Omc Notes 2
Diunggah oleh
polypeptideJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Omc Notes 2
Diunggah oleh
polypeptideHak Cipta:
Format Tersedia
CHAPTER 10 ELIMINATION REACTIONS I. Overview !
eliminationreactionscanoccurbytransferofagroupfromthe,,carbonatom of a covalent ligand and the group can transfer to the metal center or to an incoming reagent ! hydrogeneliminationprocessesareusuallyslowerthanhydrogen eliminations=>observedonlywhenhydrideelininationsareinhibitedare absent ! eliminationreactionsusuallyrequirereactionofanexternalreagent or are simply an intramolecular C-H activation process
II.
-Hydride Elimination from Metal-Alkyl Complexes ! forms a hydride and a coordinated olefin from a single alkyl group requires an open coordination site at the metal prior to the C-H bond cleavage step occurs most readily when a syn coplanar arrangement of the metal and hydride groups can be achieved
hydrogeneliminationfromsquareplanarPdandPtcomplexesusuallyoccurs by 2 pathways: one occurring after generation of an open coordination site cis to the alkyl group and one that occurs from the four coordinate square planar species
hydrogeneliminationfromthesecomplexesintheabsenceofaddedligand occurs mainly by a 3 coordinate complex (unless the complex contains PMe2Ph)
hydrogen elimination from square planar metallacyclic complexes is slower than that from acyclic alkyl complexes since the former are less able to adopt a syn coplanar geometry metallacyclic complexes are thermally more stable than their acyclic analogues anddecomposebypathwaysthatdonotincludehydride eliminations
III.
FactorsAffectingtheRateof-Hydride Elimination
the less stable isomer can be formed because of the geometric requirement of the -hydrogen elimination step
EWG slow the rate of -hydride elimination
ingeneral,anincreaseinsterichindrancedecreasestherateofhydride elimination because it disfavors the structure containing the syn coplanar arrangementofthemetal,-H, and the two linking carbons; in addition, the generation of 2 ligands from 1 increases the coordination number and thus the amount of steric hindrance
! IV.
ingeneral,anincreaseinelectrondonationincreasestherateof-H elimination
-Hydride Elimination from Metal Alkoxides and Amides ! hydrideeliminations from thiolate and phosphide complexes are rare because the C=S and C=P bonds are very weak ! ! occur by migratory deinsertion liganddissociationtogeneratea14electronintermediateisneededforboth hydrogen elimination processes to occur ! C-H bond cleavageistheRDSofhydrideeliminationfromtheamido complexes ! C-Hbondcleavagecanbereversibleorirreversibleduringhydrideelimination from Ir-OR complexes depending on the concentration of added ligand that displaces the ketone product
V.
-Hydrocarbyl Eliminations ! ! fastest from highly electrophilic early transition metal centers mostalkyleliminationsfromlatetransitionmetalsinvolvethecleavageofa strained ring
formedbyacombinationof-Heliminationand-alkyl elimination
theinitialsetof-alkyl elimination products undergo a series of transformations to generate a complex set of products
the Zr and Hf complexes react with similar rates and the differences in the enthalpic and entropic parameters offset each other
cleavage of the C-Cbondtothe-carbon of the late metal-alkyl complexes is slower and less common that that of early d0 metals
require an open coordination site
VI.
-Alkyland-Aryl Eliminations from Alkoxido and Amido Complexes ! require open coordination sites
analogous oxametallacycles containing bidentate, rather than monodentate, ligandsdonotundergo-alkyl elimination
occur via migratory deinsertions
VII.
-Halide and Alkoxide Elimination ! chlorideeliminationisparticularlyfastfrom-chloroalkyl complexes of early transition metals, Ni, Pd ! eliminationoffluorideandalkoxidegroupsisalsoknownwhenthesegroups arepresentonthecarbonsofthealkylligandsinhardd0 metal alkyl complexes
VIII.
-Hydrogen Eliminations and Abstractions ! generates a carbene and a hydride ligand from an alkyl ligand or a carbene ligand and alkane from two alkyl ligands ! most common method for generating Schrock-type alkylidene complexes (no heteroatoms) ! usuallyslowerthan-hydride eliminations and are most common for complexes thatcannotundergo-hydride eliminations (neopentyl, benzyl, methyl,etc) ! occurs most often among early transition metals such as d0 complexes of group 4 and 6 metals since these can access the high oxidation state needed for the formation of an alkylidene hydride product and the absence of d electrons does notfavorformationofastableolefincomplexresultingfrom -hydride elimination ! sterichindrancealsofacilitateshydrogeneliminationssincetheM-C-C angle increases as an alkyl group is converted to an alkylidene ligand
relief of steric hindrance
theimmediateproductof-Heliminationusuallyisntobserveddirectly reductive elimination of a hydride and an alkyl group to form an alkane frequently occurs in other cases, a carbene forms from a dialkyl complex of a d0 metal that cannot accommodate the additional valency required to form an alkylidene and a hydride ligand; this pathway requires a 4-centered transition state when the valency of the metal allows eitherpathwaytooccur,eliminations andhydrogenabstractionsarehardtodistinguish
CHAPTER 11 NUCLEOPHILIC ATTACK ON COORDINATED LIGANDS I. Fundamental Principles ! can be divided into 2 groups: nucleophilic attack at the metal center and nucleophilic attack at a ligand coordinated to the metal ! coordination of an unsaturated molecule to a transition metal reverses its reactivity from that of a weak nucleophile to that of an electrophile
CO, olefins, dienes, arenes react with nucleophiles when coordinated to metals due to the flow of electron density from the ligand to the metal ! unsaturated ligands of more electron deficient metals are more reactive towards nucleophilic attack than are unsaturated ligands of more electron rich metals ! the presenceofspectator-acceptor spectator ligands on the metal facilitate nucleophilic attack by accommodating the increase in charge ! attack at the ligand is generally favored for complexes that are coordinatively saturated or cannot accommodate an additional ligand for steric or electronic reasons
nucleophilic addition to coordinated ligands does not change the total number of electrons but may or may not change the oxidation state (no change for attack at neutral ligands, reduction for attack at anionic ligands)
II.
Nucleophilic Attack on CO and RNC Complexes ! ! ! stable products many relatively weak nucleophiles add to coordinated CO and RNC RNC ligands produce stable metal-carbene complexes but require stronger nucleophiles due to its lower electrophilicity ! most occur by direct nucleophilic attack on the coordinated ligand without prior coordination of the nucleophile to the metal
the susceptibility of M-CO ligands to nucleophilic is inversely related to the amount of backbonding rate of nucleophilic attack: [M-CO]+ >[M-CO] > [M-CO]rate decreased by EDG
organic halides and tosylates tend to react at the metal center while harder and more reactive alkylating reagents react at O to generate carbene complexes
the hydroxycarbonyl complexes generated from attack by OH- are unstable and extrude CO2 to produce metal hydrides ; when the anionic hydride is acidic enough to be deprotonated by the hydroxide, a 2 electron reduction of the metal occurs
III.
