1824 7288 38 63
Diunggah oleh
Silvi Anita Uslatu RDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
1824 7288 38 63
Diunggah oleh
Silvi Anita Uslatu RHak Cipta:
Format Tersedia
Tolone et al. Italian Journal of Pediatrics 2012, 38:63 http://www.ijponline.
net/content/38/1/63
ITALIAN JOURNAL OF PEDIATRICS
RESEARCH
Open Access
Evaluation of Helicobacter Pylori eradication in pediatric patients by triple therapy plus lactoferrin and probiotics compared to triple therapy alone
Salvatore Tolone1, Valeria Pellino2, Giovanna Vitaliti3, Angela lanzafame3 and Carlo Tolone2*
Abstract
Background: To evaluate whether the addition of a probiotic could improve Helicobacter pylori (H.P.) eradication rates and reduce the side effects of treatment in children. Methods: Between July 2008 and July 2011 all patients with a clinical, laboratory and endoscopic diagnosis of H.P. positive gastritis referred to our Unit were included in the study. Patients suffering from allergy to any of drugs used in the study, with previous attempts to eradicate H.P. and those who received antibiotics, PPIs or probiotics within 4 weeks were excluded from the present study. Patients were randomized into two therapy regimens (group A and B): both groups received standard triple treatment (omeprazole, amoxicillin and clarithromycin) while only group B patients were also given a probiotic (Probinul - Cadigroup). Patients compliance was evaluated at the end of the treatment. Successful eradication was defined as a negative 13 C-urea breath test (C13-ubt) result four weeks after therapy discontinuation. Results: A total of 68 histopathologically proven H.P.-infection children (32 male and 36 females) were included in the study. All of the patients in both groups used more than 90% of the therapies and no patients were lost at follow up. All side effects were selflimiting and disappeared once the therapy was terminated. Epigastric pain was observed in 6 (17.6%) group A vs 2 (5.8%) group B patients (P<0.05), nausea in 3 (8.8%) group A vs 1 (2.9%) group B patients (P<0.05); vomiting and diarrhea were observed in 2(5.8%) and 8 (23.5%) group A patients, respectively and never in group B (P<0.05). There was no significant difference between the two groups in terms of constipation (5.8% in group A and B). Four weeks after the completion of therapy, 56/68 patients (82.3%) tested negative for H.P. on C13-ubt. H.P. was eradicated in 26 patients (76.4%) in group A and in 30 patients (88.2%) in group B. There was no significantly difference in the rate of H.P. eradication between group A and group B (p=0.1), although the success rate for H.P. eradication was higher in group B than in group A. Conclusion: The addition of a probiotic formula to triple therapy significantly decreased the frequency of epigastric pain, nausea, vomiting and diarrhea. Keywords: H.P, H.P. eradication, Children gastritis, Probiotic
* Correspondence: carlo.tolone@libero.it 2 Department of Pediatrics, Second University of Naples, Via L De Crecchio, Naples 80138, Italy Full list of author information is available at the end of the article
2012 Tolone et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Tolone et al. Italian Journal of Pediatrics 2012, 38:63 http://www.ijponline.net/content/38/1/63
Page 2 of 5
Background Helicobacter pylori (H.P.) infection is a major cause of chronic gastritis and peptic ulcers and it is a risk factor for gastric malignancies, adenocarcinoma and low grade gastric mucosa associated lymphoid tissue (MALT) lymphoma [1]. According to the Maastricht III consensus report, H.P. eradication is recommended for patients with gastroduodenal ulcer disease, atrophic gastritis, MALT lymphoma, first degree relatives of patients with gastric cancer, patients with unexplained iron deficiency anemia and chronic idiopathic thrombocytopenic purpura. In both developed and developing countries, H.P. infection is most frequently acquired during childhood, and it is associated with family size, familial clustering, low socioeconomic status and education. The first line treatment for H.P. infection, as recommended by the Maastricht 22000 Consensus Report , is a 714 days triple therapy which includes amoxicilline, clarithromycin, OR metronidazole and a proton-pump inhibitor (PPI) [2], though new strategies may be required for treatment both in adults and children. As for adults, infanti treatment will fail in approximately 10-35% of patients, and H.P. infection will remain resulting from several factors, including nonadherence to therapy related to adverse effects or complicated dosing regimens and increasing antibiotic resistance [3-5]. To overcome this problem, both in adults than in children, alternative and adjuvant therapies have been added to conventional treatment, such as probiotics (PB) [6,7]. Although there is some controversy as to whether supplementation with probiotic improves the H.P. eradication rates [8-10], several meta-analysis and reviews have suggested that probiotics can improve the H.P. eradication rate by approximately 5-10% [10-13]. However, it is evident that not all probiotics are created equal, that the beneficial effects are strain specific, and each strain must be evaluated individually. Besides treatment studies on children are limited by the small number of infected children in each individual center [14-16], therefore this study aimed to evaluate whether the addition of a commercially multi-strain probiotics to a 7 days triple therapy in children could improve H.P. eradication rates and reduce the side effects of treatment. Methods Between July 2008 and July 2011 children referred to the Department of Pediatrics of the University of Naples with dyspeptic complaints such as heartburn, dyspepsia, nausea and epigastric pain, were enrolled in this study. The study was approved by Ethical Committee of the University of Naples. Pediatric patients with a clinical, laboratory and endoscopic diagnosis of H.P. positive gastritis and the other conditions necessary H.P. eradication for Maastricht III consensus report were included in the study. Exclusion
