CHM3102 - Condensation Polymerization Experiment
Diunggah oleh
Luqman HakimDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
CHM3102 - Condensation Polymerization Experiment
Diunggah oleh
Luqman HakimHak Cipta:
Format Tersedia
CHM3102 Polymers in Chemistry
EXPERIMENT 1 : CONDENSATION POLYMERIZATION
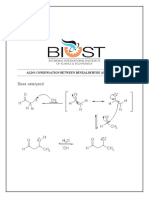
Objective : To understand by explanation about a polyamide synthesis especially by the low-temperature polycondensation method To understand the parameters used to change the polymerization conditions To better understand the molecule mass average calculation from various monoscattered samples Methodology : Set 1 5 ml sebasil chloride (B was put in a small bea!er "#1 g of soap powder was diluted in $ ml hexamethylenediamine (% (% was then mixed into (B dropwise % thin layer formed in the interface of the non-mixable solution The polymer was rolled using a glass rod or a stic! Set & 'rocedures are as that of set 1( with an exception of 5 ml of (B was mixed with 1 ml of (% with constant stirring# Set $ 'rocedures are as that of set 1( with an exception of 5 ml of (B was mixed with 1 ml of (% containing half of )a*+ with stirring# Result: Calculations : )+&)(,+& -)+& . n,l*,(,+& /,*,l Sebasil chloride +---[NH(CH2)6NHOC(CH2)8CO]n---,l . &(n-1 +,l )ylon -(1"
+examethylenediamine
,oncentration for 0% (sebasil chloride 1 "#2 0
Irwin bin Ishak - Page 1 of 4
CHM3102 Polymers in Chemistry
,oncentration for 0B (hexamethylenediamine 1 "#"- 0 3ield 4 1 weight of polymer ( number of moles ( weight of repeating unit 5eight of repeating unit 1 12#""6 . 1#""/ . -71&#"11 . &(1#""/ 8 . 12#""6 . 1#""/ . 15#999 . 1&#"11 . /71&#"11 . &(1#""/ 8 . 1&#"11 . 15#999 1 $1"#2/& g mol-1 The number of moles for % 1 0:;1""" 1 ("#2 ($#" ;1""" 1 1#& x 1"-$ moles The number of moles for B 1 0:;1""" 1 ("#"- (5#" ;1""" 1 $#" x 1"-2 moles Therefore( 3ield 4 (set 1 1 "#15&6;7(1#5x1"-$ x $1"#2/&8 x 1"" 1 $/4 3ield 4 (set & 1 "#1$1&;7(1#5x1"-$ x $1"#2/&8 x 1"" 1 &/#1/4 3ield 4 (set $ 1 "#"91&;7(1#5x1"-$ x $1"#2/&8 x 1"" 1 19#594 Discussion : <rom this experiment( students can train themselves to actually prepare a polymer( where in this experiment( )ylon( in the lab by using a method called the interface polycondensation# The hypothesis for this experiment is that the yield of )ylon for set & should be the highest( whereas for set $( the yield is the lowest# This experiment can be considered as a failure since the yield for set & was slightly lower than set 1( maybe due to minor errors that occurred while the experiment was done# =xplanation for the difference in yields got from all three sets can be made# Set & should have the highest yield form because of the stirring# This is because by stirring( not only the !inetic energy was increased( new interfaces will appear with every round of stirring# This explains why set & was more efficient than set 1# <or set $( with the presence of )a*+( appeared some sort of >competition? in the reaction# The reaction between sebasil chloride and hexamethylenediamine x 1""
Irwin bin Ishak - Page 2 of 4
CHM3102 Polymers in Chemistry
was interfered because the )a*+ will actually gain opportunity to react with both of them to form more stable compounds# Thus( the competition causes the yield to be lower# The physical properties of the polymers were also observed# The differences were caused of the different compositions used in the experiment# The nylon formed in set 1 has @uite a low elasticity( as well as the bending force and the adhering force# The same goes with set &# *n the other hand( for set $( the presence of )a*+ actually caused the polymer formed to be more elastic( has a better bending force as well as adhering force#
