Peace Corps Reactive Tuberculin Form PC-262-12 (Revised 10/2012
Diunggah oleh
Accessible Journal Media: Peace Corps DocumentsJudul Asli
Hak Cipta
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Peace Corps Reactive Tuberculin Form PC-262-12 (Revised 10/2012
Diunggah oleh
Accessible Journal Media: Peace Corps DocumentsHak Cipta:
Applicant Name ______________________________________________________________________________________________________________________
(Last, First, Middle Initial)
Date of Birth__________ /__________ /___________ Medical Case Number:________________________________________________
Reactive Tuberculin Form
OMB No.: 0420-0550
Expiration Date: 1/31/2014
(Mo/Day/Year)
REACTIVE TUBERCULIN TEST EVALUATION FORM
Dear Medical Provider,
Your patient has applied to serve with the Peace Corps and has reported a history of reactivity to tuberculosis (TB) skin
testing or a history of BCG vaccination. In order to accurately evaluate this applicants medical status, the Peace Corps needs
further information about the applicants risk of developing tuberculosis. Please answer the following questions regarding
the applicants TB status.
Privacy Act Notice
This information is collected under the authority of the Peace Corps Act, 22 U.S.C. 2501 et seq. It will be used primarily for the purpose of determining your eligibility
for Peace Corps service and, if you are invited to serve as a Peace Corps Volunteer, for the purpose of providing you with medical care during your Peace Corps
service. Your disclosure of this information is voluntary; however, your failure to provide this information will result in the rejection of your application to become
a Peace Corps Volunteer.
This information may be used for the purposes described in the Privacy Act, 5 USC 552a, including the routine uses listed in the Peace Corps System of Records.
Among other uses, this information may be used by those Peace Corps staff members who have a need for such information in the performance of their duties.
It may also be disclosed to the Office of Workers Compensation Programs in the Department of Labor in connection with claims under the Federal Employees
Compensation Act and, when necessary, to a physician, psychiatrist, clinical psychologist or other medical personnel treating you or involved in your treatment
or care. A full list of routine uses for this information can be found on the Peace Corps website at http://multimedia.peacecorps.gov/multimedia/pdf/policies/
systemofrecords.pdf.
Burden Statement:
Public reporting burden for this collection of information is estimated to average between 75 minutes to 105 minutes per applicant and 30 minutes per physician
per response. This estimate includes the time for reviewing instructions and completing the collection of information. An agency may not conduct or sponsor, and
a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden
estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to: FOIA Officer, Peace Corps, 1111 20th Street, NW,
Washington, DC 20526 ATTN: PRA (0420 - 0550). Do not return the completed form to this address..
Peace Corps Reactive Tuberculin Form
PC-262-12 (Revised 10/2012)
Page 1 of 3
Medical Case Number:
I. Current TB Test (Test must be within the past six months; Mantoux test or QuantiFeron-TB Gold) (No test is required if
there is documentation of the size of induration and there is documentation of treatment)
Select one
h Mantoux Date: ________________________________
mm of induration:________________________________
h Interferon Gamma Releasing Assay (QuantiFERON-TB Gold)
Date:________________________________ Result: h Positive h Negative
h T.SPOT TB
Date:________________________________ Result: h Positive h Negative
II. TB Test History:
h No prior TB test
h Prior TB test(s)
Date:________________________________ mm of induration:_______________________________________________________
Date:________________________________ mm of induration:_______________________________________________________
Date:________________________________ mm of induration:_______________________________________________________
h BCG vaccine (If reported by applicant, please provide) Date of vaccination:________________________________
III. Current Tests Required
h Copy of current CXR report with interpretation is required for:
Applicants with a reported induration or interval change in induration 10mm
Applicants with risk factors present (see Section V)
Applicants with a history of a prior reactive tuberculin test
Applicants with a history of BCG vaccination and a current reactive tuberculin test
h Copy of baseline Liver Functions Tests is required for:
Applicants currently being treated for latent tuberculosis infection (LTBI)
IV. Treatment History:
Note: In general, treatment of latent tuberculosis infection (LTBI) is required for all Peace Corps applicants who are candidates
for this therapy. Before an applicant can be medically cleared, and prior to departure overseas, treatment should be initiated
in accordance with Centers for Disease Control (CDC) guidelines.1 There must be a strong medical reason for not treating
preventively, e.g., high risk for hepatitis, etc.
