Explosion Safety in Ethoxylation Reactors: M Braithwaite & A Pekalski
Diunggah oleh
khali54Deskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Explosion Safety in Ethoxylation Reactors: M Braithwaite & A Pekalski
Diunggah oleh
khali54Hak Cipta:
Format Tersedia
Explosion Safety in Ethoxylation
Reactors
M Braithwaite
(1)
& A Pekalski
(3)
(1) Imperial College, London, UK
(2) Shell Global Solutions, Chester, UK
EthoxylationReactor Study (1997-2000)
many reactor types/ different bases of safety/
reactor conditions/ products
relievers
containers
Review of EO Hazards
J L Gustin, IChemE Hazards XV (April 2000)
this study solely concerned with reactor issues - ie
excludes EO/air interactions, toxicity, downstream EO handling,
liquid phase EO chemistry, corrosion etc
decomposition flame in the vessel and not detonation or events
external to the reactor is the prime concern
Combustion Characteristics of Ethylene
Oxide
Molecular Weight 44.05
Density (relative to air) 1.5
Boiling Point 10.4 Deg C at 1 ata
Stoichiometric Concentration in air 7.72 % v/v
Heat of Formation at 25 Deg C 52.7 kJ / mole
Heat of Combustion at 25 Deg C 1217 kJ /mole
Autoignition Temperature 429 Deg C at 1 ata
Flash Point Deg C <- 18 Deg C
Lower Explosion Limit 3.6 % v/v
Upper Explosion Limit 100 % v/v
Basis of Safety in EthoxylationReactors
containment
maximum pressure governed by EO concentration. T, P
inerting
use on inert (egN
2
) to render system non-reactive
relief + venting
emergency (via bursting disk)
controlled (via relief valve) - smaller capacity
suppression
rapid release of powder/ inert gas
avoidance of ignition
best practice but not a B o S
Recent Plant Accidents involving Ethylene Oxide
Year Country Company Cause
1987 Switzerland Sandoz External fire
1987 Belgium BP External hot spot
1987 Netherlands DOW Failure of heat exchanger
1989 Belgium BASF Hairline crack, EO distillation column
1989 J apan Asahi Denka EO leakage
1991 China Lu Shun Reactor malfunction
1991 USA Union Carbide Iron oxide impurities
Batch ethoxylators --- generic
chemical engineering
v v v v v v v v v v
EO / PO FEED
SUBSTRATE+CATALYST FEED
VENT TO HEADGEAR
DIFFUSIONAL MASS TRANSFER
(DISSOLUTION OF GASES)
REACTION KINETICS
HEAT TRANSFER
PRODUCT
BULK MASS TRANSFER
MIXING
DIFFUSIONAL MASS TRANSFER
(SOLUTION OF GASES)
VVV
Ethylene oxide reactions
TYPICAL RELATIVE
HEAT OF REACTION MOLAR
CHANGE KCal/Mole
HYDROLYSIS 21.4 CONTRACTION
22.4 CONTRACTION POLYMERISATION
NO CHANGE
28.1 ISOMERISATION
31.0 EXP. 1.5 --->1.75 DISPROPORTIONATION
32.7 EXP. 2.0 --->2.5 DECOMPOSITION (FLAME)
EXP. 1.1 COMBUSTION
291.2
This Study - one component
of complete engineering overview of
ethoxylationreactors (1996-2000)
revisit thermodynamic (conservative) estimates of maximum
overpressures in EO/ EO+N
2
mixtures
assess literature information of gross decomposition rates
develop simple venting model based on above to evaluate :
gaps in current understanding
needs for more experimental data
feasibility of some envisaged engineering solutions
eg N2 dilution
use of relief valves only in existing plant
alternative diluents/ operating conditions
Published EO studies
Author/ Ref GAS
Initial
Pressure
bara
Initial
Temperature
Deg C
Scale of
experiment
(litres)
Flow
conditions &
Vessel type
R Friedman & E Burke EO 0.4 - 2.0 60 140 - Laminar flame
J H Burgoyne & K E Bett
et al
EO 0.3 - 9.3 20 100 2.44 Static/ cylinder
R K J une and R F Dye EO/N
2
1.7 6.5 60 190 2.00 Static/ cylinder
R Siwek & E Rosenberg EO 0.5 4.0 40 200 20 10(3) Various/
Various
T Ogawa, A Miyake & H
Matsuo
EO/
N
2
/PO
1 5 20 180 0.765 Static/
Cylinder
M.Braithwaite, A.Pekalski
& J Zevenbergen
EO/N
2
4 100 20
Static/ turbulent
sphere
15
20
25
30
35
40
45
50
55
20 40 60 80 100 120 140 160 180 200
Initial temperature [C]
P
m
a
x
[
b
a
r
a
]
100%EO
80%EO
60%EO
50%EO
Effect of initial temperature and mixture composition on the maximum equilibrium pressure
Solid line model with soot, dashed line model without soot, Pini=4 bara, Tini=100
o
C
STANJ AN Chemical EquiibriumCode (Stanford University (Reynolds)
a
20 liter explosion sphere
Pressure-time curve:
P
max
, (dP/dt)
max
Cube-root law:
K = (dP/dt)
max
* V
1/3
= constant ?