Precaution Steps : =verything used in this experiment was assured to be absolutely clean to avoid unwanted reactions to occur Solution % must be poured into solution B dropwise to produce as much polymer as possible Conclusion : )ylon -(1" was produced in a small scale in the lab by using the low temperature interface polycondensation method# Set 1 produced $/4 amount of )ylon Set & produced &/#1/4 amount of )ylon Set $ produced 19#594 amount of )ylon =very polymer produced were observed of their featured and discussed The experiment was considered inaccurate References : A#B#:# 'ar!er( 'olymer ,hemistry( %pplied Science 'ublisher Btd# Bondon( 1962 C#5#D# ,owie( 'olymers E ,hemistry and 'hysics of 0odern 0aterial
Q& : 1# The reaction e@uilibrium for the production of )ylon -(-( nylon -(1" and )ylon -(11 are as follows E )ylon -(- E n+&)(,+& -)+& . n+**,(,+& 2,**+ )ylon -(1" E n+&)(,+& -)+& . n,l*,(,+& /,*,l )ylon -(11 E +--7--)+(,+& -)+*,(,+& /,*--8n--,l . &(n-1 +,l +--7--)+(,+& -)+*,(,+& 2,*--8n--*+ . &(n-1 +&*
Irwin bin Ishak - Page 3 of 4
CHM3102 Polymers in Chemistry
n+&)(,+& -)+& . n+**,(,+& 9,**+
+--7--)+(,+& -)+*,(,+& 9,*--8n--*+ . &(n-1 +&*
&# )+&)(,+& -)+& . +examethylenediamine
n,l*,(,+& /,*,l Sebasil chloride
+---7)+(,+& -)+*,(,+& /,*8n---,l . &(n-1 +,l )ylon -(1" 'oly (hexamethylene sebasate
$# The same stoi!iometry was not re@uired for all reactants#
Irwin bin Ishak - Page 4 of 4
Anda mungkin juga menyukai
- Christmas Around the WorldDokumen16 halamanChristmas Around the WorldVioleta Veljanovska100% (1)
- Determining Epoxide and Amine ValuesDokumen3 halamanDetermining Epoxide and Amine Valuesiqbalpec87210% (1)
- Determination of Complex Composition Using Job MethodDokumen10 halamanDetermination of Complex Composition Using Job Methodnawal200750% (8)
- O Level Biology Practice Questions And Answers EnzymesDari EverandO Level Biology Practice Questions And Answers EnzymesPenilaian: 5 dari 5 bintang5/5 (1)
- M1 Exam Portage LearningDokumen5 halamanM1 Exam Portage Learningsophia onu0% (1)
- Présentation Transportation ManagementDokumen14 halamanPrésentation Transportation ManagementHiba Hmito100% (1)
- Partial Molar Volume of Ethanol-Water SolutionsDokumen24 halamanPartial Molar Volume of Ethanol-Water SolutionsIlyas Hassan100% (1)
- Redox TitrationDokumen14 halamanRedox Titrationnorsiah100% (2)
- Distillation Column DesignDokumen20 halamanDistillation Column DesignSandeep Challa100% (1)
- Online Physical Science Class: Week 6Dokumen56 halamanOnline Physical Science Class: Week 6Camille ManlongatBelum ada peringkat
- Modeling of Soil-Structure Interaction as Finite Element Using SAP2000Dokumen5 halamanModeling of Soil-Structure Interaction as Finite Element Using SAP2000Tariq MahmoodBelum ada peringkat
- 15 Limiting ReactantsDokumen15 halaman15 Limiting ReactantsNur AmrinaBelum ada peringkat
- Percent YieldDokumen6 halamanPercent YieldDiana Jane Terez LazaroBelum ada peringkat
- Multistep Lab ReportDokumen10 halamanMultistep Lab Reportapi-508753814Belum ada peringkat
- Code: Name:: Experiment 1. The Synthesis of D, L-Phenylglycine and Its Optical Resolution Total Scores: 100 PointsDokumen5 halamanCode: Name:: Experiment 1. The Synthesis of D, L-Phenylglycine and Its Optical Resolution Total Scores: 100 Pointsthanhhotboy98Belum ada peringkat
- Format of Lab Report Example 8609Dokumen14 halamanFormat of Lab Report Example 8609herrk167% (3)
