Physics Presentation Research
Diunggah oleh
Angus LivingstoneHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Physics Presentation Research
Diunggah oleh
Angus LivingstoneHak Cipta:
Format Tersedia
Physics Presentation Research
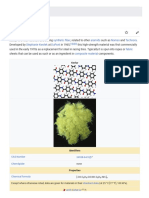
http://www.technologystudent.com/joints/kevlar2.html, V. Ryan 2011, accessed 18/11/2013 Kevlar is a material formed by combining para-phenylenediamine and terephthaloyl chloride. Aromatic polyamide (aramid) threads are the result. They are further refined, by dissolving the threads and spinning them into regular fibres. When woven, Kevlar forms a strong and flexible material. If layers of the woven Kevlar are combined with layers of resin, the resulting rigid material is light and has twenty times the strength of steel. It is also superior to specialist metal alloys. However, Kevlar is expensive due to the demands of the manufacturing process and the need for specialist equipment. Kevlar 29 is used in the manufacture of body armour (panels) for lightweight military vehicles. A good example is the US Armys Bradley Fighting Vehicle. This has been used extensively in Iraq and Afghanistan. Kevlar 29 was selected for its armour, because it is lightweight and withstands attack from RPGs. The Kevlar 29 panels protect the soldiers inside the vehicle. Kevlar 29 is ideal because it is lightweight and non-flammable and it offers protection from high temperatures (fire bombs, Molotov cocktails etc...). Kevlar 29 can also withstand the harsh environmental conditions, found in hot climates. Kevlar has a range of advantages, not only its relative low weight and high strength: Laminated Kevlar is very stable at high temperatures and it is impact and scratch resistant. Kevlar is often combined with other materials, to produce textiles with enhanced properties, such as fire resistant clothing for the Fire Services.
http://www.google.co.uk/imgres?imgurl=http://www.chm.bris.ac.uk/motm/kevlar/lock.gif &imgrefurl=http://www.chm.bris.ac.uk/motm/kevlar/kevlarh.htm&usg=__zYkrEaSFtGxeMrL xpqdiJGBlFEk=&h=600&w=816&sz=115&hl=en&start=10&zoom=1&tbnid=zmo7CYsUWf0mi M:&tbnh=106&tbnw=144&ei=OQaKUpXyJ4GShQe1lYGADQ&prev=/search%3Fq%3Dkevlar% 26safe%3Dvss%26sa%3DX%26biw%3D1600%26bih%3D805%26sout%3D1%26tbm%3Disch& itbs=1&sa=X&ved=0CD4QrQMwCQ accessed 18/11/2013
What makes Kevlar so strong? And how can it be so light at the
same time? Vlodek Gabara, September 30, 2002, via Scientific
American Kevlar is an organic fiber in the aromatic polyamide (aramid) family that combines high strength with light weight, and comfort with protection. Kevlar is five times stronger than steel on an equal weight basis and provides reliable performance and solid strength. This unique combination of attributes ensures that members of law enforcement, corrections personnel and the military will be safe from harm that can come in many forms, including bullets, knives, switchblades and shrapnel. In fact, Kevlar garments have so far saved the lives of nearly 3,000 law enforcement officials.
Using organic polymers based on "light elements"--such as carbon, nitrogen, hydrogen and oxygen--rather than "heavy elements" such as iron, gives the advantage of low-weight structures. For example, Kevlar fiber has a density of 1.4 grams per cubic centimeter compared with iron's 7.9 grams per cubic centimeter. To achieve both the strength and stiffness of Kevlar, the molecular chains within the organic fiber needed to be fully extended and perfectly aligned to make them strong, stiff and tough. Such a high degree of alignment was not easy to achieve. Kevlar fibers are based on poly-paraphenylene terephthalamide, a rigid molecule that makes it easier to realize a fully extended, or straight, chain configuration. Also, these rigid molecules will even arrange in solutions. Such solutions are called liquid crystalline, which underscores their good organization. Poly-paraphenylene terephthalamide molecules behave like uncooked spaghetti, whereas other, less rigid molecules behave more like cooked strands of spaghetti. Thus, the nature of the molecule makes it easier to achieve the desired aligned structure. In addition, polyparaphenylene terephthalamide strongly resists high temperatures and flames. Offering strength under heat, Kevlar protects against thermal hazards up to 800 degrees Farenheit. This combination of unusual properties makes Kevlar useful for a broad range of applications, such as ballistic vests, cut-resistant gloves and blast and flame barriers. Kevlar
http://www.slideshare.net/nashton/kevlar-presentation, Kevlar presentationby
nashton on Oct 19, 2010, Accessed 02/12/2013 Its molecular formula is [-CO-C6H4-CO-NH-C6H4-NH-]n
Kevlar when spun has a relative density of 1.44 and a tensile to weight ratio of 5:1. Kevlar is very heat resistant and decomposes at 675K without melting. The H-bonds between the molecules give the Kevlar its strength and durability as it has strong intermolecular forces.