0
5
10
15
20
25
30
35
0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5
Time [s]
P
[
b
a
r
a
]
Ignition
100% EO
80% EO
60% EO
Pressure-time history for EO-nitrogen mixtures of different
initial composition; Pini= 4 bara, Tini = 100 C, IE= 180 J.
Ethylene Oxide: Maximum rate of pressure
rise &
maximum pressures - from 373 K and 4
bara
Composition
EO/N2
[EO%]
Ignition
Energy
[J ]
(dP/dt)max
[bar/s]
Pmax
[bara]
60 180 25.01 17.46
80 180 36.80 25.65
100 0.72 41.98 31.83
100 180 77.80 33.65
100 540 169.6 36.38
Explosion severity data of pure EO, Pini= 4 bara,
Tin = 100
o
C, IE= 250J, V= 0.02 m3 [Bartknecht]
Mixture
status
(dP/dt)
max
[bar / s] K
d
[bar m / s]
Quiescent 96 26
Turbulent 1500 407
EO Decomposition Model -Decomposition
Flame
Laminar Flame velocity (T,P) - experimental
Simple Flamelet Model
S
eff
= S
u
x (Re/Re
c
)
S
u
- laminar burning velocity at T,P
Re
c
, - adjustable constants
Large scale studies - K
D
- experimental
K
D
- reduced maximum rate of pressure rise
EO Decomposition Model - Assumptions
ideal gas (EO, N2: EO gaseous decomposition products)
infinitessimal reaction film - 2 zone model
adiabatic process
mechanical equilibrium throughout
products in chemical equilibrium
flame velocity simulated by simple expression
spherical flame
vented gas either reactant or product - not mixed
no additional turbulence generated by venting process
Typical Pressure-Time History for EO
decomposition in a 5 m
3
vessel: initial
conditions 373 K and 4 bara: confined and
vented explosions
Time (secs)
1 2 3
P
r
e
s
s
u
r
e
(
B
a
r
a
)
0
10
20
30
40
50
Maximum Pressure in 5 m
3
vented EO
vessel: initial conditions - 373 K 4 bara
Ignition (1-base, 2-middle, 3-top)
1 2 3
P
r
e
s
s
u
r
e
(
b
a
r
a
)
30
32
34
36
38
40
Vessel Aspect Ratio
0.2 0.4 0.6 0.8 1.0
P
r
e
s
s
u
r
e
(
B
a
r
a
)
31
32
33
34
No of Relief Valves
0 1 2 3
P
r
e
s
s
u
r
e
(
B
a
r
a
)
10
15
20
25
30
35
40
45
50
EO Analysis (pre-SAFEKINEX) Conclusions
limited rate data
simple turbulence model
20 litre explosion data best available
Conservative design for explosion relief
(Containment preferred anyway for EO)
Anda mungkin juga menyukai
- Chaturvedi 2013Dokumen27 halamanChaturvedi 2013wakanda foreverBelum ada peringkat
- Jentoft Calcination 311003Dokumen70 halamanJentoft Calcination 311003Albar BudimanBelum ada peringkat
- MethaneDokumen6 halamanMethanevijay kumar honnaliBelum ada peringkat
- Final ReviewDokumen32 halamanFinal ReviewNIKHIL RAJ T RBelum ada peringkat
- Effect of Metallurgy On Pygas Fouling PDFDokumen6 halamanEffect of Metallurgy On Pygas Fouling PDFstreamtBelum ada peringkat
- AC Catalst PTDokumen5 halamanAC Catalst PTJarretBelum ada peringkat
- The Theory and Practice of Steam Reforming: By: Gerard B. Hawkins Managing Director, CEODokumen0 halamanThe Theory and Practice of Steam Reforming: By: Gerard B. Hawkins Managing Director, CEOBalaji RamanBelum ada peringkat
- Air Pollution Control Technologies: VOC IncineratorsDokumen51 halamanAir Pollution Control Technologies: VOC IncineratorsRay CBelum ada peringkat
- View ContentDokumen29 halamanView ContentMitul PrajapatiBelum ada peringkat
- Study of The Thermal Cracking During The Vacuum Distillation of Atmospheric Residue of Crude OilDokumen11 halamanStudy of The Thermal Cracking During The Vacuum Distillation of Atmospheric Residue of Crude OilPhạm QuânBelum ada peringkat
