The EditorDokumen1 halamanLetter To The EditorlanichungBelum ada peringkat
- Anthropology - Essay 1Dokumen6 halamanAnthropology - Essay 1lanichungBelum ada peringkat
- Chemistry - GraphDokumen1 halamanChemistry - GraphlanichungBelum ada peringkat
- Bible Study WorshipDokumen2 halamanBible Study WorshiplanichungBelum ada peringkat
- FHPM - Employer Mandate SummaryDokumen2 halamanFHPM - Employer Mandate SummarylanichungBelum ada peringkat
- Chemistry - GraphDokumen1 halamanChemistry - GraphlanichungBelum ada peringkat
- B'More - Photograph CritiquesDokumen1 halamanB'More - Photograph CritiqueslanichungBelum ada peringkat
- B'More - Photograph CritiquesDokumen1 halamanB'More - Photograph CritiqueslanichungBelum ada peringkat
- Biology - The Genetics of Parenthood AnalysisDokumen2 halamanBiology - The Genetics of Parenthood Analysislanichung100% (5)
- Biology - Genetics LimerickDokumen1 halamanBiology - Genetics LimericklanichungBelum ada peringkat
- TOK - EssayDokumen6 halamanTOK - EssaylanichungBelum ada peringkat
- B'More - Artist's StatementDokumen1 halamanB'More - Artist's StatementlanichungBelum ada peringkat
- Biology - Spring Break Reading AssignmentDokumen17 halamanBiology - Spring Break Reading AssignmentlanichungBelum ada peringkat
- Biology - Reaction Time Lab Analysis QuestionsDokumen3 halamanBiology - Reaction Time Lab Analysis Questionslanichung67% (3)
- Biology - The Genetics of Parenthood AnalysisDokumen2 halamanBiology - The Genetics of Parenthood Analysislanichung100% (5)
- Biology - Fly Lab Raw Data CollectionDokumen3 halamanBiology - Fly Lab Raw Data CollectionlanichungBelum ada peringkat
- Biology - North Pole Cell AnalogyDokumen1 halamanBiology - North Pole Cell AnalogylanichungBelum ada peringkat
- Biology - Genetics LimerickDokumen1 halamanBiology - Genetics LimericklanichungBelum ada peringkat
- Biology - Toothpickase LabDokumen3 halamanBiology - Toothpickase LablanichungBelum ada peringkat
- Biology - Genetics Review 2Dokumen15 halamanBiology - Genetics Review 2lanichung100% (1)
- Biology - Heart Rate Inquiry LabDokumen2 halamanBiology - Heart Rate Inquiry LablanichungBelum ada peringkat
- Biology - Fly Lab ResultsDokumen17 halamanBiology - Fly Lab ResultslanichungBelum ada peringkat
- Biology - Cell LimerickDokumen1 halamanBiology - Cell LimericklanichungBelum ada peringkat
- Biology - Enzyme Lab ConclusionDokumen4 halamanBiology - Enzyme Lab ConclusionlanichungBelum ada peringkat
- Biology - DNA MessageDokumen1 halamanBiology - DNA MessagelanichungBelum ada peringkat
- Biology - Design A FlowerDokumen1 halamanBiology - Design A FlowerlanichungBelum ada peringkat
- Expository Writing - Assignment 1.2 RevisionsDokumen3 halamanExpository Writing - Assignment 1.2 RevisionslanichungBelum ada peringkat
- Expository Writing - Writing Assignment 3.2Dokumen2 halamanExpository Writing - Writing Assignment 3.2lanichungBelum ada peringkat
- Chung Assignment 2.3Dokumen2 halamanChung Assignment 2.3lanichungBelum ada peringkat
- CHAPTER 1 2 and 3Dokumen78 halamanCHAPTER 1 2 and 3Fider Mesina Saldaña100% (1)
- Example of Critical Appraisal of Qualitative Research PaperDokumen8 halamanExample of Critical Appraisal of Qualitative Research Papergz8bjzmjBelum ada peringkat
- 4 Ways To Contribute To The Body of KnowledgeDokumen1 halaman4 Ways To Contribute To The Body of KnowledgeJoshua HernandezBelum ada peringkat