- EMT3701 Major Test 1Dokumen5 halamanEMT3701 Major Test 1infinitewealth001Belum ada peringkat
- Laboratory Report 3Dokumen5 halamanLaboratory Report 3api-355292365Belum ada peringkat
- Biodiesel Production in A Batch Reactor: 1. TheoryDokumen8 halamanBiodiesel Production in A Batch Reactor: 1. TheoryNazareno BragaBelum ada peringkat
- Robinson Annulation Synthesis of DimedoneDokumen2 halamanRobinson Annulation Synthesis of DimedoneAdhveer DomanlallBelum ada peringkat
- Investigation of CatalystsDokumen7 halamanInvestigation of CatalystsInaki Voelcker-SalaBelum ada peringkat
- Diels Alder ReactionDokumen1 halamanDiels Alder ReactionPantaleon PacisBelum ada peringkat
- Mixture and Alligation Score Up PDF For SSC Exam Dream Big Institution-CompressedDokumen19 halamanMixture and Alligation Score Up PDF For SSC Exam Dream Big Institution-CompressedSatyabrata SahuBelum ada peringkat
- Science Home Learning: L10 Atom Economy Year: 10 (Triple Only)Dokumen8 halamanScience Home Learning: L10 Atom Economy Year: 10 (Triple Only)Omar ElmasryBelum ada peringkat
- Laboratory Exercise 5.1Dokumen10 halamanLaboratory Exercise 5.1EUNICE JOI SARCONBelum ada peringkat
- Filmtec Dowex: Membranes and Ion Exchange ResinsDokumen27 halamanFilmtec Dowex: Membranes and Ion Exchange ResinsmscottgreenBelum ada peringkat
- EnzymeDokumen9 halamanEnzymepooyenpengBelum ada peringkat
- Experiment 4 SCH 213 02Dokumen11 halamanExperiment 4 SCH 213 02api-530322563Belum ada peringkat
- Emp Mol PacketDokumen7 halamanEmp Mol PacketKrizzia CarlosBelum ada peringkat
- Column Configuration and Reaction System DesignDokumen16 halamanColumn Configuration and Reaction System DesignPrashant DasBelum ada peringkat
- Effect of poly(acrylic acid) on mechanical properties of LDPE-nanoclay compositesDokumen15 halamanEffect of poly(acrylic acid) on mechanical properties of LDPE-nanoclay compositesPham Thi Thu HongBelum ada peringkat
- Stoichiometry: Angel Jane A. RoulloDokumen39 halamanStoichiometry: Angel Jane A. RoulloLoren EsguerraBelum ada peringkat
- 4.1 Exercise 1Dokumen2 halaman4.1 Exercise 1ridithaBelum ada peringkat
- 1PGenChem Learning ModuleDokumen7 halaman1PGenChem Learning ModuleAngie ReblandoBelum ada peringkat
- Model Exam Chemistry QuestionsDokumen2 halamanModel Exam Chemistry QuestionsManish GuptaBelum ada peringkat
- CHU11102 - Lab Report - Preparation of Menthene by The Dehydration of MentholDokumen7 halamanCHU11102 - Lab Report - Preparation of Menthene by The Dehydration of Mentholconorfernandez04Belum ada peringkat
- Selective Extraction of Benzene From Benzene-Cyclohexane Mixture Using 1-Ethyl-3-Methylimidazolium Tetrafluoroborate Ionic LiquidDokumen8 halamanSelective Extraction of Benzene From Benzene-Cyclohexane Mixture Using 1-Ethyl-3-Methylimidazolium Tetrafluoroborate Ionic LiquidBüşraBelum ada peringkat
- Synthesis of Vinyl Acetate-Co-Butyl Acrylate Latexes: Investigation of The Effects of Polymerization Temperature On The Latex PropertiesDokumen4 halamanSynthesis of Vinyl Acetate-Co-Butyl Acrylate Latexes: Investigation of The Effects of Polymerization Temperature On The Latex PropertiesSinan KARADEMİRBelum ada peringkat
- BCHCT-135 Chemistry IgnouDokumen8 halamanBCHCT-135 Chemistry IgnouviploveBelum ada peringkat
- #15 Lab ReportDokumen6 halaman#15 Lab ReportAli Ib TarshaBelum ada peringkat
- Chemistry Question 12sciDokumen38 halamanChemistry Question 12sciAnsari SameerBelum ada peringkat
- Chem 101 Exp3 Limiting Reagent Fall 2015Dokumen6 halamanChem 101 Exp3 Limiting Reagent Fall 2015Nahyan Akhtar MemonBelum ada peringkat