- Obtention of Tio Rutile at Room Temperature Through Direct Oxidation of TiclDokumen4 halamanObtention of Tio Rutile at Room Temperature Through Direct Oxidation of TiclWilliam Soracà OspinoBelum ada peringkat
- CT 02 - Salbidegoitia Et AlDokumen3 halamanCT 02 - Salbidegoitia Et AlJon Bisu DebnathBelum ada peringkat
- Physical Constant by Prof. Pawan Babel - Toppers ChoiceDokumen21 halamanPhysical Constant by Prof. Pawan Babel - Toppers ChoicePawan BabelBelum ada peringkat
- Temperature-Programmed Oxidation of Coked Noble Metal Catalysts After Autothermal Reforming of N-HexadecaneDokumen9 halamanTemperature-Programmed Oxidation of Coked Noble Metal Catalysts After Autothermal Reforming of N-HexadecaneImran KhanBelum ada peringkat
- Gas Explosion VentingDokumen29 halamanGas Explosion VentingMochamad SafarudinBelum ada peringkat
- Ethylene Oxide: Jump ToDokumen31 halamanEthylene Oxide: Jump ToMusa Eltayeb100% (1)
- Student Name ID: Aysha Housani 200503484 Maha Al Shehhi 200509462 Hessa Al Shehhi 200509582 Mona Thabet 200521150Dokumen78 halamanStudent Name ID: Aysha Housani 200503484 Maha Al Shehhi 200509462 Hessa Al Shehhi 200509582 Mona Thabet 200521150minumcincauBelum ada peringkat
- Electrocatalysis-of-CO-Tolerance-in-Hydrogen-Oxidation-Reaction-in-PEM-Fuel-CellsDokumen11 halamanElectrocatalysis-of-CO-Tolerance-in-Hydrogen-Oxidation-Reaction-in-PEM-Fuel-CellsFaseeh KKBelum ada peringkat
- Study of Water-Oil Emulsion Combustion in Large Pilot Power Plants For Fine Particle Matter Emission ReductionDokumen6 halamanStudy of Water-Oil Emulsion Combustion in Large Pilot Power Plants For Fine Particle Matter Emission ReductionImad AghilaBelum ada peringkat
- Dissolved Gas Analysis-1Dokumen31 halamanDissolved Gas Analysis-1Karan Tripathi100% (2)
- The Reactivity and Kinetics of Yanzhou Coal Chars From Elevated Pyrolysis Temperatures During Gasification in Steam at 900 - 120088888CDokumen9 halamanThe Reactivity and Kinetics of Yanzhou Coal Chars From Elevated Pyrolysis Temperatures During Gasification in Steam at 900 - 120088888CAlfiDahliaArofaniBelum ada peringkat
- 2007c 2 Multi-HydrationModelDokumen16 halaman2007c 2 Multi-HydrationModelHyunkyoun JinBelum ada peringkat
- Chemical Dechlorination of PCBs From Dielectric OilsDokumen6 halamanChemical Dechlorination of PCBs From Dielectric Oilsgerodot2falconBelum ada peringkat
- Carbon Dioxide Reforming of Methane Over NiAl2O3 Treated With Glow Discharge PlasmaDokumen6 halamanCarbon Dioxide Reforming of Methane Over NiAl2O3 Treated With Glow Discharge PlasmaViệtDũng TôBelum ada peringkat
- Carbon Dioxide Reforming of Methane Over NiAl2O3 Treated With Glow Discharge PlasmaDokumen6 halamanCarbon Dioxide Reforming of Methane Over NiAl2O3 Treated With Glow Discharge PlasmaViệtDũng TôBelum ada peringkat
- Ria PDFDokumen23 halamanRia PDFria andrianiBelum ada peringkat
- Formaldehyde Project Report by AbhishekDokumen98 halamanFormaldehyde Project Report by AbhishekAbhishek Kumar83% (12)
- Ethylbenzene Dehydrogenation On Fe O - CR O - K CO Catalysts Promoted With Transitional Metal OxidesDokumen10 halamanEthylbenzene Dehydrogenation On Fe O - CR O - K CO Catalysts Promoted With Transitional Metal OxidesLudmila CarballalBelum ada peringkat