criteria were: 1) allergy to any of drugs used in the study 2) previous attempts to eradicate H.P. 3) receipt of antibiotics, PPIs or probiotics within 4 weeks of the study. Informed consent was obtained from all patients and all positive 13 C-urea breath test (C13-ubt) patients underwent upper endoscopy. Two samples were taken from the gastric antrum and compass for histologic assessment, and the biopsy specimens were fixed in 10% formalin solution. Preparation were stained with hematoxylin-eosin and modified Giemsa stains and were evaluated according to updated Sydney classification. H.P. positive patients were randomized into two therapy regimens: patients in group A were given omeprazole (1 mg/kg before breakfast), amoxicillin (50 mg/kg b.i.d. after meals), clarithromycin (15 mg/kg b.i.d. after meals) for 7 days, whereas patients in group B were given the same drugs and a probiotic once a day for 7 days. Patients were instructed to take PPI 30 mins before breakfast, the antibiotics 5 mins after breakfast and dinner, and PB supplement in the afternoon. The PB supplement was taken in a commercially available form containing 5 109 Lactobacillus plantarum, 2 109 L. reuterii, 2 109 L. casei subsp. rhamnosus, 2 109 Bifidobacterium infantis and B. longum, 1 109 L. salivarius, 1 109 L. acidophilus, 5 109 Streptococcus termophilus, and 1 109 L. sporogenes (Lactobacillaceae). This PB formula (5g/dayose q.d.) (Probinul - Cadigroup) was selected because it contains high concentrations of a wide range of bacteria, as well as inuline as a prebiotic. Parents were asked to report any side effects of therapy during the treatment period and were given a possible side effect list, such as epigastric pain, nausea, diarrhea and constipation. Patients compliance was evaluated at the end of the treatment on the basis of diary that patients were asked to fill with pill count and was considered as completed if >90% of the medication had been taken. Successful eradication was defined as a negative C13ubt result four weeks after discontinuation of the therapy.
Statistical analysis
Data were collected prospectically in an electronic database (Excell Microsoft). Fishers exact test was carried out to determine the efficacy of the two treatments. Results were considered statistically significant for P values less than 0.05.
Results A total of 68 histopathologically proven H.P.-infection children (32 male and 36 females) were included in the study. The mean age of all children was 8,3 +/ 3,4 years. The patients were randomized into group A (triple therapy n 34 patients) and group B (triple therapy plus probiotic n 34 patients) for H.P. eradication. The age
Tolone et al. Italian Journal of Pediatrics 2012, 38:63 http://www.ijponline.net/content/38/1/63
Page 3 of 5
Table 1 Incidence of side effects of the treatment
Side Effects Epigastric pain Nausea Vomiting Diarrhea Constipation Group A (N=34) 6 (17.6%) 3 (8.8%) 2 (5.8%) 8 (23.5%) 2 (5.8%) Group B (N=34) 2 (5.8%) 1 (2.9%) 0 0 2 (5.8%) P value <0.05 <0.05 <0.05 <0.05 N.S.
distribution of patients and gender in group A and group B were similar. All of parents returned the daily diary filled up with side-effects and pill count. All of the patients in both groups used more than 90% of the therapies and no patients were lost at follow up. The prevalence of epigastric pain, nausea, vomiting and diarrhea was significantly higher (P<0.05) in group A than in group B . There was no significant difference between the two groups in terms of constipation. (Detailed data on side effects are summarized in Table 1). All side effects were self-limiting and disappeared once the therapy was terminated. Four weeks after the completion of therapy, 56/68 patients (82.3%) tested negative for H.P. on C13-ubt. H. P. was eradicated in 26 patients (76.4%) in group A and in 30 patients (88.2%) in group B (Figure 1). There was no significantly difference in the rate of H.P. eradication between group A and group B (p=0,1), although the success rate for H.P. eradication was higher in group B than in group A.
Discussion It is well known that childhood is an important period for acquisition of H.P. infection. Intrafamiliar trasmission of the infection , especially from mother to child, has been hypothisized as the major mode of dissemination [17]. H.P. is considered to be the major cause of chronic gastritis and duodenal ulcer in childhood and an important cofactor in the development of gastric cancer
[18]. Unfortunately eradication therapy is not always successful and reports of failed H.P. eradication therapy are increasing. Therefore, recent review studies report eradication rates of standard triple therapy in children below 75% [14,19]. Moreover this regimens have the disadvantage of risking poor compliance and causing sideeffects especially in children. Nowadays there is considerable interest in alternative therapies or adjunctive treatment against H.P. to reduce some of the drawbacks associated with the antibiotic consumption and to increase the eradication rates. Some adjuvant therapy trials both in adults and in children incorporate probiotics [20-22]. In our study H.P. eradication was achieved in 82.3% of patients. A little increase in the eradication rate and a significant reduction in side effects were observed in the group treated with triple therapy plus Probinul PB. Resistant patients were offered to second line therapy (omeprazole 1 mg/kg/d, amoxicillin 50 mg/kg/d and metronidazole 20 mg/kg/d), according to NASPGHAN guidelines, confirming the contribution of a high background resistance to clarithromycin. Also, guidelines on HP infection in children issued so far suggest that the antibiotic susceptibility test should be performed whenever available [15]. However, we did not performed susceptibility test for first line antibiotic therapy. Probiotics include viable microorganism that have a beneficial effects for the prevention and treatment of specific pathological conditions [23]. Principle mechanisms include interference with pathogenic toxins, preservation of cellular physiology , interference with pathogen attachment and interaction with normal microbiota [24,25] . In addition, stimulation or modulation of immune responses, both within the lumen and systemically, although not clearly linked to H.P. infection, may contribute [26,27]. Besides probiotics may be beneficial in reducing adverse effects and increasing tolerability of HP eradication regimens. Several studies evaluated whether probiotic supplementation might help to prevent or reduce drug-
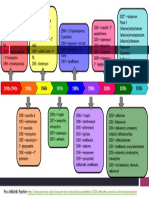
Figure 1 Patients enrollment and therapy flowchart.
