CC Lecture 1
Diunggah oleh
Merill Harrelson LibanJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
CC Lecture 1
Diunggah oleh
Merill Harrelson LibanHak Cipta:
Format Tersedia
1 CLINICAL CHEMISTRY 3 MIDTERMS :P 1/7/14 ADRENALS: Anatomy, physiology, and pathology Normal Anatomy - 2 small, yellowish bodies - Located
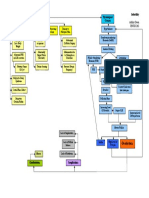
d in the perirenal space, immediately anterosuperior to the upper pole of the kidneys - Also known as the suprarenal glands - Location: at the posterior 12th thoracic vertebra - Weight: approximately 4 6 grams - 2 portions: adrenal cortex and adrenal medulla 2. Zona fasciculata - Middle zone (75%), thickest and located between the zona glomerulosa and zona reticularis. - Primary secretion: Glucocorticoids -> involved in increasing blood glucose levels. - Have additional effects in protein and fat metabolism. - The naturally synthesized glucocorticoids of most importance is cortisol. * DHEA: partly released in this zone 3. Zona reticularis - Innermost zone (10%), in between the zona fasciculata and medulla; interior layer - Primary secretion: Androgen - Main androgen is Dehydroepiandrosterone sulfate (DHEAS) 4. Capsule (5%) - Lining of adrenals not capable of secreting hormones. Hormones of the Adrenal Cortex - All adrenal cortex hormones are steroids - Not stored, synthesized only when needed. (needs stimulation -> production -> effect) * Cortex steroidogenesis
Layers: A. Adrenal cortex - Outer layer - Responsible for the regulation of salt, sugar, and sexual characteristics. - Hormones produced are known as corticosteroids. * Components of Corticosteroids: a. Mineralocorticoids: regulate salt b. Glucocorticoids: regulate sugar c. Androgens: regulate sexual characteristics - Cholesterol serve as the precursor for the production of corticosteroids. Microscopic Regions of the Adrenal Cortex: 1. Zona glomerulosa - Outermost zone (10%), located just below the adrenal cortex. - Secretes mineralocorticoids * Mineralocorticoids are aptly termed as they are involved in the regulation of electrolytes in ECF. - The naturally synthesized mineralocorticoids of most importance is aldosterone.
CRH (Hypothalamus) - Stimuli: - Serum cortisol - Circadian signals - Stress
ACTH (Anterior pituitary) - Stimulates transport of free cholesterol into adrenal mitochondria
Adrenal parenchymal cells Cholesterol enters into mitochondria
Cholesterol (Mitochondria)
Minus 6 carbons (CYP450 enzyme)
Pregnenolone
MHML
2 G Blood Pressure - mineralocorticoids - Serum Potassium Pregnenolone 3 Progesterone 21 F Blood Pressure - glucocorticoids - Glucose 17-OH Pregnenolone 3 17-OHase 3 c. Enhances reabsorption of sodium from the intestine especially in colon absence of aldosterone leads to diarrhea - no Na reabsorption -> continuous excretion of sodium with water * Regulation and control a. Renin Angiotensin System Stimulus: blood volume -> Renin secretion by juxtaglomerular cells -> reacts with angiotensinogen -> angiotensin I production (liver) -> passes through the lungs -> angiotensin converting enzyme (ACE) changes it to active form: angiotensin II: vasoconstriction, Na reabsorption by PCT, release Aldosterone Na reabsorption at DCT-> Angiotensin III -> more aldosterone release b. Potassium: K = aldosterone synthesis c. ACTH more pregnenolone = more progesterone d. Natriuretic Peptide (heart) - inhibits aldosterone secretion, promotes natriuresis by the kidneys. - decrease potassium = production by heart - decreases aldosterone to allow potassium reabsorption and sodium secretion * Disorders of Aldosterone a. Natural increase (Physiologic response) - deficiency in Na and plenty of K in the diet - heavy sweat (decreased Na) b. Natural decrease - deficiency in the diet - having large amounts of water and drinks c. Addisons disease - occurs when about 90% of cortex cells are damaged, very rare (8/million person) MHML R Vinlization - androgens - sex DHEA (S) * Effects of Aldosterone: a. Renal and circulatory effects (ECF volume regulation, sodium and potassium ECF concentration) b. Promotes reabsorption of sodium from the ducts of sweat and salivary glands during excessive sweat or saliva loss.