Nucleophilic Attack on Carbene and Carbyne Complexes
cationic carbene complexes undergo simple addition processes to generate neutral products while neutral complexes react either by addition or by addition + eliminations
nucleophilic additions to carbenes are restricted to cationic Fischer carbene complexes or Fischer carbene complexes that possess ancillary ligands that stabilize negative charges high valent, early metal Schrock type carbenes are usually nucleophilic at the carbene carbon and thus react with Lewis bases and nucleophiles
IV.
Nucleophilic Cleavage of M-CBonds ! nucleophilicattackonboundalkyl ligands is slower than attack on unsaturated ligands bound through a single atom or on unsaturated ligands bound through multiple atoms lack of low-lying LUMO at the alkyl ligand and the partial negative charge that typically lies at carbon bound to a transition metal ! reaction rate depends on the amount of electron density at or the electrophilicity of the alkyl carbon and on the stability of the metal fragment that would serve as the leaving group
attack of a nucleophile on an alkyl group is most common when the alkyl group is bound to an electron poor and high valent metal center occur by mechanisms similar to SN2 sensitive to solvent polarity, steric effects, and produces an inversion of configuration
metal benzyl complexes also react with nucleophiles with an inversion of configuration
metal acyl complexes react with nucleophiles to produce carboxylic acid derivatives
the fastest reactions occur with acyl groups bound to transition metals that are electrophilic and that are good leaving groups ! allylgroupsundergomorerapidnucleophilicattackthananyofthe-bonded groups1 allylgroupsaremuchlessreactivetowardsnucleophilesthan3 allyl groups
V.
NucleophilicAttackon2 Unsaturated Hydrocarbon Ligands ! coordination of an olefin to an electron poor metal causes the coordinated olefin to react with nucleophiles because coordination leads to a net flow of electron density from the unsaturated hydrocarbon to the metal
nucleophilic attack occurs most readily on unsaturated hydrocarbons bound to metal centers in relatively high oxidation states possessing a positive charge or EW ancillary ligands
an increase in reactivity of unsaturated hydrocarbons with electrophiles is observed upon coordination to electron rich metal centers their olefin complexes are best described as metallacyclopropane complexes large degree of M-Cbondcharacter react with electrophiles
the Davies-Green-Mingos guidelines predict the most kinetically favorable position for nucleophilic attack on coordinated, unsaturated hydrocarbons in 18e cationic complexes evenoroddaccordington open or closed according to acyclic or cyclic nucleophilic attack on open polyenes tends to occur at a terminal carbon atom
VI.
NucleophilicAttackon2 Olefin Complexes ! usually, nucleophilic attack occurs onto the face of the olefin that is opposite the metal without coordination of nucleophile to the metal prior to attack ! symmetric metal olefin complexes are deactivated towards nucleophilic attack but slippage of the olefin creates a LUMO that can interact with the incoming nucleophile ! nucleophilic attack occurs mostly at the more substituted terminus of substituted olefins since these carbons bear more positive charge
limited to mono or disubstituted olefins since more highly substituted olefins coordinate only weakly to metals displacement of the more substituted olefins from the metal by the nucleophile is a major competing reactions (esp. if the nucleophile is also a good ligand for the metal)
VII.
Nucleophilic Attack on Square Planar Pd(II) Diene and Allene Complexes ! dienescanbindtometalsina2 or4 fashion
VIII.
NucleophilicAttackon2-Alkyene Complexes ! ! produces-vinyl complexes there are fewer stable alkyne complexes of higher oxidation state or cationic metals than olefin complexes since they are most susceptible to nucleophilic attack
thetransstereochemistryofthe-vinyl group suggests that nucleophilic attack occurs external to the metal and does not involve prior coordination of MeOH trans attack of the nucleophile on the coordinated acetylene
IX.
Reactionsof2-Arene Complexes ! 2 coordination leads to de-aromatization of the arene, thus isolating the diene unit for reactions thechemistryof2-arene complexes resemble those of dienes
X.
Nucleophilic Attack on Imine and Aldehyde Complexes ! coordination of imines and aldehydes to Lewis acids enhances their reactivity with nucleophiles ! rearrangementofthe2-aldehydetothe1 form precedes attack of CN- at the aldehydic carbon
hydride additions occur with high stereoselectivity for addition to one face of the aldehyde over the other to form an amido complex as the major product; deprotonation of the N-H hydrogen produces an iminyl or azavinylidene as the minor product
XI.
NucleophilicAttackonPolyhapto(3- 6) Ligands ! usually occurs at the face of the allyl ligand opposite the metal to produce a neutral2 olefin complex in which the metal has been reduced by 2 electrons
! !
reaction occurs most readily for electron poor allyl complexes the ligands trans to the two termini of an allyl group affect the rate of attack attack often occurs trans to the ligand that is more donating or has a higher trans influence lengthening of the M-C bond trans to this ligand and a greater positive charge of the carbon more weakly bound to the metal
in accord with the Davies-Green-Mingos rule, attack occurs at the terminal carbon of the allyl group and occurs at the open allyl group rather than at the closed5 Cp group
even though products are usually formed from attack at the less substituted position, the regiochemistry can be directed to the more substituted position by electronic effects on the allyl group imparted by steric properties, bite angle, donor atoms and ancillary ligands
complexes generated from electron accepting ligands form the most branched product
nucleophilic addition to the central carbon is also favored by the presence of significant positive charge at this site
XII.
NucleophilicAttackon4 Diene Complexes ! cationic4 diene complexes should be among the most reactive substrates towards nucleophilic attack and should undergo preferential attack at a terminal position according to the DGM rule
XIII.
NucleophilicAttackon5 Dienyl Complexes ! ! reactions of electrophiles at Cp ligands are more common reactionoccursexclusivelyattheterminalcarbontogenerateanew4 diene complex ! attack occurs exclusively from the face opposite the metal and exclusively at the terminal position of the dienyl system
XIV.
Nucleophilic Attackon6 Arene and Cycloheptatrienyl Complexes ! coordination to EW metals makes the arene more electron poor and converts it from a nucleophile to an electrophile coordination lowers the pKa for deprotonation of both arene and benzylic hydrogens
coordination makes unsaturated groups conjugated with the arene more electrophilic benzylic carbocations and benzylic radicals of coordinated arenes are more stable than benzylic carbocations and radicals of free arenes allows nucleophilic attack to occur on the arene unit
the steric effect of the metal fragment leads to attack on the side of the arene opposite the metal can be used to control the relative stereochemistry of the substituents in the product the position of attack is controlled by the electronic properties of the substitutents (meta vs para) steric effects discourage ortho attack
unstabilized nucleophiles like organolithium reagents tend to add irreversibly while stabilized ones like malonates tend to add reversibly
as predicted by the DMG rule, nucleophilic attack on cationic metal complexes occurs on the complexed arene, not on the cycloheptadienyl ligand
CHAPTER 14 !