Tolone et al. Italian Journal of Pediatrics 2012, 38:63 http://www.ijponline.net/content/38/1/63
Page 4 of 5
related side effects during H.P. eradication therapy in adults [28-30]. Up to date, in the pediatric population, few randomized studies have evaluated whether consumption of probiotics could increase H.P. eradication rates and reduce the side effects of treatment [31-33]. In fact, in a recent trial by Szajewska et al. [33] was identified that the use of Lactobacillus GG along with standard triple therapy didnt resulted in an increased eradication rate and decreased overall therapy-related side effects. Some of these trials do not provide evidence on the beneficial effect in children of supplementation of probiotics to triple therapy for eradicating H.P. infection nor for positively affecting therapy related symptoms and overall treatment tolerance [34]. However other investigators have shown that in symptomatic H.P. positive children, the occurrence of antibiotic associated side-effects was significantly reduced by the addition of probiotics compared with the placebo supplemented group [31]. H.P. eradication depends on a number of factor, including patients compliance, adverse effects, bacterial resistance, poor drug distribution and concentration, socioeconomic conditions and geographic differences. So antibiotic related side effects may be depending on different probiotic strains taken during the H.P. eradication therapy. In our study the choice of the Probinul probiotic formula was determinated by the fact that it contains high concentration of a wide range of bacteria, as well as inuline as prebiotic. Previously we have demonstrated that this probiotic formula was able to reduce antibiotic gastro-intestinal related side effects in children treated with amoxicillin therapy for pneumonia infections [35]. Besides De Bortoli et al. have demonstrated that the addition of bovine lactoferrin and this probiotic formula to standard triple eradication therapy could improve the H.P. eradication rate and reduce side effects in adult [36]. In this study the combination of standard triple therapy + probiotic + bovine lactoferrin was more effective than triple therapy alone. This could be explained by the combined effect of the bactericidal and bacteriostatic properties of bovine lactoferrin and the mechanism of the probiotics including their direct, nonspecific, bacteriostatic activity and their enhancement of immunoglobulin A production [37]. Probiotics may act through both immunological as well as non-immunological mechanism in H.P. eradication. The latest mechanism includes providing antimicrobial substances, competing with H.P. for adhesion and providing a mucosal barrier. When there are decreases in side effects, compliance improves [26]. The efficacy of probiotic supplementation for reducing side effects during the course of anti HP regimens appears to be dependent of which probiotic species are used. In fact probiotics
must be metabolically active in the intestinal lumen, where they should survive but not persist after the therapy regimen has been completed. They must be acid and bile resistant and should be antagonist to pathogenic bacteria [38-40]. The present study has shown that the addiction of the Probinul probiotic formula to standard antibiotic treatment reduced in children H.P. therapy-associated side effects (occurrence of side effects: 61.5% in group A vs 14.5% in group B). The H.P. eradication was achieved in 26 of 34 patients in group A (76.4%) and in 30 of 34 patients (88.2%) in group B. Although the success rate was higher in group A than in group B, the different was not significant. Also, we preferred to use the conventional 7-days triple therapy to better highlight the efficacy of probiotic supplement; furthermore extending the duration of therapy form 7 to 14 days is not clearly associated with an increased eradication rate [41]. However, our study presents some limitations, as a relative small number of patients and absence of a placebo-control group for the probiotics; also, the treatment group with probiotics is likely to be superior to the control group if the sample size is larger. In conclusion our study suggests that the addition of this probiotic formula to triple therapy did not increase (significantly) the H.P. eradication rates; however it significantly decreased the frequency of epigastric pain, nausea, vomiting and diarrhea. In children with H.P. infection, we think, there is evidence to recommend the use of this probiotic formula along with standard triple therapy as an option for decreasing overall therapy related side effects and slightly increasing the eradication rates.
Competing interests The authors declare that they have no competing interests. Authors contributions TS designed the study and wrote the manuscript; PV, VG and LA provided data collection and were involved in editing the manuscript; TC designed the study, wrote the manuscript and gave final approval of the version to be published. All authors read and approved the final manuscript. Author details 1 Division of General and Bariatric Surgery, Second University of Naples, Via Pansini 5, Naples 80131, Italy. 2Department of Pediatrics, Second University of Naples, Via L De Crecchio, Naples 80138, Italy. 3Department of Medical and Pediatrics Science, Via SSofia n.78, Catania 95123, Italy. Received: 14 July 2012 Accepted: 17 October 2012 Published: 31 October 2012 References 1. Suerbaum S, Michetti P: Helicobacter pylori infection. N Engl J Med 2002, 347:11751186. 2. Malfertheiner P, Mgraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G, European Helicobacter Pylori Study Group (EHPSG): Current concepts in the management of Helicobacter pylori infection the Maastricht 22000 Consensus Report. Aliment Pharmacol Ther 2002, 16:167180.