- Parametric Sensitivity Study of A Butyl Acrylate Emulsion Polymerization ModelDokumen6 halamanParametric Sensitivity Study of A Butyl Acrylate Emulsion Polymerization ModelInternational Journal of Innovative Science and Research TechnologyBelum ada peringkat
- LE 005 007 General Chemistry 1 Continuation .Updated FinalDokumen26 halamanLE 005 007 General Chemistry 1 Continuation .Updated FinalShaman KingBelum ada peringkat
- Autumn Chemistry Online Tournament Multiple Choice QuestionsDokumen12 halamanAutumn Chemistry Online Tournament Multiple Choice QuestionsAnju GuptaBelum ada peringkat
- CHM 3402 Experiment 2Dokumen13 halamanCHM 3402 Experiment 2Luqman HakimBelum ada peringkat
- Preparation of Monodisperse Silica Particles With Controllable Size and ShapeDokumen5 halamanPreparation of Monodisperse Silica Particles With Controllable Size and ShapeBenni WewokBelum ada peringkat
- Lab Report WittigDokumen5 halamanLab Report WittigBaizhen Zhu100% (2)
- Pre LabghvyicDokumen6 halamanPre LabghvyicGobe JamBelum ada peringkat
- Effect of Compatibiliser, Curing Sequence and Ageing On The Thermal Stability of Silicone Rubber, EPDM Rubber and Their BlendsDokumen8 halamanEffect of Compatibiliser, Curing Sequence and Ageing On The Thermal Stability of Silicone Rubber, EPDM Rubber and Their BlendsThai KhangBelum ada peringkat
- Classification Tests For Hydroxyl and Carbonyl Containing CompoundsDokumen5 halamanClassification Tests For Hydroxyl and Carbonyl Containing CompoundsliaprielaBelum ada peringkat
- Chemistry Paper 1 Questions and AnswersDokumen24 halamanChemistry Paper 1 Questions and AnswersSueBelum ada peringkat
- Process Variables I: University of The Philippines DilimanDokumen4 halamanProcess Variables I: University of The Philippines DilimanAcademicBMBelum ada peringkat
- Chem/Biochem 471 Exam 2 11/14/07: NameDokumen7 halamanChem/Biochem 471 Exam 2 11/14/07: Namedaravar1Belum ada peringkat
- Percent Yield: Chemfile Mini-Guide To Problem SolvingDokumen11 halamanPercent Yield: Chemfile Mini-Guide To Problem SolvingdhavaleshBelum ada peringkat
- Enzyme Kinetics Examples and ProblemsDokumen4 halamanEnzyme Kinetics Examples and Problemskiiadizon07100% (1)
- Chemical Mole WorkDokumen5 halamanChemical Mole WorkMiekBelum ada peringkat
- CH141 Exam 1 Practice QuestionsDokumen5 halamanCH141 Exam 1 Practice QuestionsHarrison SawyerBelum ada peringkat
- Bromination ExperimentDokumen9 halamanBromination Experimentch_ymyaaBelum ada peringkat
- Matriculation Chemistry Polymers PDFDokumen19 halamanMatriculation Chemistry Polymers PDFiki292Belum ada peringkat
- Chapter 5 Lec 4Dokumen3 halamanChapter 5 Lec 4IsaBelum ada peringkat
- Sustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeDari EverandSustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeBelum ada peringkat
- Self-Assembly: From Surfactants to NanoparticlesDari EverandSelf-Assembly: From Surfactants to NanoparticlesRamanathan NagarajanBelum ada peringkat
- Exp 8.doctoronDokumen5 halamanExp 8.doctoronLuqman HakimBelum ada peringkat
- Module 4: Understanding Complex TitrationDokumen18 halamanModule 4: Understanding Complex TitrationLuqman HakimBelum ada peringkat
- Determination of pKa of Bromothymol BlueDokumen9 halamanDetermination of pKa of Bromothymol BlueLuqman HakimBelum ada peringkat
- CHM 3402 Experiment 2Dokumen13 halamanCHM 3402 Experiment 2Luqman HakimBelum ada peringkat
- Module 4: Understanding Complex TitrationDokumen18 halamanModule 4: Understanding Complex TitrationLuqman HakimBelum ada peringkat