PRINCIPLES OF CATALYSIS a catalyst is a substance that increases the rate of a chemical reaction without itself being consumed
it is eventually regenerated after a series of transformations and can thus be used in substoichiometric amounts
catalysts reduce the free energy of the highest energy transition states and thereby increase the rate of reaction they dont change the energies of the reactants and products they only change the reaction rates, not the thermodynamics or equilibrium constants
catalysts decrease the activation energy relative to that of the uncatalyzed reaction by stabilizing the transition state binds one or more of the reactants and remains bound through the transition state; dissociation of the product either regenerates the starting catalyst directly or generate a species that will be converted to the starting catalyst
catalysts can also lower the energy of the transition state by creating a completely new reaction pathway
in catalyzed reactions, the reaction typically occurs by more steps but the activation energy of each of the individual steps is lower than the activation energy of the uncatalyzed process => lower overall
a catalyst precursor is a complex or mixture of compounds that gives rise to a complex that lies on the catalytic reaction pathway; drawn off the catalytic cycle because it leads to the true catalyst
irreversible decomposition of the catalyst is drawn in a step external to the catalytic cycle
reactions outside the cycle can retard the catalytic process by siphoning the catalyst away from the productive steps; often, dissociation of dative ligands must occur for stable complexes to enter the catalytic cycle
cocatalysts or promoters are additional reaction components that are added in small amounts to enhance the rates and selectivities of catalytic reactions
the efficiency of a catalytic reaction can be quantified by the TON (turnover number) and TOF (turnover number) the TON is the number of moles of product generated per mole of catalyst the TOF is the TON per unit time
the rate constants of different steps of a catalytic cycle are different, but the net rates of each step of a catalytic process are identical once the system has reached steady state the rate of each step is then proportional to the rate constant and the concentrations of reagents and species that lie on the catalytic cycle
the step with the smallest pseudo first order rate constant is the turnover limiting step and controls the efficiency of the catalytic process since the rate cannot exceed k[cat][reagent]
a homogeneous catalyst in the same phase as the reactants => discrete, molecular species
heterogeneous catalysts are in a different phase as the reagents => solid HETEROGENEOUS CATALYSTS low cost, easy separation from products, high stability, recyclability often catalyze oxidation and reduction reactions higher thermal stability easy separation slow rates because of low effective concentration lack of precise 3D architecture and nonuniformity in the local structures and properties of catalytic sites low selectivity
HOMOGENEOUS CATALYSTS rapid rates for the diffusion of reagents and heat in the solution phase ability to initiate reactions with discrete compounds that can be characterized by solution phase methods ability to control a 3D architecture created by ligand geometries => higher regioselectivity, diastereoselectivity, enantioselectivity more reactive difficult separation inability to regenerate decomposed catalyst low thermal stability potential catalyst contamination !
it is hard to definitively distinguish homogeneous catalysts from heterogeneous ones
light scattering and TEM can be used to identify the presence of nanoclusters in solution that appear to be homogeneous
elemental Hg has been shown to poison heterogeneous catalysts by blocking pores of high surface area solids reactions catalyzed by heterogeneous catalysts are typically retarded by adding Hg whereas those catalyzed by homogeneous catalysts are unaffected suggestive but not definitive
dibenzo[a,e]cyclooctetraene binds to soluble complexes to poison their catalytic activity but does not bind as well to metal surfaces reactions catalyzed by homogeneous catalysts are unaffected by this additive while those catalyzed by heterogeneous catalysts are suggestive, but not definitive
kinetic measurements provide the most definitive data to distinguish between homogeneous and heterogeneous catalysts when the particulate matter can be observed visually via light scattering or TEM if the observed solid or nanoclusters catalyze the reaction, then the reaction will occur with a sigmoidal profile in which the rate increases as the solid or nanoclusters form
there is no correlation between reaction at the Si and Re face and the (S) and (R) configurations or between reaction at a pro-(S) and a pro-(R) group and the (S) and (R) configuration
stereoselectivity can be quantified with optical rotation, %ee, er
the reaction of a prochiral substrate with achiral reagents in the presence of an asymmetric catalyst occurs preferentially at a prochiral face, at one of two enantiotopic groups or with one of two enantiomers in a racemic mixture
the reaction of a chiral catalyst with a prochiral substrate gives rise to diastereomeric transition states different spatial relationships between the substrate, reagent, and catalyst result in diastereomeric transition states that have unequal energies
small changes in activation energies results in dramatic differences in %ee 1.38kcal/mol difference gives rise to a 10:1 ratio of products
the enantioselectivity determining step controls the enantioselectivity and occurs in the first irreversible step that takes place through diastereomeric transition states
asymmetric induction can occur when a chiral catalyst reacts directly with a prochiral substrate without substrate coordination to the catalyst prior to the enantioselectivity determining step 2 diastereomeric steps
when prochiral substrates bind to chiral catalysts in step separate from the enantioselectivity determining step, diastereomeric complexes possessing different energies result
Curtin Hammett conditions: when interconversion of diastereomeric complexes is significantly faster than their reaction to form product, the enantioselectivity determining step is the reaction to form product
Curtin Hammett principle: when competing reaction pathways begin from rapidly interconverting isomers, the product ratio is determined by the relative heights of the highest energy barriers leading to the 2 different products enantioselectivity is controlled by the relative energies of the two diastereomeric transition states rather than by the stabilities of the two diastereomeric intermediates
asymmetry is transmitted when chiral catalysts bind substrate and react preferentially with one of its prochiral faces or when it prevents reaction at one of the prochiral faces by blocking it (steric biasing)
catalysts containing C2 symmetric ligands are most selective because of the small number of metal substrate adducts and transition states available to them due to their symmetry
quadrant diagrams are models used to predict the facial stereoselectivity in catalyst substrate complexes and transition states subject to steric biasing
in the quadrant model, the environment around the metal is divided into quandrants in which the horizontal dividing line is congruent with a plane in the catalyst; the shaded diagonal quadrants are spaces occupied by substituents on the ligands that extend forward while unshaded areas correspond to less hindered space => conformational preferences
the means by which metal complexes of chiral ligands block quadrants depends on the nature of the ligand and the M-L adduct
CHAPTER 15 ! [Rh(PPh3)3Cl] is Wilkinsons catalyst and it catalyzes the cis hydrogenation of alkenes ! mildly oxygen sensitive => some of the PPh3 reacts with oxygen to become POPh3 and the resulting catalyst becomes dinuclear [Rh(PPh3)2Cl]2 with bridging Cl, producing different rates, regioselectivities, and stereoselectivities ! steric effects affect olefin binding constants but when the olefin binds too strongly, it can siphon the catalyst away from the cycle and change the mechanism
undergoes hydrogenation without isotopic scrambling
involves a dihydride intermediate in which both hydrides are transferred to the same alkene and a migratory insertion => cis
selective olefin reduction in the presence of esters, ketones, and nitroarenes
cationic Rh complexes with the general formula [RhL2(H)2(S)2]+ also catalyze the hydrogenation of olefins
cationic Rh complexes with more ED ligands and bidendate ligands are more active
the active species is generated from [Rh(alkene)2(H)2]+ by hydrogenation of the coordinated alkene
the cationic catalyst generates products with little olefin isomerization while the neutral catalyst produced isomerization products
Crabtrees catalyst is [Ir(COD)py(PCy3)]+ and is a precatalyst in which hydrogen reduces COD to form cationic Ir(III) hydride complexes ; unaffected by oxygen and its activity relies on the use of relatively noncoordinating solvents activity improved by using bulky, extremely weakly coordinating anions
it catalyzes the hydrogenation of hindered olefins in reduces them in the presence of many other functional groups
Crabtrees catalyst is much more reactive than the neutral, more hindered Rh and Ru catalysts towards more substituted alkenes ; cationic Ir complexes are unusually reactive towards tetrasubstituted alkenes ! [Ru(PR3)n(H)(Cl)] catalysts selectively catalyze the hydrogenation of terminal alkenes
Typical Hydrogenation Mechanisms
Rh complexes containing phosphine ligands catalyze the hydrogenation of olefins by mechanisms that involve dihydride intermediates
the accumulation of products outside the dotted enclosure reduces the rate of the catalytic reaction
as can be seen above, the overall hydrogenation reaction by Wilkinsons catalyst can be divided into: addition of hydrogen to RhClL3, the reaction of RhH2ClL3 with the olefin by migratory insertion, and reductive elimination of the reduced product => hydrogen first pathway
CHAPTER 22 POLYMERIZATION AND OLIGOMERIZATION OF OLEFINS
the physical properties of polypropylene depend heavily on the relative configurations of the methyl groups in the polymer chain
! ! !