Tolone et al. Italian Journal of Pediatrics 2012, 38:63 http://www.ijponline.net/content/38/1/63
Page 5 of 5
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
Chey WD, Wong BC, Practice Parameters Committee of the American College of Gastroenterology: American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007, 102:18081825. Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ: Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007, 56:772781. Mamori S, Higashida A, Kawara F, Ohnishi K, Takeda A, Senda E, Ashida C, Yamada H: Age-dependent eradication of Helicobacter pylori in Japanese patients. World J Gastroenterol 2010, 16:41764179. Gotteland M, Brunser O, Cruchet S: Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther 2006, 23:10771086. Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD: Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2007, 25:155168. Ozdil K, Calhan T, Sahin A, Senates E, Kahraman R, Yzbasioglu B, Demirdag H, Demirsoy H, Skmen MH: Levofloxacin based sequential and triple therapy compared with standard plus probiotic combination for Helicobacter pylori eradication. Hepatogastroenterology 2011, 58:11481152. Tursi A, Brandimarte G, Giorgetti GM, Modeo ME: Effect of Lactobacillus casei supplementation on the effectiveness and tolerability of a new second-line 10-day quadruple therapy after failure of a first attempt to cure Helicobacter pylori infection. Med Sci Monit 2004, 10:CR662CR666. Medeiros JA, Gonalves TM, Boyanova L, Pereira MI, de Carvalho JN, Pereira AM, Cabrita AM: Evaluation of Helicobacter pylori eradication by triple therapy plus Lactobacillus acidophilus compared to triple therapy alone. Eur J Clin Microbiol Infect Dis 2011, 30:555559. Szajewska H, Horvath A, Piwowarczyk A: Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther 2010, 32:10691079. Chenoll E, Casinos B, Bataller E, Astals P, Echevarra J, Iglesias JR, Balbarie P, Ramn D, Genovs S: Novel probiotic Bifidobacterium bifidum CECT 7366 strain active against the pathogenic bacterium Helicobacter pylori. Appl Environ Microbiol 2011, 77:13351343. Wilhelm SM, Johnson JL, Kale-Pradhan PB: Treating bugs with bugs: the role of probiotics as adjunctive therapy for Helicobacter pylori. Ann Pharmacother 2011, 45:960966. Francavilla R, Lionetti E, Castellaneta SP, Magist AM, Boscarelli G, Piscitelli D, Amoruso A, Di Leo A, Miniello VL, Francavilla A, et al: Improved efficacy of 10-Day sequential treatment for Helicobacter pylori eradication in children: a randomized trial. Gastroenterology 2005, 129:14141419. Oderda G, Shcherbakov P, Bontems P, Urruzuno P, Romano C, Gottrand F, Gmez MJ, Ravelli A, Gandullia P, Roma E, European Pediatric Task Force on Helicobacter pylori, et al: Results from the pediatric European register for treatment of Helicobacter pylori (PERTH). Helicobacter 2007, 12:150156. Lionetti E, Indrio F, Pavone L, Borrelli G, Cavallo L, Francavilla R: Role of probiotics in pediatric patients with Helicobacter pylori infection: a comprehensive review of the literature. Helicobacter 2010, 15:7987. Kivi M, Tindberg Y, Srberg M, Casswall TH, Befrits R, Hellstrm PM, Bengtsson C, Engstrand L, Granstrm M: Concordance of Helicobacter pylori strains within families. J Clin Microbiol 2003, 41:56045608. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ: Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001, 345:784789. Kato S, Konno M, Maisawa S, Tajiri H, Yoshimura N, Shimizu T, Toyoda S, Nakayama Y, Iinuma K: Results of triple eradication therapy in Japanese children: a retrospective multicenter study. J Gastroenterol 2004, 39:838843. Song MJ, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI: The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter 2010, 15:206213. Kim MN, Kim N, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Kim JS, Jung HC, Song IS: The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter 2008, 13:261268. Chiesa C, Pacifico L, Anania C, Poggiogalle E, Chiarelli F, Osborn JF: Helicobacter pylori therapy in children: overview and challenges. Int J Immunopathol Pharmacol 2010, 23:405416.