- Au-Pt/Co O Catalyst For Methane Combustion: LetterDokumen4 halamanAu-Pt/Co O Catalyst For Methane Combustion: LetterfafaporkBelum ada peringkat
- Table of Specific Heat Capacities: List of Thermal ConductivitiesDokumen34 halamanTable of Specific Heat Capacities: List of Thermal Conductivitiesduta_nugraha0% (1)
- Kinetic Modeling of The Methanol To Olefins Process. 2. Experimental Results, Model Discrimination, and Parameter EstimationDokumen10 halamanKinetic Modeling of The Methanol To Olefins Process. 2. Experimental Results, Model Discrimination, and Parameter Estimationamerico molinaBelum ada peringkat
- Duval TriangleDokumen5 halamanDuval TriangleVictor Jr QuijanoBelum ada peringkat
- United States: (12) Patent Application Publication (10) Pub. No.: US 2007/0284285 A1Dokumen6 halamanUnited States: (12) Patent Application Publication (10) Pub. No.: US 2007/0284285 A1deni.sttnBelum ada peringkat
- Ash ReinjectionDokumen22 halamanAsh Reinjectiongaol_bird009Belum ada peringkat
- Steam Cracking of Naphtha in Packed Bed ReactorsDokumen6 halamanSteam Cracking of Naphtha in Packed Bed Reactorscandidater100% (1)
- Aging of Polymeric MaterialsDokumen29 halamanAging of Polymeric MaterialspapanadnadBelum ada peringkat
- J Jcat 2004 10 020Dokumen9 halamanJ Jcat 2004 10 020valeBelum ada peringkat
- Antti Vuori ANPSG2001Dokumen29 halamanAntti Vuori ANPSG2001Eduardo MagallonBelum ada peringkat
- 4571 Chap14 Catalysis IntroDokumen14 halaman4571 Chap14 Catalysis IntroSankar SasmalBelum ada peringkat
- Metal Dusting Corrosion Initiation in Conversion of Natural Gas To Synthesis GasDokumen24 halamanMetal Dusting Corrosion Initiation in Conversion of Natural Gas To Synthesis GasPrasanna RajaBelum ada peringkat
- Coatings Against High Temperature Corrosion Deposited by Metal-Organic Low Pressure Chemical Vapour DepositionDokumen6 halamanCoatings Against High Temperature Corrosion Deposited by Metal-Organic Low Pressure Chemical Vapour DepositionmileivandaBelum ada peringkat
- Methane: Jump To Navigation Jump To Search Ethane CH4 (Disambiguation)Dokumen14 halamanMethane: Jump To Navigation Jump To Search Ethane CH4 (Disambiguation)GeorgeBelum ada peringkat
- Combustion Engineering 2Dokumen137 halamanCombustion Engineering 2Trương HàoBelum ada peringkat
- Petroleum Refinery Lab. Report No.3Dokumen13 halamanPetroleum Refinery Lab. Report No.3Mohammed IhsanBelum ada peringkat
- Cement Kilns-Chlorine Impact On ProcessDokumen27 halamanCement Kilns-Chlorine Impact On Processmuhaisen2009100% (4)
- Thermal Decomposition Kinetics Unsaturated Polyester and Unsaturated Polyester Reinforcement by Toner Carbon Nano Powder (TCNP) CompositesDokumen8 halamanThermal Decomposition Kinetics Unsaturated Polyester and Unsaturated Polyester Reinforcement by Toner Carbon Nano Powder (TCNP) CompositesInternational Journal of Application or Innovation in Engineering & ManagementBelum ada peringkat
- The Influences of Alloy Elements On The Carburized Layer in Steels Using Vacuum Carburization in An Acetylene AtmosphereDokumen7 halamanThe Influences of Alloy Elements On The Carburized Layer in Steels Using Vacuum Carburization in An Acetylene AtmosphereSumit KumarBelum ada peringkat
- Lec. 10 SI Engine Combustion IIDokumen20 halamanLec. 10 SI Engine Combustion IIrajeev50588Belum ada peringkat
- Styrene Design ProblemDokumen4 halamanStyrene Design ProblemAli AbdullahBelum ada peringkat