http://prezi.com/snk_g2pslmsd/kevlar/ Kevlar, Courtney Pasco, April 2013, accessed 02/12/2013
http://inventors.about.com/library/inventors/blkevlar.htm http://en.wikipedia.org/wiki/File:Kevlar-3D-balls.png
Anda mungkin juga menyukai
- A Comprehensive Guide to Composites: Processes & Procedures from the ProfessionalsDari EverandA Comprehensive Guide to Composites: Processes & Procedures from the ProfessionalsPenilaian: 5 dari 5 bintang5/5 (2)
- Service ManualDokumen582 halamanService ManualBogdan Popescu100% (5)
- Synthesis of KevlarDokumen5 halamanSynthesis of KevlarAimee Ruth Inuguidan Dagupon100% (2)
- ENIRAM - Guide To Dynamic Trim Optimization 280611 PDFDokumen14 halamanENIRAM - Guide To Dynamic Trim Optimization 280611 PDFPhineas MagellanBelum ada peringkat
- Ultimate Electronics - Book - CircuitLabDokumen3 halamanUltimate Electronics - Book - CircuitLabEldon50% (2)
- Catalogo Perylsa CompletoDokumen221 halamanCatalogo Perylsa CompletoAlvaro Diaz0% (1)
- Kevlar: Synthetic Fiber Aramids Nomex Technora Stephanie Kwolek Dupont Fabric Composite MaterialDokumen12 halamanKevlar: Synthetic Fiber Aramids Nomex Technora Stephanie Kwolek Dupont Fabric Composite MaterialFaizan AlamBelum ada peringkat
- Kevlar by Abhishek JaguessarDokumen5 halamanKevlar by Abhishek Jaguessarreedoye21Belum ada peringkat
- KevlarDokumen6 halamanKevlarsunitbhaumikBelum ada peringkat
- Consumer Chemistry (CH 419)Dokumen9 halamanConsumer Chemistry (CH 419)Mihir Kumar MechBelum ada peringkat
- Kevlar Fiber: Devansh GuptaDokumen23 halamanKevlar Fiber: Devansh Guptagopal rao sirBelum ada peringkat
- KEVLAR2SUBBUDokumen17 halamanKEVLAR2SUBBUSubbu SureshBelum ada peringkat
- KevlarDokumen8 halamanKevlarapi-282592950Belum ada peringkat
- KevlarDokumen14 halamanKevlarKhaledIssamAl-qudah0% (1)
- Kevlar Is A Heat-Resistant and Strong: paraDokumen1 halamanKevlar Is A Heat-Resistant and Strong: parasubhamBelum ada peringkat
- Kevlar: From Wikipedia, The Free EncyclopediaDokumen9 halamanKevlar: From Wikipedia, The Free EncyclopediaMust WatchBelum ada peringkat
- Du Pont Kevlar Aramid Und Us Trial Fiber Abridged)Dokumen16 halamanDu Pont Kevlar Aramid Und Us Trial Fiber Abridged)canhconvsip100% (1)
- Polymeric Composite Materials-5.2Dokumen23 halamanPolymeric Composite Materials-5.2HALİM BOZTEPEBelum ada peringkat
- Additive Manufacturing AssignmentDokumen4 halamanAdditive Manufacturing AssignmentArenBelum ada peringkat
- 8.kevlar-A New Fibre Reinforcement For Conveyor BeltingDokumen11 halaman8.kevlar-A New Fibre Reinforcement For Conveyor BeltingLejo VargheseBelum ada peringkat
- Kevlar-The Super Tough Fiber: Engr. Reashad Bin Kabir, Engr. Nasrin FerdousDokumen5 halamanKevlar-The Super Tough Fiber: Engr. Reashad Bin Kabir, Engr. Nasrin FerdousBabtista EzraBelum ada peringkat
- 2-Aircraft Materials CompositeDokumen81 halaman2-Aircraft Materials CompositeIrfan Yusoff100% (2)
- Composite Material ApplicationsDokumen35 halamanComposite Material ApplicationsTejas shastrakarBelum ada peringkat
- Aircraft Material and Processes 1006Dokumen12 halamanAircraft Material and Processes 1006Gokul Raam GBelum ada peringkat