isotactic => all syn, highly crystalline syndiotactic => all anti, highly crystalline atactic => random relative stereochemistry, amorphous
! !
olefins can join in a head to head or head to tail fashion the stereochemistry of the polymer involves the relative configuration of diad units and can be controlled by catalyst or the last inserted monomer
"#$%&'()*(&*
)()*(&*
&*+
&*+
ansa-metallocene systems (two indenyl groups linked by a bridge) are used to obtain highly stereoregular polymers ; the linker between the two indenyl groups restricts ligand rotation and forces a C2 or Cs orientation of the indenyl groups
polymerization catalysts can be converted to oligomerization catalysts by reducing their steric properties
olefin oligomerization by group 4 metal catalysts are among the most used industrially
stronger electron donation increases the rate of -elimination versus olefin insertion and oligomers are formed instead of polymer
olefin dimerization can occur by two general mechanisms, one involving olefin insertion and the other involving a metallacyclic intermediate
the coupling of acrylates to form predominantly linear dimers occurs in the presence of a variety of Pd, Ru, and Rh catalysts
for all catalysts, the polymerization and oligomerization of conjugated dienes are thought to proceed through (3-allyl) metal complexes as intermediates; these intermediates can be formed by insertion of the diene into the M-H or M-R bond
the equilibrium between the 3 and 1 forms depends strongly on the nature of the other ligands and is a major factor in determining the course of the reaction
many complexes with low valent metals (esp. Ni(0) and Pd(0)) couple butadiene to form the corresponding bis-( 3-allyl) complex
Ziegler type catalysts are most commonly used for the commercial production of polybutadiene and polyisoprene; the selectivity of these processes are strongly metal-dependent; however, all involve (3-allyl)metal complexes as the active species
the cyclotrimerization of butadiene to cyclododecatriene is also catalyzed by Ni(0)
the insertion can occur from an 1-allyl complex or from an 3-allyl complex the formation of 1,2 or 1,4-polybutadiene depends on whether migratory insertion occurs at the terminal or internal carbon of the 3-allyl ligand
CHAPTER 21 METATHESIS OF OLEFINS AND ALKYNES ! olefin metathesis reactions cleave C=C bonds or triple bonds and reassemble them to generate products with new C=C bonds or triple bonds ; requires catalyst and is largely controlled by thermodynamics
there are six classes of olefin metathesis
! !
Schrock catalysts contain Mo Grubbs catalysts contain Ru
olefin metathesis occurs by a sequence of [2+2] cycloaddition and cycloreversion reactions
cyclic olefins possessing ring strain are used as monomers for olefin metathesis because the thermodynamics will favor the ring-opened materials
the overall mechanism for ROMP is the reverse of RCMP
alkyne metathesis cleaves the C-C triple bonds in two alkynes to form two new alkynes; it is used to make conjugated polymers with interesting electronic properties and to synthesize macrocycles with E or Z alkenes after reduction ; it is catalyzed by metal alkylidenes
the intermediate of alkyne metathesis involves a metallacyclobutadiene complex
alkyne cross metathesis can be used to synthesize unsymmetrical alkynes
ring closing alkyne metathesis + reduction can be used to synthesize macrocyclic natural products containing cis olefins
enyne metathesis generates dienes from alkynes and alkenes by splitting the alkene in half and adding them across the alkyne ; high heterofunction tolerance when used with Grubbs catalyst
enyne metathesis can be used with olefin metathesis to form bicyclic products
the mechanism of enyne metathesis involves the formation of an alkylidene complex and the subsequent formation of a metallocyclobutene complex
CHAPTER 17 CATALYTIC CARBONYLATION ! all the group 9 and 10 metals, in combination with iodide, are active catalysts for methanol carbonylation
3rd row transition metals are less commonly used in industrial processes because they form stronger M-L bonds, which decreases the rate of migratory insertion of CO
! !
=> promoters required however, the stronger M-L bonds also means that the rate of oxidative addition is faster
hydroformylation is a metal catalyzed reaction in which an olefin, CO, and H2 react to produce an aldehyde; also called the oxo process addition of formaldehyde elements across the C=C bond
common side reactions include alkene hydrogenation, aldehyde hydrogenation, and alkene isomerization
to obtain desired product is n-butyraldehyde, the n:i (normal to iso or the l/b for linear to branched ratio) ratio of aldehydes is maximized
hydroformylations catalyzed by HCo(CO)4 are typically run at high temperatures and high pressures of a 1:1 mixture of CO:H2 (syn gas)
HCo(CO)4 is exceptionally acidic, TBP 18e- complex that is synthesized by reacting H2 and Co2(CO)8
high CO pressure is needed to prevent the formation of higher Co clusters and of metallic Co
the rate of hydroformylation depends only on [CO]-1 and [H2]
increasing the pressure of the 1:1 syn gas mixture has little effect on the rate but prevents catalyst decomposition ! the catalytic cycle involves a succession of 18e and 16e species
hydroformylation of terminal alkenes catalyzed by HCo(CO)4 in the absence of any added ligands usually occurs to form a 3-4:1 n:i ratio the regioselectivities and rates can be improved by adding phosphines the electron donating property of phosphine ligands increases the Co-CO bond strength by increasing backbonding, allowing the reaction to be conducted at lower CO pressures and higher temperatures without catalyst decomposition higher n:i ratios
however, the electron donating property of the added phosphine ligand also increases the rate of hydrogenation of the initially produced aldehydes to form alcohols react much more slowly and produce hydrogenated alkene products as significant side products different catalytic species (no alkylcobalt complexes; only phosphine substituted cobalt carbonyl dimers and hydrides complexes)
the formation of linear aldehydes from internal olefins occurs by insertion of the olefin into a Co-H to form a branched alkyl species, followed by isomerization of the branched alkyl species to the linear alkyl
the phosphine modified cobalt systems are good catalysts for alkene isomerization, producing n:i ratios that are the same as those for reactions initiated with terminal or internal alkenes
in the absence of added phosphorus ligands, the Rh-catalyzed hydroformylation of alkenes is very fast but unselective (1:1 n:i ratio)
Wilkinsons catalyst could be used as a catalyst precursor for hydroformylation at room temperature and atmospheric pressure and was highly selective for n-
aldehydes (n:i >10); no alkene hydrogenation, alcohol formation or alkene isomerization reactions were observed; the active catalyst is HRh(CO)2(PPh3)2
water soluble Rh-triarylphosphine catalysts circumvent the problem of separating and recycling the valuable Rh catalyst the reaction is carried out in a biphasic system in which the catalyst resides in the aqueous phase and the product resides in the organic phase and can be removed from the catalyst without distillation the catalyst is generated from the product of sulfonation of PPh3 to produce P(C6H4-m-SO3-Na+)3, which binds to Rh to generate a highly water soluble catalyst easy separation of catalyst and product without distillation at temperatures that might decompose the Rh catalyst
the hydroformylation of geminally disubstituted alkenes has been shown to form linear products with high selectivity
reactions of many olefins containing EWG at the C=C bond react to form branched products since the branched alkyl complex is more stable when it bears an EWG on the carbon
! !