- Industrial Management Ehu601Dokumen2 halamanIndustrial Management Ehu601AyushiBelum ada peringkat
- The Bender Gestalt Test: An Investigation Into Problems Concerning Administration and Scoring and Its Application To Low-Educated AdultsDokumen284 halamanThe Bender Gestalt Test: An Investigation Into Problems Concerning Administration and Scoring and Its Application To Low-Educated AdultsJOY POMERBelum ada peringkat
- Research Process Notes PDFDokumen18 halamanResearch Process Notes PDFCLAUDINE MUGABEKAZIBelum ada peringkat
- Class Size and Teaching Competency Template 1 1Dokumen8 halamanClass Size and Teaching Competency Template 1 1noelroajrBelum ada peringkat
- A Study On Customer Satisfaction Towards Organised and Unorganised RetailingDokumen5 halamanA Study On Customer Satisfaction Towards Organised and Unorganised RetailingMohammed WaseemBelum ada peringkat
- Nursing Research1: Dr. Josielyn P. Maligalig.,RnDokumen27 halamanNursing Research1: Dr. Josielyn P. Maligalig.,RnKatherine Gayle Guia100% (1)
- Midas Civil - Analysis ReferenceDokumen400 halamanMidas Civil - Analysis ReferenceMohd FaizalBelum ada peringkat
- A Systematic Guideline For Developing The Best Real-Time PCR PrimersDokumen9 halamanA Systematic Guideline For Developing The Best Real-Time PCR PrimersSamBelum ada peringkat
- Research Paper Accounting CareerDokumen8 halamanResearch Paper Accounting Careerefhs1rd0100% (1)
- Iso Asme 14414-2015Dokumen62 halamanIso Asme 14414-2015Kuya Fabio VidalBelum ada peringkat
- AGMA 2015 915-1-A02Dokumen108 halamanAGMA 2015 915-1-A02danielk32100% (1)
- Rubrics For Solving Complex Engineering Problem: Unsatisfactory 0 Satisfactory 1 Good 2 Very Good 3 Comprehensive 4Dokumen2 halamanRubrics For Solving Complex Engineering Problem: Unsatisfactory 0 Satisfactory 1 Good 2 Very Good 3 Comprehensive 4Asfahan AliBelum ada peringkat
- GD&T Versus Geometrical Product Spec PDFDokumen14 halamanGD&T Versus Geometrical Product Spec PDFHusen Taufiq100% (2)
- MATH 141 Webworks .Set1Dokumen2 halamanMATH 141 Webworks .Set1razanmk961214Belum ada peringkat
- Basics of Statistical Inference: Drawing Inferences From A Starbucks SurveyDokumen28 halamanBasics of Statistical Inference: Drawing Inferences From A Starbucks SurveyDipesh MundhraBelum ada peringkat
- 3is 2Dokumen39 halaman3is 2Dyan PerezBelum ada peringkat
- P&S Unit-I 2023-24Dokumen4 halamanP&S Unit-I 2023-24kartheekmurala19Belum ada peringkat
- Red Label Tea: Group 4Dokumen9 halamanRed Label Tea: Group 4shruti100% (2)
- Human Growth Development and NutritionDokumen13 halamanHuman Growth Development and Nutritionm_anindoBelum ada peringkat
- Lived Experience of Orphan ChildrenDokumen89 halamanLived Experience of Orphan Childrenlord19_s100% (1)
- Module 7 Sampling DistributionDokumen22 halamanModule 7 Sampling DistributionvanessaBelum ada peringkat
- Trends in MarketingDokumen10 halamanTrends in Marketingnaushadali18Belum ada peringkat
- Statistics 101Dokumen28 halamanStatistics 101Carlo Carlo100% (1)
- Research Lecture NotesDokumen4 halamanResearch Lecture NotesNelsie DuhilagBelum ada peringkat
- External Auditing As A Supporting Tool For ManagementDokumen54 halamanExternal Auditing As A Supporting Tool For ManagementTasmiaBelum ada peringkat
- Proficiency StageDokumen10 halamanProficiency StageFLORITA SERRANO50% (2)
- 103Dokumen9 halaman103ieom2012Belum ada peringkat