23. Jonkers D, Stockbrgger R: Review article: Probiotics in gastrointestinal and liver diseases. Aliment Pharmacol Ther 2007, 26:133148. 24. Leonardi S, Miraglia del Giudice M, La Rosa M, Bellanti J: Atopic disease, immune and environment. Allergy Asthma Proc 2007, 28:410417. 25. del Giudice MM, Rocco A, Capristo C: Probiotics in the atopic march: highlights and new insights. Dig Liv Dis 2006, 38:S288S290. 26. McFarland LV: Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol 2010, 16:22022222. 27. Franceschi F, Cazzato A, Nista EC, Scarpellini E, Roccarina D, Gigante G, Gasbarrini G, Gasbarrini A: Role of probiotics in patients with Helicobacter pylori infection. Helicobacter 2007, 12:5963. 28. Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P, et al: The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2001, 15:163169. 29. Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A: Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol 2002, 97:27442749. 30. Myllyluoma E, Veijola L, Ahlroos T, Tynkkynen S, Kankuri E, Vapaatalo H, Rautelin H, Korpela R: Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapya placebo-controlled, doubleblind randomized pilot study. Aliment Pharmacol Ther 2005, 21:12631272. 31. Lionetti E, Miniello VL, Castellaneta SP, Magist AM, de Canio A, Maurogiovanni G, Ierardi E, Cavallo L, Francavilla R: Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther 2006, 24:14611468. 32. Hurduc V, Plesca D, Dragomir D, Sajin M, Vandenplas Y: A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Paediatr 2009, 98:127131. 33. Szajewska H, Albrecht P, Topczewska-Cabanek A: Randomized, doubleblind, placebo-controlled trial: effect of lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr 2009, 48:431436. 34. Goldman CG, Barrado DA, Balcarce N, Rua EC, Oshiro M, Calcagno ML, Janjetic M, Fuda J, Weill R, Salgueiro MJ, et al: Effect of a probiotic food as an adjuvant to triple therapy for eradication of Helicobacter pylori infection in children. Nutrition 2006, 22:984988. 35. Tolone C: Efficacy of Probinul in reduction of antibiotic gastro-intestinal related side effects in children with respiratory infections. In Proceeding of 62 National Congress of Italian Society of Pediatrics; 2006. 36. de Bortoli N, Leonardi G, Ciancia E, Merlo A, Bellini M, Costa F, Mumolo MG, Ricchiuti A, Cristiani F, Santi S, et al: Helicobacter pylori eradication: a randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am J Gastroenterol 2007, 102:951956. 37. Fukushima Y, Kawata Y, Hara H, Terada A, Mitsuoka T: Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol 1998, 42:3944. 38. Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD: Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol 2000, 165:10221029. 39. del Giudice M, De Luca MG: The role of probiotics in the clinical management of food allergy and atopic dermatitis. J Clin Gastroenterol 2004, 38(6 Suppl):S84S85. 40. Del Giudice MM, Leonardi S, Maiello N, Brunese FP: Food allergy and probiotics in childhood. J Clin Gastroenterol 2010, 44:S22S25. 41. Hsu PI, Wu DC, Wu JY, Graham DY: Is there a benefit extending the duration of Helicobacter Pylori sequential therapy to 14 days? Helicobacter 2011, 16:146152.
doi:10.1186/1824-7288-38-63 Cite this article as: Tolone et al.: Evaluation of Helicobacter Pylori eradication in pediatric patients by triple therapy plus lactoferrin and probiotics compared to triple therapy alone. Italian Journal of Pediatrics 2012 38:63.
Anda mungkin juga menyukai
- Lactobacillus Reuteri Helicobacter PyloriDokumen8 halamanLactobacillus Reuteri Helicobacter PyloriSrisailan KrishnamurthyBelum ada peringkat
- Randomized Controlled Trial Comparing Standard Triple and Sequential Regimens For HelicobacterDokumen7 halamanRandomized Controlled Trial Comparing Standard Triple and Sequential Regimens For HelicobacterGantuya BoldbaatarBelum ada peringkat
- 2 PDFDokumen5 halaman2 PDFBangladesh Academy of SciencesBelum ada peringkat
- Celinski 2011 - Effects of Melatonin and Tryptophan On Healing of Gastric and Duodenal Ulcers With Helicobacter PyloriDokumen6 halamanCelinski 2011 - Effects of Melatonin and Tryptophan On Healing of Gastric and Duodenal Ulcers With Helicobacter PyloriElluzBelum ada peringkat
- Lactobacillus Reuteri Versus Triple Therapy For TH PDFDokumen4 halamanLactobacillus Reuteri Versus Triple Therapy For TH PDFRonny BudimanBelum ada peringkat
- Enterogermina Wilhelm 2011Dokumen7 halamanEnterogermina Wilhelm 2011Natália LopesBelum ada peringkat
- Journal of Diabetes and Its ComplicationsDokumen14 halamanJournal of Diabetes and Its ComplicationsRyanIndraSaputraBelum ada peringkat
- Helicobacter PyloriDokumen8 halamanHelicobacter PyloriQuỳnh ThưBelum ada peringkat
- HP Toronto Consensus 2016 PDFDokumen33 halamanHP Toronto Consensus 2016 PDFhoangducnamBelum ada peringkat
- Decreased Serum Ghrelin Following Helicobacter Pylori EradicationDokumen10 halamanDecreased Serum Ghrelin Following Helicobacter Pylori Eradicationps piasBelum ada peringkat