- Project - Aramid FibersDokumen22 halamanProject - Aramid FibersMohammed Anass EL MAROUANIBelum ada peringkat
- ScriptDokumen4 halamanScriptJoel KhouriBelum ada peringkat
- Aramid Fibres and Trade Names PDFDokumen6 halamanAramid Fibres and Trade Names PDFKali MuthuBelum ada peringkat
- KevlarDokumen11 halamanKevlarLisbeth Damian SamameBelum ada peringkat
- Hi Performance FibersDokumen13 halamanHi Performance Fibersahmer adnanBelum ada peringkat
- Types of Fiber ReinforcementDokumen9 halamanTypes of Fiber ReinforcementNebyat YazachewBelum ada peringkat
- CHDX04 Module 1 MaterialsDokumen18 halamanCHDX04 Module 1 Materialsargiun20Belum ada peringkat
- Arlon 55NT PCB Laminate Identification & FunctionalityDokumen5 halamanArlon 55NT PCB Laminate Identification & FunctionalityjackBelum ada peringkat
- Articulo de Kevlar-29 Con Grafeno y NanosheetsDokumen6 halamanArticulo de Kevlar-29 Con Grafeno y NanosheetsSoulklaxBelum ada peringkat
- Advanced Composite Materials in Typical Aerospace Applications-LibreDokumen10 halamanAdvanced Composite Materials in Typical Aerospace Applications-LibrezaujahaminBelum ada peringkat
- Composite-Reinforcements v2Dokumen5 halamanComposite-Reinforcements v2rafaelengemecanicoBelum ada peringkat
- Fsa Jury Assignment Kirti PatelDokumen18 halamanFsa Jury Assignment Kirti PatelKIRTI PATELBelum ada peringkat
- Kevlar Bullet Proof: Kevlar Is A Material That Is Used in Making Bulletproof Jackets and VestsDokumen2 halamanKevlar Bullet Proof: Kevlar Is A Material That Is Used in Making Bulletproof Jackets and Vestsashishtelang2013Belum ada peringkat
- Kevlar ProjedtDokumen11 halamanKevlar Projedtapi-345347907Belum ada peringkat
- PHY303-Smart Materials & Devices: SBSR, Sharda University, G. Noida, India E Mail: Ph. No.:+91-9971729840Dokumen25 halamanPHY303-Smart Materials & Devices: SBSR, Sharda University, G. Noida, India E Mail: Ph. No.:+91-9971729840Prashant KumarBelum ada peringkat
- KEVLAR Technical GuideDokumen32 halamanKEVLAR Technical GuideZuhair MahmudBelum ada peringkat
- Ballistic Impact Analysis of Graphene Nanosheets Reinforced Kevlar-29Dokumen6 halamanBallistic Impact Analysis of Graphene Nanosheets Reinforced Kevlar-29Venny Damayanti PuahaBelum ada peringkat
- Chapter 03 Synthetic Fiber RopesDokumen12 halamanChapter 03 Synthetic Fiber RopesCaptIsqanBelum ada peringkat
- Introduction To Boat Building in CompositesDokumen4 halamanIntroduction To Boat Building in CompositesMax BeeksBelum ada peringkat
- Common Fiber Reinforcement of A Glass, Carbon and Kevlar Common Fiber Reinforcement of A Glass, Carbon and KevlarDokumen28 halamanCommon Fiber Reinforcement of A Glass, Carbon and Kevlar Common Fiber Reinforcement of A Glass, Carbon and KevlarCedrick LandichoBelum ada peringkat
- DuPont Kalrez Brochure 2011 PDFDokumen12 halamanDuPont Kalrez Brochure 2011 PDFTim kuBelum ada peringkat
- SAFE Boats CatalogDokumen11 halamanSAFE Boats CatalogalraqimBelum ada peringkat
- Composite MaterialDokumen92 halamanComposite MaterialAparna RBelum ada peringkat
- Literature Review of Glass FibreDokumen5 halamanLiterature Review of Glass Fibreaflspbnyu100% (1)
- TegrisDokumen3 halamanTegrisRanjan ChaturvediBelum ada peringkat