h No treatment received
h INH therapy received:
Date treatment initiated:______________________________________________________________ Ongoing: ___________________________________________________
Date treatment completed:__________________________________________________________
1 Core Curriculum on Tuberculosis: What the Clinician Should Know, 4th Edition, 2000. U.S. Department of Health and Human Services and Centers for Disease Control and Prevention.
Peace Corps Reactive Tuberculin Form
PC-262-12 (Revised 10/2012)
Page 2 of 3
Medical Case Number:
h Full-course of other treatment:
Drug regimen:_______________________________________________________________________________ Date treatment initiated:___________________________
Ongoing: ______________________________________________________________________________________
Date treatment completed:__________________________________________________________
h Full-course or treatment not received:
Please explain:______________________________________________________________________________________________________________________________________________________________________________
______________________________________________________________________________________________________________________________________________________________________________________________________
______________________________________________________________________________________________________________________________________________________________________________________________________
______________________________________________________________________________________________________________________________________________________________________________________________________
______________________________________________________________________________________________________________________________________________________________________________________________________
V. RISK ASSESSMENT FOR DEVELOPING ACTIVE TB (Please check yes or no):
YES
NO
Person infected with the human immunodeficiency virus.
h
h
Close contact (i.e., those sharing the same household or other enclosed environments) of person(s) known or_
suspected to have tuberculosis.
h
h
Foreign-born person who has recently arrived (within five years) from a country that has a high incidence or_
prevalence of tuberculosis (includes most countries in Asia, Africa, and Latin America).
h
h
Resident or employee of high-risk congregate setting (e.g., correctional institution, nursing home, mental_
institution, or shelter for the homeless).
Person who injects illicit drugs or uses other high-risk substances (e.g., crack cocaine).
Health care worker who is exposed to high-risk clients or is/has been mycobacteriology laboratory personnel.
VI. CURRENT TB SYMPTOMS:
YES
NO
Cough lasting longer than three weeks
Night sweats (drenching bed clothes that last more than one week)
Unexplained weight loss of 10 pounds or more than 10 percent of normal weight
Fatigue/malaise lasting longer than two weeks
Loss of appetite > two weeks
Fever > 100 degrees lasting > one week
VII. RECOMMENDATIONS FOR FURTHER EVALUATION AND TREATMENT:____________________________________________________________
____________________________________________________________________________________________________________________________________________________________________________________________________________
____________________________________________________________________________________________________________________________________________________________________________________________________________
____________________________________________________________________________________________________________________________________________________________________________________________________________
____________________________________________________________________________________________________________________________________________________________________________________________________________
Name of Physician ___________________________________________________________Signature______________________________________________________________Date____________________
Peace Corps Reactive Tuberculin Form
PC-262-12 (Revised 10/2012)
Page 3 of 3
Anda mungkin juga menyukai