asymmetric hydroformylation uses C2 symmetric biphosphites the atropisomeric ligand (S,R)-BINAPHOS generates a Rh complex that catalyzes the hydroformylation of vinylarenes with high n:i selectivities and high %ee
the copolymerization of CO and olefins forms polyketones
CATALYSTS FOR ASYMMETRIC HYDROGENATION I. Aromatic Bisphospines with Axial Chiral Backbones - the first enantioselective hydrogenations were achieved with P-chiral bisphosphines - the scope was increased with the use of aromatic phosphines with axial chiral backbones
the dihedral angle of the backbone of the ligand can influence enantioselectivity
II.
the substituents on the P-bound aryl groups (esp. on the 3rd and 5th positions) can increase the enantioselectivity by enhancing gearing of these groups and limiting the degrees of freedom - the electronic properties of the backbone can affect selectivities Compounds with Chiral Ferrocenyl Backbones - ferrocene units have planar chirality when they have 2 different substituents on the same Cp ligand - these are highly active and selective catalysts for many enantioselective processes; they do so without the C2 symmetry of P-chiral ligands and BINAP - these ligands contain a carbon stereocenter and a chiral ferrocene -
III.
P-chiral phosphines
SHARPLESS EPOXIDATION
diethyl tartrate
an enantioselective reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols the stereochemistry of the resulting epoxide is determined by the diastereomer of the chiral tartrate diester used t-butyl hydroperoxide is used as the oxidizing agent titanium tetra(isopropoxide) as catalyst high %ee drawback: requires allylic alcohol
the structure of the catalyst is still uncertain the putative catalyst was determined using X-ray techniques
selectivity decreases with larger R1 but increases with larger R2 and R3 the product from 1,2-allylic diols cannot be predicted with this model a given dialkyl tartrate will preferentially add to the same face independent of substitution on the olefin
the Sharpless epoxidation can also give kinetic resolution of a racemic mixture of secondary 2,3-epoxyalcohols
Examples
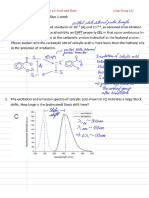
FLUXIONALITY I. Ring Whizzing
3 1H NMR resonances are seen at low T (AABB multiplet for the 4 olefinic protons at 6-6.5ppm, a singlet for the 5-Cp ring at 3-3.5ppm, and another for the C1 proton) only 2 singlets of almost equal intesntiy are seen at room T => ring whizzing of the 1-Cp ring around Fe
not because of dissociation + recombination 1,2 or 1,3-shifts
the indenyl system would rearrange rapidly if a direct 1,3-shift occurs via a 3-allyl intermediate or transition state a direct 1,2-shift would be suppressed in the indenyl system since it would have to go through a high energy o-xylene intermediate therefore, if the molecule is fluxional, it would do so via the 1,3 process; however, the molecule is nonfluxional, which means that the 1,2-shift is the pathway for the 1-Cp rings fluxionality
II.
Interchange of 1-Cp and 5-Cp Rings
at low T, 2 sharp singlets are observed at 5.9ppm and 5.2ppm; both broaden when T is increased => the two 1-Cp rings undergo ring whizzing to give 1 peak and the 5-Cp ring gives the other peak at high T, only 1 sharp singlet is observed => the 5-Cp and 1-Cp rings are exchanging the molecule can undergo 5-Cp and 1-Cp ring exchange because it is coordinately unsaturated (16e) => does not occur in 18e complexes
the exchange is also observed when one of the ligands in the complex has the ability to shuttle between two structures having a difference of 2 electrons (such as NO)
III. Allyl Complexes - anti protons (1-3ppm) are closer to the metal => shielded and high field relative to the syn (2-5ppm) protons; the proton at the middle is at 4-6.5ppm - since the molecule is rigid, the 3 types of protons produce distinctive chemical shifts - the syn and anti protons do not couple with each other but couple with the proton on the central carbon => the syn and anti protons appear as doublets while the central proton appears at a multiplet - upon raising the temperature, the two doublets collapse into one and the multiplet becomes a quintet => only 2 types of protons (4 terminal and 1 nonterminal hydrogen)
IV. -
Allene Complexes 3 signals : the lowest value is for the a and b protons while the highest is for the c proton the coupling constant between a and b is smaller than in free allene 4 signals may be observed if the symmetry is lower
V. Scrambling of CO groups - the interconversion of permutational isomers and geometric isomers in mononuclear complexes (example: Fe(CO)5) - the interconversion of bridging and terminal arrangements and the migration and scrambling of CO groups in binuclear and polynuclear complexes => arises because the energy of the M2(CO)2 system does not vary much over an entire range of configurations
2 signals at low T (cis, trans) and 1 signal at R.T => interconversion between the cis and trans forms at R.T
the cis/trans interconversion results in an overall exchange of bridging and terminal CO groups
the bridging COs are inequivalent because one is cis to Cp and one is cis to CO; the terminal CO should appear as a doublet since it is coupled to one Rh; the bridging CO should appear as a triplet since they are coupled to two Rh atoms
WILKINSONS CATALYST classes of hydrogenation catalysts: those without M-H bond (initiated by OA of H2), those with M-H bond (not initiated by OA of H2), and f block/early transition metal hydrides (no OA)
terminal alkynes are hydrogenated more rapidly than terminal alkenes conjugated dienes are hydrogenated more slowly than isolated alkenes internal and branched alkenes/alkynes are hydrogenated more slowly than terminal ones substrates containing polar functional groups are hydrogenated more rapidly than those without since polar functional groups help the olefin coordinate better with the catalyst