- 1.1C Fiber Used in Technical TextilesDokumen10 halaman1.1C Fiber Used in Technical TextilesDhrubo AdhikaryBelum ada peringkat
- 1 Introduction To Glass Fiber-Based Composites and StructuresDokumen16 halaman1 Introduction To Glass Fiber-Based Composites and StructuresAjin vijayanBelum ada peringkat
- Wind Turbine Blade Production - New Products Keep Pace As Scale IncreasesDokumen8 halamanWind Turbine Blade Production - New Products Keep Pace As Scale IncreasesSandeep BandyopadhyayBelum ada peringkat
- E Glass Fibre PDFDokumen4 halamanE Glass Fibre PDFEsteban GabrielBelum ada peringkat
- Processing of Polymers and Composites: Lecture 19 - 27/02/2020Dokumen17 halamanProcessing of Polymers and Composites: Lecture 19 - 27/02/2020MK SaravananBelum ada peringkat
- Ultra High Molecular Weight PolyethyleneDokumen7 halamanUltra High Molecular Weight PolyethyleneAliceAlormenuBelum ada peringkat
- Examples of Condensation PolymersDokumen55 halamanExamples of Condensation PolymersJhunel Antonio RomanBelum ada peringkat
- Fiberglassing - About ReinforcementsDokumen2 halamanFiberglassing - About ReinforcementsMata Bana NajouBelum ada peringkat
- Muehlen Sohn 2 Thermal CharactericticsDokumen1 halamanMuehlen Sohn 2 Thermal CharactericticsChrystal BakerBelum ada peringkat
- Design of A Bullet-Proof Vest Using Shear Thickening FluidDokumen11 halamanDesign of A Bullet-Proof Vest Using Shear Thickening FluidSatish Raja DhulipalaBelum ada peringkat
- UHMWPEDokumen6 halamanUHMWPEtechdocuBelum ada peringkat
- High Performance Plastics Materials Guide: Craftech Industries'Dokumen20 halamanHigh Performance Plastics Materials Guide: Craftech Industries'akirloskarBelum ada peringkat
- 2019 CatalogDokumen120 halaman2019 CatalogMasterr100% (1)
- Yadav Khagendra K. M-Tech (AME) Hindustan UniversityDokumen28 halamanYadav Khagendra K. M-Tech (AME) Hindustan Universityمحمد قديشيBelum ada peringkat
- Montgomery Law QuestionnaireDokumen1 halamanMontgomery Law QuestionnaireAngus LivingstoneBelum ada peringkat
- Alternative Careers in MedicineDokumen8 halamanAlternative Careers in MedicineAngus LivingstoneBelum ada peringkat
- Montgomery ExplainedDokumen1 halamanMontgomery ExplainedAngus LivingstoneBelum ada peringkat
- Pub QuizDokumen1 halamanPub QuizAngus LivingstoneBelum ada peringkat
- Pub QuizDokumen1 halamanPub QuizAngus LivingstoneBelum ada peringkat
- International Drinking RulesDokumen3 halamanInternational Drinking RulesAngus LivingstoneBelum ada peringkat
- Sports ReportDokumen1 halamanSports ReportAngus LivingstoneBelum ada peringkat
- Succinic Dehydrogenase: Angus LivingstoneDokumen2 halamanSuccinic Dehydrogenase: Angus LivingstoneAngus LivingstoneBelum ada peringkat
- Physics Coursework Results 2Dokumen9 halamanPhysics Coursework Results 2Angus LivingstoneBelum ada peringkat
- NC Error PropagationDokumen12 halamanNC Error PropagationSalman KhanBelum ada peringkat
- Karyn Corbett Pedagogy Letter To SelfDokumen2 halamanKaryn Corbett Pedagogy Letter To Selfapi-513861296Belum ada peringkat