- Fluconazole Versus Nystatin in The Prevention of CandidaDokumen25 halamanFluconazole Versus Nystatin in The Prevention of CandidaMutia SesunanBelum ada peringkat
- The Oncologist 1998 Goumas 50 3Dokumen4 halamanThe Oncologist 1998 Goumas 50 3Kammi HumedBelum ada peringkat
- Ref 9 - García AlmeidaDokumen2 halamanRef 9 - García AlmeidajavierBelum ada peringkat
- Efficacy of Omeprazole and Amoxicillin With Either Clarithromycin or Metronidazole On Eradication of in Chinese Peptic Ulcer PatientsDokumen5 halamanEfficacy of Omeprazole and Amoxicillin With Either Clarithromycin or Metronidazole On Eradication of in Chinese Peptic Ulcer PatientsSyukron MakmunBelum ada peringkat
- Real-World Data of Helicobacter Pylori Prevalence, Eradicationregimens, and Antibiotic Resistance in Thailand, 2013-201Dokumen5 halamanReal-World Data of Helicobacter Pylori Prevalence, Eradicationregimens, and Antibiotic Resistance in Thailand, 2013-201andi kulokBelum ada peringkat
- Helicobacter Pylori: Eradication - An Update On The Latest TherapiesDokumen5 halamanHelicobacter Pylori: Eradication - An Update On The Latest TherapiesBaim FarmaBelum ada peringkat
- Case-Finding in Primary Care For Coeliac Disease: Accuracy and Cost-Effectiveness of A Rapid Point-Of-Care TestDokumen11 halamanCase-Finding in Primary Care For Coeliac Disease: Accuracy and Cost-Effectiveness of A Rapid Point-Of-Care TestmarcosBelum ada peringkat
- Nutrients 10 01678Dokumen11 halamanNutrients 10 01678zulfa nadiaBelum ada peringkat
- PHAR Article 108090 en 1Dokumen9 halamanPHAR Article 108090 en 1xvallejos58Belum ada peringkat
- Helicobacter Pylori Eradication RacgpDokumen6 halamanHelicobacter Pylori Eradication Racgpnoogu100% (1)
- DR - Rama ChaudhryDokumen49 halamanDR - Rama Chaudhryjbabu123Belum ada peringkat
- 1-s2 0-S1010660x14000020-MainDokumen6 halaman1-s2 0-S1010660x14000020-Mainapi-271060009Belum ada peringkat
- Jpen 1987Dokumen30 halamanJpen 1987J CBelum ada peringkat
- Lactobacillus Reuteri in The Treatment of Helicobacter Pylori InfectionDokumen6 halamanLactobacillus Reuteri in The Treatment of Helicobacter Pylori InfectionHuy Tân NguyễnBelum ada peringkat
- Comparison of Octreotide and Hyoscine in Controlling Gastrointestinal Symptoms Due To Malignant Inoperable Bowel ObstructionDokumen4 halamanComparison of Octreotide and Hyoscine in Controlling Gastrointestinal Symptoms Due To Malignant Inoperable Bowel ObstructionThiago GomesBelum ada peringkat
- Grila de Evaluare A Unui Articol StiintificDokumen4 halamanGrila de Evaluare A Unui Articol StiintificAndrei DamaschinBelum ada peringkat
- Proktitis 1Dokumen2 halamanProktitis 1adi chandraBelum ada peringkat
- Probiotics For The Prevention of Pediatric Antibiotic-Associated Diarrhea (Review)Dokumen4 halamanProbiotics For The Prevention of Pediatric Antibiotic-Associated Diarrhea (Review)EvelynRuizBelum ada peringkat
- Fang2018 PDFDokumen11 halamanFang2018 PDFRosiBelum ada peringkat
- Clinical Indications ForDokumen7 halamanClinical Indications Forronywijaya7Belum ada peringkat
- Gemcitabine Vs BCGDokumen8 halamanGemcitabine Vs BCGmulkikimulBelum ada peringkat
- Lactobacillus Reuteri Helicobacter Pylori: Clinical StudyDokumen7 halamanLactobacillus Reuteri Helicobacter Pylori: Clinical StudyHuy Tân NguyễnBelum ada peringkat
- Oral Administration of Tannins and Flavonoids in Children With Acute Diarrhea: A Pilot, Randomized, Control-Case StudyDokumen6 halamanOral Administration of Tannins and Flavonoids in Children With Acute Diarrhea: A Pilot, Randomized, Control-Case StudyAnonymous jM0QFnr0Belum ada peringkat
- Egypt J Med Microbiol 2009 18 4 165 178Dokumen14 halamanEgypt J Med Microbiol 2009 18 4 165 178Cristina RaduleaBelum ada peringkat
- Pylori BiogaiaDokumen8 halamanPylori BiogaiaAlejandro NavarreteBelum ada peringkat
- Biopharmaceutical Studies On Gastroretentive Oral Dosage Forms For Eradication of Helicobacter PyloriDokumen14 halamanBiopharmaceutical Studies On Gastroretentive Oral Dosage Forms For Eradication of Helicobacter PyloriFarhan IqbalBelum ada peringkat
- Divya Thesis Final (30th Jan)Dokumen26 halamanDivya Thesis Final (30th Jan)smalaparaBelum ada peringkat
- Cancer Epidemiol Biomarkers Prev 2004 Ley 4 10Dokumen8 halamanCancer Epidemiol Biomarkers Prev 2004 Ley 4 10Cristian BorrelliBelum ada peringkat
- 5 Jurnal PI - Farmasi Josan2 1 - 2Dokumen5 halaman5 Jurnal PI - Farmasi Josan2 1 - 2Serley WulandariBelum ada peringkat
- 388Dokumen6 halaman388anhiramdhaniBelum ada peringkat
- 2008 - Nancy M Tofil - Histamine2receptorantagonistsvsintravenousprotonpu (Retrieved 2017-02-01)Dokumen6 halaman2008 - Nancy M Tofil - Histamine2receptorantagonistsvsintravenousprotonpu (Retrieved 2017-02-01)adsadassddBelum ada peringkat
- Dokazano Dejstvo Nigelle Sative (Crnog Kumina) U Eliminaciji Helicobacter PyloriDokumen11 halamanDokazano Dejstvo Nigelle Sative (Crnog Kumina) U Eliminaciji Helicobacter PylorirbcollegeBelum ada peringkat
- Jurnal GastroDokumen16 halamanJurnal GastroDaniel TariganBelum ada peringkat