17-OH Progesterone 21
Deoxycorticosterone 11- Deoxycortisol Androstenedione Androstenediol 11 Corticosterone 18 OH ALDOSTERONE (15 20 mg/day) Cortisone (waste product) ESTRADIOL 11 CORTISOL 3 TESTOSTERONE
* Regulation - only free cholesterol can enter steroidogenesis - the availability is regulated by ACTH (positively) and LDL (negatively) - F-layer glucocorticoids powerfully suppress ACTH release, thus cortisol is the primary feedback regulation of ACTH - ACTH does not significantly impact G-layer aldosterone synthesis HORMONES 1. Aldosterone - Normal level: 4-9 mg/100 mL of blood - Discarded in urine at about 2-18 mg daily - Responsible for regulating Sodium reabsorption - Target cells are called principal (P) cells - Stimulates synthesis of more Sodium/Potassium ATPase pumps.
3 - Addisonian has a remarkable depression in aldosterone level - accompanied by a low BP, high temp., weight loss, decreased blood Na, and increased blood K. o Signs and symptoms of Addisons disease: - fatigue - weight loss - anorexia why? -> function of cortisol - changes in skin pigment (small black freckles, decrease in cortisol -> increased ACTH -> increased MSH) - muscular weakness (cortisol helps muscles maintain contraction and avoid fatigue) Formed in the F zone (essentially) and R zone of adrenal cortex. In circulation, cortisol is bound to corticosteroid-binding globulin (CBG) or transcortin. Exhibits diurnal variation -> highest concentration at 8 AM, lowest at late evening. Free cortisol -> biologically active form Glucocorticoids have no target gland; exert their influence throughout the body.
* Physiological actions of cortisol 1/9/14 * Anti-inflammatory effects of cortisol Reduces phagocytic action of WBC Reduces fever Suppresses allergic reactions Wide spread therapeutic use Promotes gluconeogenesis Promotes breakdown of skeletal muscle proteins Enhances fat breakdown (lipolysis) Suppresses immune system (immune system uses a lot of sugar) Breakdown of bone matrix (high doses)
d. Conns syndrome - Primary: due to tumor (adenoma), nodularity, hyperplasia - Secondary: due to excess stimulation by angiotensin - Most common cause: Aldosterone producing adenoma -> not controlled by negative feedback - Incidence: Females > Males, 30-60 y/o, 7% of patients investigated for hypertension. * Investigations a. Blood - Na and K levels - Plasma aldosterone - Plasma renin activity b. Urine - urinary potassium and sodium c. Imaging - ultrasound - CAT scan - MRI - Iodo-cholesterol isotope scan
2. Cortisol - Major glucocorticoid hormone - Secretion: 25 mg/day; no normal range because production depends on the need.
* Regulation of Cortisol Release > Enhanced release can be caused by: - Physical trauma - Infection - Extreme heat and cold - Exercise to the point of exhaustion - Extreme mental anxiety * Disorders of Cortisol a. Glucocorticoid Deficiency i. Loss of cortisol - disruption of glucose concentration - reduction in metabolism of fats and proteins - patient is susceptible to different types of stress MHML
4 - sluggishness of energy mobilization result in weak muscle even when glucose and other nutrients are available cortisol is needed for metabolic function ii. Hyperadrenalism Cushings syndrome - caused by exogenous glucocorticoids and by tumors (adrenal/pituitary) - zona glomerulosa tumor increases aldosterone -> increased sodium, blood pressure -> 80% suffer from hypertension - zona reticularis tumor increases cortisol -> excess protein catabolism, redistribution of fat o Characteristics Buffalo torso - Redistribution of fat from lower parts of the body to the thoracic and upper abdominal areas. Moon face - Edematous appearance of face - Acne and hirsutism (excess growth of facial hair) b. Dehydroepiandrosterone (DHEA) c. Androstenedione Small amounts of testosterone (T) and Dihydrotestosterone (DHT) Peak production of androgens between 20 30 years old, then falls off gradually DHEA and DHEAS levels decrease during illness, depression and other stresses (impotency and infertility)
* Disorders of Androgens a. Androgen Excess - Androgen stimulates organ development and linear growth and epiphyseal fusion. - Virilization in boys include penile enlargement, androgen dependent hair growth and other secondary sex characteristics. - Girls develop hirsutism, acne, and clitorimegaly * Diagnosis of Androgen Excess - High DHEA production strongly suggests adrenal hyperandrogenism. - Elevated testosterone values are seen with either adrenal or gonadal hyperandrogenism. - Plasma DHEA or urine 17 Ketosteroids can identify adrenal causes of pathologic masculinization and feminization.