- Oxidation CO Over CuODokumen4 halamanOxidation CO Over CuOncaothachBelum ada peringkat
- Hidratación de Oxido de Etileno Cinética PDFDokumen5 halamanHidratación de Oxido de Etileno Cinética PDFangieBelum ada peringkat
- Thermal Analysis 462 PresDokumen23 halamanThermal Analysis 462 PresMaddie BoyerBelum ada peringkat
- A Transient Response Study of The Selective Catalytic Oxidation of Ammonia To Nitrogen On Pt-CuO-Al2O3 Olofsson Et Al Chem. Eng. Sci. 2004Dokumen11 halamanA Transient Response Study of The Selective Catalytic Oxidation of Ammonia To Nitrogen On Pt-CuO-Al2O3 Olofsson Et Al Chem. Eng. Sci. 2004juan davidBelum ada peringkat
- CATALYSTSDokumen21 halamanCATALYSTSJhon Jairo Rico CerqueraBelum ada peringkat
- Simulation of Ethane Steam Cracking With SeverityDokumen7 halamanSimulation of Ethane Steam Cracking With Severity9uhBelum ada peringkat
- Production of Pure Hydrogen by Ethanol DehydrogenationDokumen9 halamanProduction of Pure Hydrogen by Ethanol DehydrogenationAbdulwahid SultanBelum ada peringkat
- Water-Gas Shift ReactionDokumen68 halamanWater-Gas Shift Reactiondejla670% (1)
- Fundamentals of Fluidized-Bed Chemical Processes: Butterworths Monographs in Chemical EngineeringDari EverandFundamentals of Fluidized-Bed Chemical Processes: Butterworths Monographs in Chemical EngineeringBelum ada peringkat
- Worked Problems in Heat, Thermodynamics and Kinetic Theory for Physics Students: The Commonwealth and International Library: Physics DivisionDari EverandWorked Problems in Heat, Thermodynamics and Kinetic Theory for Physics Students: The Commonwealth and International Library: Physics DivisionPenilaian: 4 dari 5 bintang4/5 (3)
- OSHA To Inspect Plants Like Ga. Refinery: News NationDokumen2 halamanOSHA To Inspect Plants Like Ga. Refinery: News Nationkhali54Belum ada peringkat
- The Call of The Wild Illustrated - Cs PDFDokumen108 halamanThe Call of The Wild Illustrated - Cs PDFkhali54Belum ada peringkat
- Calculating The Probability of Failure On Demand (PFD) of Complex Structures by Means of Markov ModelsDokumen5 halamanCalculating The Probability of Failure On Demand (PFD) of Complex Structures by Means of Markov ModelsfoamtrailerBelum ada peringkat
- DEC09ts PDFDokumen19 halamanDEC09ts PDFBoomdayBelum ada peringkat
- Pumping Fluids and Getting Fluid To The PumpDokumen3 halamanPumping Fluids and Getting Fluid To The PumpTeng Chuan OngBelum ada peringkat
- Valeur KV Calcul de Vanne PDFDokumen2 halamanValeur KV Calcul de Vanne PDFkhali54Belum ada peringkat
- Valeur KV Calcul de Vanne PDFDokumen2 halamanValeur KV Calcul de Vanne PDFkhali54Belum ada peringkat
- 1.1. ObjectiveDokumen2 halaman1.1. Objectivekhali54Belum ada peringkat
- Loss Prevention in Heavy Industry Risk Assessment of LargeDokumen8 halamanLoss Prevention in Heavy Industry Risk Assessment of Largekhali54Belum ada peringkat
- Chapter 14D1 15Dokumen15 halamanChapter 14D1 15khali54Belum ada peringkat
- Barrières KletzDokumen12 halamanBarrières Kletzkhali54Belum ada peringkat
- CatastropheDokumen16 halamanCatastrophekhali54Belum ada peringkat
- Dust Explosion Incidents and Regulations in The United StatesDokumen7 halamanDust Explosion Incidents and Regulations in The United Stateskhali54Belum ada peringkat
- Hyster H40-70FTDokumen552 halamanHyster H40-70FTmarcelo75% (4)