- Fiitjee All India Test Series: Concept Recapitulation Test - Iv JEE (Advanced) - 2019Dokumen13 halamanFiitjee All India Test Series: Concept Recapitulation Test - Iv JEE (Advanced) - 2019Raj KumarBelum ada peringkat
- Grade 8 - EnglishDokumen2 halamanGrade 8 - EnglishTCHR KIMBelum ada peringkat
- Exposition Text Exercise ZenowskyDokumen8 halamanExposition Text Exercise ZenowskyZenowsky Wira Efrata SianturiBelum ada peringkat
- Permeability Estimation PDFDokumen10 halamanPermeability Estimation PDFEdison Javier Acevedo ArismendiBelum ada peringkat
- FINAL ReportDokumen48 halamanFINAL ReportMythri RangaswamyBelum ada peringkat
- 3-Storeyed 31-3-2015-Schedule PDFDokumen1 halaman3-Storeyed 31-3-2015-Schedule PDFSi Thu AungBelum ada peringkat
- Studi Tentang Pelayanan Terhadap Kapal Perikanan Di Pelabuhan Perikanan Pantai (PPP) Tumumpa Kota ManadoDokumen9 halamanStudi Tentang Pelayanan Terhadap Kapal Perikanan Di Pelabuhan Perikanan Pantai (PPP) Tumumpa Kota ManadoAri WibowoBelum ada peringkat
- UntitledDokumen37 halamanUntitledUnknown UserBelum ada peringkat
- Kick-Ass Customer Service-Part 1Dokumen3 halamanKick-Ass Customer Service-Part 1Mahfuzul Haque SujanBelum ada peringkat
- PienaDokumen1 halamanPienaMika Flores PedroBelum ada peringkat
- Response 2000 IntroductionDokumen24 halamanResponse 2000 IntroductionRory Cristian Cordero RojoBelum ada peringkat
- Submission Letter To LBUDokumen46 halamanSubmission Letter To LBUramesh bajracharyaBelum ada peringkat
- ENGLISH TOEFL Structure (3rd Exercise)Dokumen5 halamanENGLISH TOEFL Structure (3rd Exercise)susannnnnnBelum ada peringkat
- Books & PeriodicalsDokumen1 halamanBooks & PeriodicalsDebabrat MishraBelum ada peringkat
- (English (Auto-Generated) ) Intraday Trading On Nifty (2nd September, 2021) 8 Lakhs Profit Shreyas Bandi Trade Ideas Live (DownSub - Com)Dokumen41 halaman(English (Auto-Generated) ) Intraday Trading On Nifty (2nd September, 2021) 8 Lakhs Profit Shreyas Bandi Trade Ideas Live (DownSub - Com)YaaroBelum ada peringkat
- Malraux Anti-MemoiresDokumen9 halamanMalraux Anti-MemoiresNevenaRistićBelum ada peringkat
- Focus On Teaching - Jim KnightDokumen213 halamanFocus On Teaching - Jim KnightFernando TeixeiraBelum ada peringkat
- Effect of Liquidity Risk On Performance of Deposit Money Banks in NigeriaDokumen6 halamanEffect of Liquidity Risk On Performance of Deposit Money Banks in NigeriaEditor IJTSRDBelum ada peringkat
- Chapter 01 Fundamental and Derived QuantitiesDokumen7 halamanChapter 01 Fundamental and Derived QuantitiesAlicia WilliamsBelum ada peringkat
- Beamforming For 4.9G/5G Networks: Exploiting Massive MIMO and Active Antenna TechnologiesDokumen12 halamanBeamforming For 4.9G/5G Networks: Exploiting Massive MIMO and Active Antenna TechnologiesAymen Ben zinebBelum ada peringkat
- Capital Budgeting and Capital Budgeting and Risk Analysis Risk AnalysisDokumen16 halamanCapital Budgeting and Capital Budgeting and Risk Analysis Risk AnalysisHaris FendiarBelum ada peringkat
- Casio AT 1 Service ManualDokumen28 halamanCasio AT 1 Service ManualMario Gabriel MoralliBelum ada peringkat
- LogDokumen67 halamanLogRudhi Al GhaisanBelum ada peringkat
- Xgenus X-Ray PDFDokumen61 halamanXgenus X-Ray PDFAli NuriBelum ada peringkat