b. Effects on carbohydrate metabolism i. Adrenal Diabetes - hypertension of cortisol results in increase blood glucose levels up to 2x normal (200mg/dL) - prolonged oversecretion of insulin burns out the Beta cells of the pancreas resulting in life long DM. c. Other effects i. High blood pressure (secondary) ii. Osteoporosis due to increased protein breakdown. * Diagnosis a. 24 - hours urine free cortisol or salivary cortisol b. Low dose dexamethasone suppression test c. Plasma ACTH d. Imaging studies 3. Adrenal Androgens - Predominant androgens produced at the adrenal cortex are: a. Dehydroepiandrosterone sulfate (DHEAS)
B. Adrenal Medulla - Devoted to synthesis of cathecolamines which includes adrenaline (epinephrine) and noradrenaline (norepinephrine) - Both hormones are secreted in stressful situations - Function as an atypical sympathetic ganglion - Its products serve as first responders to stress by acting within seconds to promote a fight flight response.
MHML
5 Physiologic Effects of Cathecolamines - Cathecolamines causes general physiological changes that prepare the body for physical activities. - In case of (fight or flight), cathecolamines cause: Elevation of blood pressure Increasing blood sugar Increasing heart rate Increased metabolic rate Affects peripheral nervous system Cathecolamines Production Cytoplasm Phenylalanine -> Tyrosine -> DOPA -> Dopamine -> Vesicle transporters (VMAT) -> Dopamine in lipid vesicles Norepinephrine VMAT Epinephrine in secretory vesicles (active form) * Diagnosis PNMT Cortisol 24 hour urine VMA (metabolite of epinephrine) Urinary cathecolamines Plasma cathecolamines Urinary metanephrine Plasma metanephrine CT to locate tumor failed to involute after birth and secretes excessive amounts of cathecolamines. Extra adrenal paragangliomas (extra adrenal pheochromocytomas) are closely related, less common.
* Symptomatology - symptoms may be sporadic and paroxysmal against a background of continuing high concentration of cathecolamines and chronic physiological changes such as: Hypovolaemia Palpitation Hypertension Sweating and pallor Anxiety Chest pain and weakness
Cathecolamine Degradation 3 Mechanisms: a. Reuptake into secretory vesicles b. Uptake in nonneuronal cells (mostly liver) c. Degradation -> metabolites such as metanephrines and Vanillylmandelic acid (VMA) Metabolites and free cathecolamines are eliminated by direct filtration into urine. Epinephrine is converted from norepinephrine by renal PNMT enzyme.
b. Adrenal Incidentaloma - Incidental finding of adrenal masses during diagnostics - Usually non-functioning and benign ..
Disorder of the Adrenal Medulla a. Pheochromocytoma Neuroendocrine tumor of the medulla originating in the chromaffin cells or extra adrenal chromaffin tissue that MHML
Anda mungkin juga menyukai
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Para PBL CompleteDokumen9 halamanPara PBL CompleteMerill Harrelson LibanBelum ada peringkat
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- Nelson Pediatrics Review (MCQS) 17edDokumen631 halamanNelson Pediatrics Review (MCQS) 17edGabrielle Maycock75% (8)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- Research StrawberryDokumen30 halamanResearch StrawberryMerill Harrelson LibanBelum ada peringkat
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- Systemic Pathology Problem Based LearningDokumen1 halamanSystemic Pathology Problem Based LearningMerill Harrelson LibanBelum ada peringkat
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- #2 IncDokumen1 halaman#2 IncMerill Harrelson LibanBelum ada peringkat
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Interpretation of Results ReportingDokumen7 halamanInterpretation of Results ReportingMerill Harrelson LibanBelum ada peringkat
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- QFR, Expt12, #1bDokumen1 halamanQFR, Expt12, #1bMerill Harrelson LibanBelum ada peringkat
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- QFR Expt8#4Dokumen2 halamanQFR Expt8#4Merill Harrelson LibanBelum ada peringkat
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- cc2 #4&6Dokumen2 halamancc2 #4&6Merill Harrelson LibanBelum ada peringkat
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Sero QFR 1Dokumen2 halamanSero QFR 1Merill Harrelson LibanBelum ada peringkat
- Parasitqfr 4Dokumen1 halamanParasitqfr 4Merill Harrelson LibanBelum ada peringkat