- Le ChatelierDokumen2 halamanLe ChatelierLuqman HakimBelum ada peringkat
- Preparation and Precipitation of Lyophobic ColloidDokumen12 halamanPreparation and Precipitation of Lyophobic ColloidLuqman Hakim50% (2)
- Topic+5 SolutionDokumen59 halamanTopic+5 SolutionLuqman HakimBelum ada peringkat
- TGN Level 1 No. 5 Derivation of Snow LoadDokumen4 halamanTGN Level 1 No. 5 Derivation of Snow LoadjeddijBelum ada peringkat
- Iso 1924 2 2008Dokumen11 halamanIso 1924 2 2008Pawan Kumar SahaBelum ada peringkat
- Experimental Design and Optimization MethodsDokumen38 halamanExperimental Design and Optimization MethodssudalaiyandiBelum ada peringkat
- Iot Finals Clap Switch Group 5Dokumen15 halamanIot Finals Clap Switch Group 5RICHYBOY SALACBelum ada peringkat
- Automatic Transaxle PDFDokumen50 halamanAutomatic Transaxle PDFdemos70100% (1)
- Colistimethate Sodium 1 Million I.U. Powder For Solution For Injection - Colistin - (Emc)Dokumen8 halamanColistimethate Sodium 1 Million I.U. Powder For Solution For Injection - Colistin - (Emc)hakim shaikhBelum ada peringkat
- 43-101 Technical Report Quimsacocha, February 2009Dokumen187 halaman43-101 Technical Report Quimsacocha, February 2009Marco Vinicio SotoBelum ada peringkat
- CodigosDokumen73 halamanCodigosEnzo Miguel Sarabia MontesBelum ada peringkat
- Research PaperDokumen13 halamanResearch PaperHamid ElmyBelum ada peringkat
- Omcmle Physiology Workbook Part 5 PDFDokumen63 halamanOmcmle Physiology Workbook Part 5 PDFloiuse shepiralBelum ada peringkat
- Spcr-TagbayaganDokumen76 halamanSpcr-TagbayaganReycia Vic QuintanaBelum ada peringkat
- Pharmaceutics | Water Solubility and Dissolution RateDokumen11 halamanPharmaceutics | Water Solubility and Dissolution RateAnnisa AgustinaBelum ada peringkat
- LearnEnglish Video Zone How These Women Changed Science ForeverDokumen3 halamanLearnEnglish Video Zone How These Women Changed Science ForeverDaniella MensatoBelum ada peringkat
- Exercise Questions (Materials) .: BFT 112 Introduction To EngineeringDokumen1 halamanExercise Questions (Materials) .: BFT 112 Introduction To EngineeringSK DarsyanaaBelum ada peringkat
- AkzoNobel-Trigonox 239Dokumen6 halamanAkzoNobel-Trigonox 239Wafa AjiliBelum ada peringkat
- Error Correction - Test 1Dokumen4 halamanError Correction - Test 1phucnguyen0429Belum ada peringkat
- IB Chemistry HL Test 2nd FEBDokumen13 halamanIB Chemistry HL Test 2nd FEBprasad100% (1)
- Personal Care Na Hair GuideDokumen8 halamanPersonal Care Na Hair GuideIsabellaBelum ada peringkat
- Well Serve CingDokumen140 halamanWell Serve CingYounes MakBelum ada peringkat
- ElectrochemistryDokumen24 halamanElectrochemistryZainul AbedeenBelum ada peringkat
- JJ309 Fluid Mechanics Unit 6Dokumen30 halamanJJ309 Fluid Mechanics Unit 6Adib AzharBelum ada peringkat
- CalderaDokumen56 halamanCalderaEsteban TapiaBelum ada peringkat
- Calculator For SW Density Changes - Effect of List On Ships DraftDokumen3 halamanCalculator For SW Density Changes - Effect of List On Ships DraftHein Thurein KyawBelum ada peringkat
- Systematic Literature Review and Mapping of The Prediction of Pile CapacitiesDokumen12 halamanSystematic Literature Review and Mapping of The Prediction of Pile CapacitiesCaio Augusto Lemke CostaBelum ada peringkat
- Anorexia NervosaDokumen2 halamanAnorexia NervosaDhea Mae MadisBelum ada peringkat
- Tenofovir Disoproxil Fumarate: Riefing - Nfrared BsorptionDokumen4 halamanTenofovir Disoproxil Fumarate: Riefing - Nfrared BsorptionMostofa RubalBelum ada peringkat
- Telemark PulloverDokumen2 halamanTelemark Pulloverkidknits100% (1)