- Faecal Microbial Flora and Disease Activity in Rheumatoid Arthritis During A Vegan DietDokumen5 halamanFaecal Microbial Flora and Disease Activity in Rheumatoid Arthritis During A Vegan DietHendra SudaryonoBelum ada peringkat
- 1741 7015 8 30Dokumen8 halaman1741 7015 8 30Andres HernándezBelum ada peringkat
- Study Consipation Howaru-TransitDokumen8 halamanStudy Consipation Howaru-TransitMộc MộcBelum ada peringkat
- S0301211519303719 Viscerale Aiuta Contro ChemioDokumen23 halamanS0301211519303719 Viscerale Aiuta Contro Chemiomyname myselfBelum ada peringkat
- Rescue Therapies For The Management of HelicobacDokumen19 halamanRescue Therapies For The Management of Helicobacps piasBelum ada peringkat
- 5 Fu 2Dokumen10 halaman5 Fu 2Luiis LimaBelum ada peringkat
- Functional Constipation in Children: Investigation and Management of Anorectal MotilityDokumen4 halamanFunctional Constipation in Children: Investigation and Management of Anorectal MotilityWinny Herya UtamiBelum ada peringkat
- 11 JCO-2005-Ravasco-1431-8Dokumen8 halaman11 JCO-2005-Ravasco-1431-8Samuel Kyei-BoatengBelum ada peringkat
- Original Articles Gestational Trophoblastic Disease Following Complete Hydatidi-Form Mole in Mulago Hospital, Kampala, UgandaDokumen5 halamanOriginal Articles Gestational Trophoblastic Disease Following Complete Hydatidi-Form Mole in Mulago Hospital, Kampala, UgandadianaristinugraheniBelum ada peringkat
- Probiotics in Adolescent Prediabetes: A Pilot RCT On Glycemic Control and Intestinal BacteriomeDokumen14 halamanProbiotics in Adolescent Prediabetes: A Pilot RCT On Glycemic Control and Intestinal BacteriomeKarina EkawidyaniBelum ada peringkat
- Evaluating The Quality of Life of Adult Patients oDokumen1 halamanEvaluating The Quality of Life of Adult Patients opspd anakBelum ada peringkat
- Helicobacter Pylori - The Latest in Diagnosis and TreatmentDokumen5 halamanHelicobacter Pylori - The Latest in Diagnosis and TreatmentekramsBelum ada peringkat
- Probiotics in Cystic Fibrosis Patients A Double Blind CrossoveDokumen7 halamanProbiotics in Cystic Fibrosis Patients A Double Blind CrossoveDarwinBelum ada peringkat
- Terapia Sistémica en Carcinoma Metastásico de Colon Y Recto: Mauricio Lema Medina MDDokumen14 halamanTerapia Sistémica en Carcinoma Metastásico de Colon Y Recto: Mauricio Lema Medina MDapi-26302710Belum ada peringkat
- 81-IBP y MicrobiotaDokumen11 halaman81-IBP y MicrobiotaAlfonso De la FuenteBelum ada peringkat
- Recurrence of Helicobacter Pylori Infection 1 YearDokumen7 halamanRecurrence of Helicobacter Pylori Infection 1 YearRoaaBelum ada peringkat
- Microbiology ProfileDokumen52 halamanMicrobiology ProfileRushikesh LadBelum ada peringkat
- Secundum Artem: Compounding For Otic DisordersDokumen0 halamanSecundum Artem: Compounding For Otic Disordersdebieyolanda_7180456Belum ada peringkat
- Antibiotics & Antibiotic ResistanceDokumen53 halamanAntibiotics & Antibiotic ResistanceLeenoos RayapanBelum ada peringkat
- Injeksi & Oplosan ObatDokumen16 halamanInjeksi & Oplosan ObatFebriani RatnaBelum ada peringkat
- Nutrients A-Z (gnv64) PDFDokumen354 halamanNutrients A-Z (gnv64) PDFAnil Penumacha100% (1)
- Golden Age MIcrobiologyDokumen3 halamanGolden Age MIcrobiologyBecca TayBelum ada peringkat
- MCQs Saudia Pharmacy Registration ExamDokumen7 halamanMCQs Saudia Pharmacy Registration ExamAli ButtBelum ada peringkat
- AntibioticsDokumen32 halamanAntibioticsapi-3868619100% (1)
- Medicinal Chemistry - PenicillinDokumen39 halamanMedicinal Chemistry - PenicillinV G Viju KumarBelum ada peringkat
- BACTERIOCIN in Hurdle TechnologyDokumen31 halamanBACTERIOCIN in Hurdle TechnologyAjidhaslin Dhass0% (1)
- Antibiotic Approval TimelineDokumen1 halamanAntibiotic Approval TimelineMartin CuellarBelum ada peringkat
- Sensibilidad y Especificidad Del Gammagrama Ciprofloxacino-Tc99M Sensibilidad y Especificidad en Osteomielitis Infantil (AOM 2010)Dokumen4 halamanSensibilidad y Especificidad Del Gammagrama Ciprofloxacino-Tc99M Sensibilidad y Especificidad en Osteomielitis Infantil (AOM 2010)Simon ChangBelum ada peringkat
- Ampicillin/sulbactamDokumen0 halamanAmpicillin/sulbactamputri_agustina_1Belum ada peringkat
- Medication: Expected Pharmacological Action Therapeutic UseDokumen1 halamanMedication: Expected Pharmacological Action Therapeutic UseMaria MarquezBelum ada peringkat
- 41 How To Cure DiseaseDokumen60 halaman41 How To Cure DiseaseEdie M Murgia50% (2)
- 1a..antimicrobials IntroductionDokumen84 halaman1a..antimicrobials IntroductionAnonymous RlDDdTAsKJBelum ada peringkat
- Ats2946 Sample Exam s2 2017Dokumen7 halamanAts2946 Sample Exam s2 2017hafsatutuBelum ada peringkat
- Enrofloxacin: Therapeutic ReviewDokumen4 halamanEnrofloxacin: Therapeutic ReviewAndi Dytha Pramitha SamBelum ada peringkat
- Oritavancin Orbactiv MonographDokumen11 halamanOritavancin Orbactiv MonographDani11Belum ada peringkat
- Dry Powder Injectables TheonDokumen1 halamanDry Powder Injectables TheonshugarcontrollBelum ada peringkat
- Drug Study 1Dokumen4 halamanDrug Study 1bibet_martijaBelum ada peringkat