- PDF 44201231009PMSHM-SHVTDokumen53 halamanPDF 44201231009PMSHM-SHVTabhayundale100% (1)
- 5 - GP CompletionsDokumen37 halaman5 - GP Completionsweldsv1Belum ada peringkat
- ST Energy Balance 2015Dokumen106 halamanST Energy Balance 2015jayavishnuBelum ada peringkat
- Duliajan - 786 602, AssamDokumen2 halamanDuliajan - 786 602, AssamJeshiBelum ada peringkat
- Temperature Effects On Sulphur Catalyzed Dimerization of Fluted Pumpkin (Telfairia Occidentalis)Dokumen4 halamanTemperature Effects On Sulphur Catalyzed Dimerization of Fluted Pumpkin (Telfairia Occidentalis)International Organization of Scientific Research (IOSR)Belum ada peringkat
- 2Dokumen35 halaman2Avinash Katragadda100% (1)
- Refinery Process: Application Solutions GuideDokumen168 halamanRefinery Process: Application Solutions Guide최승원Belum ada peringkat
- Hybrid Electric Vehicle: Presented By: Nikhil V Dhote Guided By: Prof C. R. Patil SirDokumen24 halamanHybrid Electric Vehicle: Presented By: Nikhil V Dhote Guided By: Prof C. R. Patil SirRushikesh TajneBelum ada peringkat
- Enclosures Catalogue ITA ENGDokumen156 halamanEnclosures Catalogue ITA ENGAbrakain69Belum ada peringkat
- Westerbeke 4,5bcgtc Part ListDokumen77 halamanWesterbeke 4,5bcgtc Part Listcengiz kutukcu100% (1)
- Hatlapa L220, L270, L350Dokumen2 halamanHatlapa L220, L270, L350hao0% (1)
- NSTM 262 OilsDokumen110 halamanNSTM 262 OilsMaria Gabriela BusteloBelum ada peringkat
- A2 Chem Unit 5Dokumen47 halamanA2 Chem Unit 5Bill CipherBelum ada peringkat
- Miller XMT 304Dokumen40 halamanMiller XMT 304Alexis OrganisBelum ada peringkat
- Chemistry Level) (CIE) Paper 2Dokumen302 halamanChemistry Level) (CIE) Paper 2Mohamed Akkash67% (3)
- EssarDokumen118 halamanEssarinnocentshah100% (1)
- CATOFIN® Dehydrogenation: Process Features Process BenefitsDokumen2 halamanCATOFIN® Dehydrogenation: Process Features Process BenefitsJay ShuklaBelum ada peringkat
- Delphi Passenger Car Light Duty Truck Emissions Brochure 2012 2013Dokumen100 halamanDelphi Passenger Car Light Duty Truck Emissions Brochure 2012 2013danplaiBelum ada peringkat
- Green Chemistry Bio Diesel PROJECTDokumen5 halamanGreen Chemistry Bio Diesel PROJECTSuriyavelu M. S.Belum ada peringkat
- Over View On 5 S TechnicDokumen14 halamanOver View On 5 S TechnicSachleen Singh BajwaBelum ada peringkat
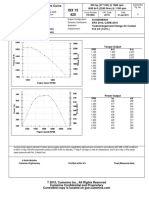
- KTA19-DM Performance CurveDokumen2 halamanKTA19-DM Performance CurveVincen Lim100% (2)
- Product Loading - Marine Loading ArmDokumen9 halamanProduct Loading - Marine Loading Arminvilink87Belum ada peringkat
- Engine Performance Curve: Linehaul ApplicationDokumen2 halamanEngine Performance Curve: Linehaul ApplicationCarlosBelum ada peringkat
- Powermate Pm0103002 User Manual and Parts List (Pramac)Dokumen36 halamanPowermate Pm0103002 User Manual and Parts List (Pramac)Adrian Fraga FernandezBelum ada peringkat
- STD Spec - Pig TrapsDokumen10 halamanSTD Spec - Pig TrapsArun MishraBelum ada peringkat
- Installation Manual: BECIN - 2000 BECIN - 3000 BEC - 1500 BEC - 2500Dokumen41 halamanInstallation Manual: BECIN - 2000 BECIN - 3000 BEC - 1500 BEC - 2500Marcus Miller100% (1)
- BoilerDokumen48 halamanBoilerAbdallah Mansour100% (2)
- Iocl Project For 7th SemDokumen14 halamanIocl Project For 7th SemAbhisek PurohitBelum ada peringkat
- LNG & LPGDokumen66 halamanLNG & LPGsujit das100% (2)