cationic Rh complexes are more active than Wilkinsons catalyst => more electrophilic and thus favors alkene coordination the active form of the cationic Rh complexes is generated after COD is hydrogenated and then detaches from the metal, freeing up 2 coordination sites that are then weakly coordinated by solvent molecules
the cis isomer is preferentially hydrogenated
homogeneous catalysts provide much higher selectivity than heterogeneous catalysts
phosphine ligands used for asymmetric hydrogenations can be categorized into the following types: diphosphines with chiral phosphorus atoms (DIPAMP), diphosphines with chiral backbones (DIOP), atropisomeric ligands (BINAP), those with chiral substituents on P (DuPhos), and ligands with planar symmetry (Josiphos) atropisomers are stereoisomers resulting from hindered rotation about single bonds where the steric strain energy barrier to rotation is high enough to allow for isolation of the conformers (tropos = turn, a=not); they display axial/planar chirality and have ortho substituents that cause steric repulsion
the enantioselective step is the OA of hydrogen to the square planar diastereomeric substrate complex that are in rapid dissociation equilibrium; the major enantiomer of the product arises from the minor substrate catalyst diastereomer
HYDROFORMYLATION the aldehyde produced is the kinetic product; alkanes are the thermodynamic product
d[aldehyde]/dt = k[alkene][Co][pH2]/[pCO] the inverse dependence on CO concentration agrees with the mechanistic requirement of CO dissociation from the 18e complex HCo(CO)4 however, if CO pressure is not high enough, metallic Co becomes deposited; increasing the CO pressure decreases the rate of hydroformylation and reduces the extent of alkene isomerization
the general relative reactivity of alkenes for hydroformylation is as follows:
substituting CO for an electron donating PR3 group increases thermal stability of the catalyst and reduces the CO pressure needed; however, it also increases the hydridic nature of H, which could reduce the aldehyde to an alcohol halides inhibit hydroformylation Rh phosphine catalysts for hydroformylation have the following rate dependence: R=[alkene][Rh][pH2]
larger phosphine ligand bite angles favored higher n/iso ratios the use of phosphites instead of phosphines also lead to higher n/iso ratios
chiral aldehydes are formed only when the addition of H2/CO to the alkene occurs in the Markovnikov manner in enantioselective hydroformylation, higher iso/n ratios are preferred because the n isomer would be achiral
THE ACETIC ACID PROCESS & WACKER PROCESSES
the OA of Me-I is the rate determining step; CO insertion is faster than OA
in the Cativa processes substitutes Rh for Ir; the Ir catalyst is more stable and more soluble in the reaction medium
an iodide ligand is transferred from the anionic Ir complex to the promoter, creating a free site on the catalyst so that the CO can bind; in addition, the OA of R-X is much faster than to Rh
in the Wacker process, the rate follows the equation: R= k[PdCl42-][C2H4]/[Cl-]2[H+]
OLEFIN METATHESIS the Mo catalyst (Schrocks catalyst) has high activity, allowing it to react with internal and terminal olefins and open low strain cyclic olefins; however, it is highly oxophilic, moisture and oxygen sensitive, and has low functional group tolerance the Ru catalyst (Grubbs catalyst) has a higher preference for C=C bonds and have higher functional group tolerance; in addition, they do not require air and moisture free conditions
the first generation Grubbs catalyst has phosphine groups while the second generation Grubbs catalyst has a NHC group => more reactive
the cycloaddition between two alkenes to give cyclobutanes is symmetry forbidden and occurs only photochemically => d orbitals on the metal alkylidene fragment breaks this symmetry and the reaction becomes facile
the driving force for ROM is the release of ring strain
the driving force for RCM is the formation of a thermodynamically stable ring
in ROMP, strained cyclic olefins to produce stereoregular and monodisperse polymers and copolymers; proceeds by the same mechanism as normal olefin metathesis except that the new olefin generated remains attached to the catalyst as part of a growing polymer chain
enyne metathesis, is a reaction between an alkyne and an alkene that forms a 1,3 diene product; can be either intermolecular and intramolecular
intermolecular EM
intramolecular EM
mechanism
example
Anda mungkin juga menyukai
- Types of ReactionsDokumen33 halamanTypes of ReactionsNicksonBelum ada peringkat
- ReactionsDokumen33 halamanReactionsNicksonBelum ada peringkat
- Alkyl, Aryl, Carbene, Alkylidene & Carbyne LigandsDokumen22 halamanAlkyl, Aryl, Carbene, Alkylidene & Carbyne LigandsNorah AltayyarBelum ada peringkat
- Advances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookDari EverandAdvances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookArmando J. L. PombeiroPenilaian: 5 dari 5 bintang5/5 (1)
- Part 4Dokumen4 halamanPart 4Nhu TranBelum ada peringkat
- Organometallic ReactionDokumen22 halamanOrganometallic ReactionFikri Zacky AditamaBelum ada peringkat
- Lecture 2 Reactions of Aldehydes and KetonesDokumen12 halamanLecture 2 Reactions of Aldehydes and KetonesKoki KingBelum ada peringkat
- Manuel C. Francisco: Notes in Organometallic CompoundsDokumen10 halamanManuel C. Francisco: Notes in Organometallic CompoundsManuel FranciscoBelum ada peringkat
- 2 Leaching MechanismsDokumen42 halaman2 Leaching MechanismsThamara Cienfuegos MondragonBelum ada peringkat
- Oxidative AdditionDokumen7 halamanOxidative AdditionMuhammad Hassan ZiaBelum ada peringkat
- ElectrochemistryDokumen43 halamanElectrochemistryShiloh FrederickBelum ada peringkat
- Chapter 6 (Electrolysis) Form 4Dokumen9 halamanChapter 6 (Electrolysis) Form 4AliahYusriBelum ada peringkat
- Chapter 5Dokumen17 halamanChapter 5Atie IekahBelum ada peringkat
- Advanced Inorganic Chemistry (CHM4301) : Alkenes and AlkynesDokumen19 halamanAdvanced Inorganic Chemistry (CHM4301) : Alkenes and AlkynesAnonymous lClX1bBelum ada peringkat