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- CC ReviewerDokumen2 halamanCC ReviewerMerill Harrelson LibanBelum ada peringkat
- El FiliDokumen2 halamanEl FiliMerill Harrelson LibanBelum ada peringkat
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- The Song of RolandDokumen10 halamanThe Song of RolandMerill Harrelson Liban100% (1)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- Hypothyrodism in PregnancyDokumen32 halamanHypothyrodism in Pregnancymohammed makki0% (1)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Evaluation Thyroid NoduleDokumen35 halamanEvaluation Thyroid Nodulehendra nuraminBelum ada peringkat
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Metabolic and Endocrine Disorders PDFDokumen14 halamanMetabolic and Endocrine Disorders PDFPascal St Peter Nwaorgu100% (2)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Lactation Concept MapDokumen1 halamanLactation Concept Mapapi-392216729100% (1)
- Thyroid Function TestsDokumen1 halamanThyroid Function TestsWail hassan ShahadaBelum ada peringkat
- Burman Thyroid Disorders and Diseases PDFDokumen242 halamanBurman Thyroid Disorders and Diseases PDFxguerratBelum ada peringkat
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Quantum Yoga PDFDokumen241 halamanQuantum Yoga PDFMohd AnowarBelum ada peringkat
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- Clinicopathological Conference: Presenter: Dr. Bilal Ahmad Facilitator: Dr. Ghulam SiddiqDokumen21 halamanClinicopathological Conference: Presenter: Dr. Bilal Ahmad Facilitator: Dr. Ghulam SiddiqFaiza Hashim SoomroBelum ada peringkat
- Thyroid Crisis... FinalDokumen53 halamanThyroid Crisis... FinalYhanaAdarneBelum ada peringkat
- Infertility Concept MapDokumen1 halamanInfertility Concept Mapashleydean0% (1)
- P9mi9p6lx - Lesson 3.3-The Integumentary System 3Dokumen23 halamanP9mi9p6lx - Lesson 3.3-The Integumentary System 3Precious RoxasBelum ada peringkat
- 1-Anatomy of The Pituitary Gland (Updated)Dokumen14 halaman1-Anatomy of The Pituitary Gland (Updated)LyssahBelum ada peringkat
- Hypothalamo HypophyseDokumen58 halamanHypothalamo Hypophyseyinuo94Belum ada peringkat
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- Jawaban Lo Kelompok (JLK) Scenario 3 Kelompok Tutorial 5Dokumen9 halamanJawaban Lo Kelompok (JLK) Scenario 3 Kelompok Tutorial 5NOVI UMAMI UMAMIBelum ada peringkat
- MCN 313 1HDokumen4 halamanMCN 313 1HJon EricBelum ada peringkat
- Homoeopathic Perspective of Thyroid DisordersDokumen20 halamanHomoeopathic Perspective of Thyroid DisordersSaurav AroraBelum ada peringkat
- OB 1.03C Placental HormonesDokumen2 halamanOB 1.03C Placental HormonesCandice SongcoBelum ada peringkat
- WORKSHEET 12.4: Reflex Arc: Name: - Chapter 12 Coordination and ResponseDokumen3 halamanWORKSHEET 12.4: Reflex Arc: Name: - Chapter 12 Coordination and ResponseVinBelum ada peringkat
- Human Male Reproductive SystemDokumen45 halamanHuman Male Reproductive Systemcyber secBelum ada peringkat
- TSHDokumen25 halamanTSHFlorea RodicaBelum ada peringkat
- (1479683X - European Journal of Endocrinology) ENDOCRINE OBESITY - Pituitary Dysfunction in ObesityDokumen14 halaman(1479683X - European Journal of Endocrinology) ENDOCRINE OBESITY - Pituitary Dysfunction in ObesityAnonymous nRufcMpvNBelum ada peringkat
- HPA Axis Terkait Dengan Gangguan Jiwa (Dr. Hadi) .PPSXDokumen19 halamanHPA Axis Terkait Dengan Gangguan Jiwa (Dr. Hadi) .PPSXMbak RockerBelum ada peringkat
- Pituitary DisordersDokumen65 halamanPituitary Disordersgema disiyuna100% (1)
- SB7.1p HormonesDokumen9 halamanSB7.1p HormonesHisokagenBelum ada peringkat
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- ANAPHY LAB Worksheet 8.1 Endocrine SystemDokumen3 halamanANAPHY LAB Worksheet 8.1 Endocrine SystemKristine Lorainne DelamideBelum ada peringkat
- The Missing Key To Thyroid HealthDokumen22 halamanThe Missing Key To Thyroid HealthEmily Williams0% (1)
- Endocrinology Question 1Dokumen16 halamanEndocrinology Question 1BasirQidwai100% (4)
- Leaflet Final Ann PERKENI 2023Dokumen2 halamanLeaflet Final Ann PERKENI 2023ngurahardhi88Belum ada peringkat
- Recurrent Laryngeal Nerve Injury in Thyroid SurgeryDokumen5 halamanRecurrent Laryngeal Nerve Injury in Thyroid SurgeryDiornald MogiBelum ada peringkat
- Menstrual CycleDokumen17 halamanMenstrual CycleBala MuruganBelum ada peringkat