- International Medical Guide For Ships (Quantification Addendum) Third EditionDokumen58 halamanInternational Medical Guide For Ships (Quantification Addendum) Third EditionΔΗΜΗΤΡΗΣΧΑΛΑΤΣΗΣ100% (1)
- PediamedsDokumen9 halamanPediamedssven stantonBelum ada peringkat
- Cephalosporins Quick ReviewDokumen19 halamanCephalosporins Quick ReviewErinson Custodio PlasenciaBelum ada peringkat
- DR Lie Khie Chen - Management of MDR Infection in Sepsis Jade 2016Dokumen33 halamanDR Lie Khie Chen - Management of MDR Infection in Sepsis Jade 2016Astria PermanaBelum ada peringkat
- Pharmacokinetic and PharmacodynamicDokumen5 halamanPharmacokinetic and PharmacodynamicdrblondyBelum ada peringkat
- (1875855X - Asian Biomedicine) Identifications of Hordeolum Pathogens and Its Susceptibility To Antimicrobial Agents in Topical and Oral MedicationsDokumen6 halaman(1875855X - Asian Biomedicine) Identifications of Hordeolum Pathogens and Its Susceptibility To Antimicrobial Agents in Topical and Oral MedicationsSilvia Dwi AgustinBelum ada peringkat
- Algal Bioactive Diversities Against Pathogenic MicrobesDokumen8 halamanAlgal Bioactive Diversities Against Pathogenic Microbesmy.dear.sirBelum ada peringkat
- Updated IDSA - ATS Guidelines On Management of Adults With HAP and VAPDokumen11 halamanUpdated IDSA - ATS Guidelines On Management of Adults With HAP and VAPr dwiandiniBelum ada peringkat
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Dari EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Penilaian: 3 dari 5 bintang3/5 (1)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionDari EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionPenilaian: 4 dari 5 bintang4/5 (404)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsDari EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsBelum ada peringkat
- The Age of Magical Overthinking: Notes on Modern IrrationalityDari EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityPenilaian: 4 dari 5 bintang4/5 (32)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisDari EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisPenilaian: 4.5 dari 5 bintang4.5/5 (42)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDDari EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDPenilaian: 5 dari 5 bintang5/5 (3)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDari EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BePenilaian: 2 dari 5 bintang2/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedDari EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedPenilaian: 4.5 dari 5 bintang4.5/5 (82)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryDari EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryPenilaian: 4 dari 5 bintang4/5 (46)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaDari EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsDari EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsPenilaian: 4 dari 5 bintang4/5 (4)
- The Obesity Code: Unlocking the Secrets of Weight LossDari EverandThe Obesity Code: Unlocking the Secrets of Weight LossPenilaian: 4 dari 5 bintang4/5 (6)
- Manipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesDari EverandManipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesPenilaian: 4.5 dari 5 bintang4.5/5 (1412)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsDari EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsPenilaian: 5 dari 5 bintang5/5 (1)
- The Comfort of Crows: A Backyard YearDari EverandThe Comfort of Crows: A Backyard YearPenilaian: 4.5 dari 5 bintang4.5/5 (23)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Dari EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Penilaian: 4.5 dari 5 bintang4.5/5 (110)
- Why We Die: The New Science of Aging and the Quest for ImmortalityDari EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityPenilaian: 4 dari 5 bintang4/5 (5)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsDari EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsPenilaian: 4.5 dari 5 bintang4.5/5 (170)
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisDari EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisPenilaian: 5 dari 5 bintang5/5 (8)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisDari EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisPenilaian: 3.5 dari 5 bintang3.5/5 (2)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessDari EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessPenilaian: 4.5 dari 5 bintang4.5/5 (328)
- Troubled: A Memoir of Foster Care, Family, and Social ClassDari EverandTroubled: A Memoir of Foster Care, Family, and Social ClassPenilaian: 4.5 dari 5 bintang4.5/5 (27)
- Dark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingDari EverandDark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingPenilaian: 4 dari 5 bintang4/5 (1138)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeDari EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifePenilaian: 4.5 dari 5 bintang4.5/5 (254)