- Key Concept Chapter 10 ElectrolysisDokumen20 halamanKey Concept Chapter 10 ElectrolysisKim KatBelum ada peringkat
- KorosikuliahDokumen20 halamanKorosikuliahWahyu Candra JatmikaBelum ada peringkat
- Classification of Organometallic CompoundsDokumen28 halamanClassification of Organometallic CompoundsDingetegna GodanaBelum ada peringkat
- ATOOCV1 12 0 Addition To Carbon Hetero Multiple BondsDokumen65 halamanATOOCV1 12 0 Addition To Carbon Hetero Multiple BondsSIMARAN JAISWAL 41 M3SBelum ada peringkat
- Oxidative Addition and Reductive EliminationDokumen25 halamanOxidative Addition and Reductive EliminationRafi NovalBelum ada peringkat
- Unit1 Mod 3 Group IV ElementsDokumen9 halamanUnit1 Mod 3 Group IV ElementsNkemzi Elias NzetengenleBelum ada peringkat
- AlkeneDokumen3 halamanAlkeneRichard MohammedBelum ada peringkat
- G-12 - Chapter-2-HandoutDokumen16 halamanG-12 - Chapter-2-Handoutbrook debebeBelum ada peringkat
- Chapter 10 Electrochemistry Text Book ExerciseDokumen31 halamanChapter 10 Electrochemistry Text Book ExerciseshahidkakaBelum ada peringkat
- Electrochemistry Lecture Notes 2018-2019Dokumen69 halamanElectrochemistry Lecture Notes 2018-2019syed100% (1)
- Infrared (IR) Spectroscopy and C O Bond StrengthDokumen29 halamanInfrared (IR) Spectroscopy and C O Bond StrengthSniper ArcheryBelum ada peringkat
- Reductive EliminationDokumen14 halamanReductive EliminationAɞdȗl NąvêêdBelum ada peringkat
- Organometallics - Part 2Dokumen33 halamanOrganometallics - Part 2Rohan Bhupen Shah ae22b052Belum ada peringkat
- C C, C N, C O CouplingDokumen67 halamanC C, C N, C O CouplingAnonymous vRpzQ2BLBelum ada peringkat
- Chemistry Homework Material (Electrolysis) by Adaugo Olaedo UbahDokumen3 halamanChemistry Homework Material (Electrolysis) by Adaugo Olaedo UbahAdaugo UbahBelum ada peringkat
- Chemistry - ElectrolysisDokumen51 halamanChemistry - Electrolysisjoannavera2020Belum ada peringkat
- Inorganic Reactions and Methods, The Formation of Bonds to Elements of Group IVB (C, Si, Ge, Sn, Pb) (Part 4)Dari EverandInorganic Reactions and Methods, The Formation of Bonds to Elements of Group IVB (C, Si, Ge, Sn, Pb) (Part 4)A. P. HagenBelum ada peringkat
- Gist 2 Xii Chemistry 2023Dokumen15 halamanGist 2 Xii Chemistry 2023sroy191006Belum ada peringkat
- Name: Pranav Arun Patil Roll No: A007Dokumen20 halamanName: Pranav Arun Patil Roll No: A007Pranav PatilBelum ada peringkat
- Organic ReactionsDokumen44 halamanOrganic ReactionsJanhavi PatilBelum ada peringkat
- Electricity and ChemistryDokumen13 halamanElectricity and Chemistrysalman ahsanBelum ada peringkat
- AdisiDokumen107 halamanAdisiazizgagabBelum ada peringkat
- of HydrocarbonsDokumen45 halamanof HydrocarbonsSneha KediaBelum ada peringkat
- Organometallic ChemistryDokumen14 halamanOrganometallic ChemistrySelva Mani100% (1)
- Screenshot 2022-06-19 at 3.39.23 PMDokumen47 halamanScreenshot 2022-06-19 at 3.39.23 PMWalaa AdelBelum ada peringkat
- AldehydeKetonesNotessee PDFDokumen7 halamanAldehydeKetonesNotessee PDFSubhabrata MabhaiBelum ada peringkat
- C-H ActivationDokumen24 halamanC-H ActivationSuman MondalBelum ada peringkat
- Electrolytic ConductionDokumen38 halamanElectrolytic ConductionVishwanath ReddyBelum ada peringkat
- Department of Chemistry, Ben-Gurion University of The Negev, Beer-Sheva, Israel Department of Biological Chemistry, The College of Judea and Samaria, Ariel, IsraelDokumen19 halamanDepartment of Chemistry, Ben-Gurion University of The Negev, Beer-Sheva, Israel Department of Biological Chemistry, The College of Judea and Samaria, Ariel, IsraelRafael Ricardo Celin ManceraBelum ada peringkat
- ElectrolysisDokumen17 halamanElectrolysisSuhaan HussainBelum ada peringkat
- Chemistry Lesson23 (Electrochemistry3)Dokumen23 halamanChemistry Lesson23 (Electrochemistry3)Siang DanielBelum ada peringkat
- Aldehydes, Ketones and Carboxylic Acids: Teacher OrientationDokumen9 halamanAldehydes, Ketones and Carboxylic Acids: Teacher OrientationAnand RawatBelum ada peringkat
- 9 Ligand SubstitutionDokumen89 halaman9 Ligand SubstitutionJuan Carlos MedinaBelum ada peringkat
- Part 7 Redox Reactions, Chemical Cells and Electrolysis - Part 2Dokumen37 halamanPart 7 Redox Reactions, Chemical Cells and Electrolysis - Part 2冰雪樱恋Belum ada peringkat
- Group IV ElementsDokumen41 halamanGroup IV ElementsNomi KhattakBelum ada peringkat
- BT1 Inorganic Chem Solutions 2012Dokumen40 halamanBT1 Inorganic Chem Solutions 2012Shrabonti MohammedBelum ada peringkat
- Kendriya Vidyalaya Bowenpally: Chemistry Investigatory Project 2020-21Dokumen10 halamanKendriya Vidyalaya Bowenpally: Chemistry Investigatory Project 2020-21koustubhBelum ada peringkat
- Haloalkanes and Haloarenes NotesDokumen18 halamanHaloalkanes and Haloarenes NotesAnkitha shajiBelum ada peringkat
- Ch13 ElectrolysisDokumen35 halamanCh13 ElectrolysishahaBelum ada peringkat
- Presentation KPTDokumen79 halamanPresentation KPTNor Fatihah Ab Malek100% (1)
- ELECTROCHEMISTRY WorksheetDokumen83 halamanELECTROCHEMISTRY WorksheetbhargavintnaiduBelum ada peringkat
- 32 DR Zhou Lecture 32 PDFDokumen23 halaman32 DR Zhou Lecture 32 PDFBUCH203Belum ada peringkat
- Electrolyte SolutionsDokumen167 halamanElectrolyte Solutionszatty kimBelum ada peringkat
- Electricity and ChemistryDokumen5 halamanElectricity and Chemistrymohamed komiBelum ada peringkat
- ElectrochemistryDokumen58 halamanElectrochemistryWatan SahuBelum ada peringkat
- NMR Yield CalculationDokumen4 halamanNMR Yield CalculationpolypeptideBelum ada peringkat
- Units of Measurement: Introduction To ChemistryDokumen4 halamanUnits of Measurement: Introduction To ChemistrypolypeptideBelum ada peringkat
- Glass Care Safe Handling PDFDokumen16 halamanGlass Care Safe Handling PDFAdinda SaraswatiBelum ada peringkat
- Glass Care Safe Handling PDFDokumen16 halamanGlass Care Safe Handling PDFAdinda SaraswatiBelum ada peringkat
- Homework 3Dokumen13 halamanHomework 3polypeptideBelum ada peringkat
- Homework 3Dokumen13 halamanHomework 3polypeptideBelum ada peringkat
- Chem 14A Discussion Section: Coordination ChemistryDokumen3 halamanChem 14A Discussion Section: Coordination ChemistrypolypeptideBelum ada peringkat
- Chemical Equilibrium (Final Review)Dokumen20 halamanChemical Equilibrium (Final Review)polypeptideBelum ada peringkat
- Mechanism of Microbial InfectionsDokumen122 halamanMechanism of Microbial InfectionspolypeptideBelum ada peringkat
- Viral Diseases - Mechanisms of Microbial InfectionsDokumen105 halamanViral Diseases - Mechanisms of Microbial InfectionspolypeptideBelum ada peringkat
- Lecture of Zoonoses at NTU 2015Dokumen159 halamanLecture of Zoonoses at NTU 2015polypeptideBelum ada peringkat
- Midterm RecitationDokumen12 halamanMidterm RecitationpolypeptideBelum ada peringkat
- Animal ConditioningDokumen27 halamanAnimal ConditioningpolypeptideBelum ada peringkat
- Aic I HW7Dokumen2 halamanAic I HW7polypeptideBelum ada peringkat
- Chapter24 Materials ChemDokumen25 halamanChapter24 Materials ChempolypeptideBelum ada peringkat
- Chemistry GRE SampleDokumen0 halamanChemistry GRE Sampleyoostan100% (2)
- Pka TablesDokumen6 halamanPka TablesAlessandro MoreniBelum ada peringkat
- General Chemistry C Homework 6 Due On 12/11 Name: ID: DepartmentDokumen2 halamanGeneral Chemistry C Homework 6 Due On 12/11 Name: ID: DepartmentpolypeptideBelum ada peringkat
- Chapter 28 LCDokumen5 halamanChapter 28 LCpolypeptideBelum ada peringkat
- Functional Groups in Organic ChemistryDokumen1 halamanFunctional Groups in Organic ChemistrygznmemberBelum ada peringkat
- Protective GroupDokumen26 halamanProtective Groupchhan4Belum ada peringkat
- Chapter 7 Intro To Optical InstrumentsDokumen10 halamanChapter 7 Intro To Optical InstrumentspolypeptideBelum ada peringkat
- Chapter1s StatMechDokumen22 halamanChapter1s StatMechpolypeptideBelum ada peringkat
- C H ActivationDokumen55 halamanC H Activationpolypeptide100% (1)
- E26 QuestionsDokumen5 halamanE26 QuestionspolypeptideBelum ada peringkat
- FLUXIONALITYDokumen7 halamanFLUXIONALITYpolypeptideBelum ada peringkat
- Est-Ce QueDokumen6 halamanEst-Ce QuepolypeptideBelum ada peringkat
- Alkyne Metathesis & Enyne MetathesisDokumen3 halamanAlkyne Metathesis & Enyne MetathesispolypeptideBelum ada peringkat
- Literature Report 2Dokumen12 halamanLiterature Report 2polypeptideBelum ada peringkat
- Test Taking StrategiesDokumen193 halamanTest Taking StrategiesAmy Kennedy100% (1)
- Flow Master V7 Release NotesDokumen6 halamanFlow Master V7 Release NotesGabriel ValdiviaBelum ada peringkat
- Pbat 10 eDokumen16 halamanPbat 10 eapi-290101261Belum ada peringkat
- Paper Insulated Distribution CablesDokumen10 halamanPaper Insulated Distribution Cablesdeepak2628Belum ada peringkat
- ChE ThermodynamicsDokumen49 halamanChE ThermodynamicsMiguel FelisildaBelum ada peringkat
- Aspen Plus IGCC ModelDokumen12 halamanAspen Plus IGCC ModelHAFIZ IMRAN AKHTERBelum ada peringkat
- Instrumentation ITPDokumen19 halamanInstrumentation ITPMohammad IrfanBelum ada peringkat
- 3RH29111FA22 Datasheet enDokumen6 halaman3RH29111FA22 Datasheet enFlavioBelum ada peringkat
- Karlsson Capacity Control Residential HP Heating Systems TESIDokumen115 halamanKarlsson Capacity Control Residential HP Heating Systems TESIGiovanniCuocoBelum ada peringkat
- KI1101-2012-KD Lec07 IntermolecularAttractionsDokumen128 halamanKI1101-2012-KD Lec07 IntermolecularAttractionsAchmad RochliadiBelum ada peringkat
- Natco Dual Frequency: Electrostatic TreaterDokumen2 halamanNatco Dual Frequency: Electrostatic TreaterHERNANDO CASTAÑOBelum ada peringkat
- PowerTHRU BrochureDokumen4 halamanPowerTHRU BrochureNesa VijinBelum ada peringkat
- Doosan DX225LCA Electric Circuit 110705Dokumen1 halamanDoosan DX225LCA Electric Circuit 110705Eduardo Ariel Bernal97% (30)
- International Actor CPs - Northwestern 2014Dokumen215 halamanInternational Actor CPs - Northwestern 2014DerekBelum ada peringkat
- In Touch With The Medium: Level Monitoring SensorsDokumen24 halamanIn Touch With The Medium: Level Monitoring Sensorsm_najmanBelum ada peringkat
- Practice Questions Chs 21-24 1Dokumen40 halamanPractice Questions Chs 21-24 1jevanjunior0% (1)
- The Chemical Engineer - Issue 945 - March 2020Dokumen68 halamanThe Chemical Engineer - Issue 945 - March 2020George Isaac McQuilesBelum ada peringkat
- ELSD IQ OQ ProtocolDokumen7 halamanELSD IQ OQ ProtocolRajan RamaswamiBelum ada peringkat
- Plug in Electric Vehicles in Smart Grids Integration TechniquesDokumen355 halamanPlug in Electric Vehicles in Smart Grids Integration Techniqueseng_alhemyari100% (3)
- App H - Evaluation of Sludge Dryer and Dewatering FacilitiesDokumen77 halamanApp H - Evaluation of Sludge Dryer and Dewatering FacilitiesRajan BhosaleBelum ada peringkat
- Bangui Wind FarmDokumen5 halamanBangui Wind FarmChristopher YsitBelum ada peringkat
- BS EN ISO 9934-1-Type of MagnetizationDokumen3 halamanBS EN ISO 9934-1-Type of Magnetizationbhavin178Belum ada peringkat
- PT7Dokumen2 halamanPT7Anonymous j5apk2AumBelum ada peringkat
- KD2250-F-SDMO (Alternator Data Sheet SDMO 2000KVA Prime Generator Set)Dokumen6 halamanKD2250-F-SDMO (Alternator Data Sheet SDMO 2000KVA Prime Generator Set)schraz4575Belum ada peringkat
- Aeroacoustic Testing of Wind Turbine Air PDFDokumen271 halamanAeroacoustic Testing of Wind Turbine Air PDFAlexandre FariaBelum ada peringkat
- GA-I (H) D 1000 Series ROBOTDokumen1 halamanGA-I (H) D 1000 Series ROBOTmfarrukhkBelum ada peringkat
- Aalborg OL: The Large Capacity Modular Boiler PlantDokumen2 halamanAalborg OL: The Large Capacity Modular Boiler Plantthlim19078656Belum ada peringkat
- Solved Problems On RectifiersDokumen12 halamanSolved Problems On RectifiersAravind KarthikBelum ada peringkat
- Instruction Manual Professional Timing Light Instruction Manual Professional Timing LightDokumen1 halamanInstruction Manual Professional Timing Light Instruction Manual Professional Timing LightJohn DePBelum ada peringkat
- Electrical Engineering Portal - Com Principles For Controlling HarmonicsDokumen3 halamanElectrical Engineering Portal - Com Principles For Controlling HarmonicsRobert GalarzaBelum ada peringkat