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Peace Corps Counterparts and Supervisors T0121 Section 3 of 3 (2002)Dokumen52 halamanPeace Corps Counterparts and Supervisors T0121 Section 3 of 3 (2002)Accessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- Peace Corps Executive Order 13985 Equity SummaryDokumen6 halamanPeace Corps Executive Order 13985 Equity SummaryAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- Peace Corps Counterparts and Supervisors T0121 Section 1 & 2, (2002)Dokumen174 halamanPeace Corps Counterparts and Supervisors T0121 Section 1 & 2, (2002)Accessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Global Counterpart Survey 2015 QuestionnaireDokumen6 halamanGlobal Counterpart Survey 2015 QuestionnaireAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- Senate Letter To Peace Corps Acting Director On The Pacific IslandsJuly 19, 2022Dokumen3 halamanSenate Letter To Peace Corps Acting Director On The Pacific IslandsJuly 19, 2022Accessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- First US Peace Corps Volunteers Since War Arrive in Vietnam - Radio Free AsiaDokumen1 halamanFirst US Peace Corps Volunteers Since War Arrive in Vietnam - Radio Free AsiaAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- Peace Corps Volunteer Handbook 2022Dokumen54 halamanPeace Corps Volunteer Handbook 2022Accessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Volunteer Handbook Global Peace Corps Policy 2020 DecemberDokumen52 halamanVolunteer Handbook Global Peace Corps Policy 2020 DecemberAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- FAQs For 2020 Peace Corps Evacuees (COVID) Allowances and BenefitsDokumen7 halamanFAQs For 2020 Peace Corps Evacuees (COVID) Allowances and BenefitsAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
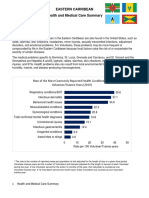
- Eastern Caribbean Peace Corps OHS Health and Medical Care SummaryDokumen3 halamanEastern Caribbean Peace Corps OHS Health and Medical Care SummaryAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
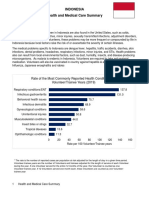
- Indonesia Peace Corps OHS Health and Medical Care SummaryDokumen2 halamanIndonesia Peace Corps OHS Health and Medical Care SummaryAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Peace Corps SPIGA - InternshipOpportunityDokumen3 halamanPeace Corps SPIGA - InternshipOpportunityAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Peace Corps Overview and Issues 2021Dokumen23 halamanPeace Corps Overview and Issues 2021Accessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- Global Volunteer Policy Handbook 2021Dokumen54 halamanGlobal Volunteer Policy Handbook 2021Accessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (345)
- Volunteer Handbook Global Peace Corps Policy 2020 DecemberDokumen52 halamanVolunteer Handbook Global Peace Corps Policy 2020 DecemberAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Volunteer Assignment Description VAD Peace CorpsDokumen10 halamanVolunteer Assignment Description VAD Peace CorpsAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Guide To Peace Corps Records SchedulesDokumen44 halamanGuide To Peace Corps Records SchedulesAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Peace Corps Stylebook Updated May 2013Dokumen70 halamanPeace Corps Stylebook Updated May 2013Accessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Peace Corps Benefits Onesheets BenefitsDokumen2 halamanPeace Corps Benefits Onesheets BenefitsAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Volunteer Assignment Description VAD Peace CorpsDokumen10 halamanVolunteer Assignment Description VAD Peace CorpsAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Peace Corps Primary Literacy AdvisorDokumen4 halamanPeace Corps Primary Literacy AdvisorAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Peace Corps Monitoring and Evaluation Specialist For The Match Research Unit (MRU) in South AfricaDokumen3 halamanPeace Corps Monitoring and Evaluation Specialist For The Match Research Unit (MRU) in South AfricaAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Peace Corps in Country Host Country Contributions MS 722 ProceduresDokumen6 halamanPeace Corps in Country Host Country Contributions MS 722 ProceduresAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Peace Corps Komoros Welcome Book 2016 - AprilDokumen39 halamanPeace Corps Komoros Welcome Book 2016 - AprilAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- Colombia Welcome Book - 2016 JuneDokumen43 halamanColombia Welcome Book - 2016 JuneAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Peace Corps Technical Trainer Assistant For Community Health and Malaria Prevention CHAMPDokumen1 halamanPeace Corps Technical Trainer Assistant For Community Health and Malaria Prevention CHAMPAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Peace Corps Works Cross-Sectional Analysis of 21 Host Country Impact Studies 2016Dokumen76 halamanPeace Corps Works Cross-Sectional Analysis of 21 Host Country Impact Studies 2016Accessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Final Report On The Audit of Peace Corps Panama IG-18-01-ADokumen32 halamanFinal Report On The Audit of Peace Corps Panama IG-18-01-AAccessible Journal Media: Peace Corps DocumentsBelum ada peringkat
- Malaria Rapid Diagnostic Tests (RDTS)Dokumen37 halamanMalaria Rapid Diagnostic Tests (RDTS)MegbaruBelum ada peringkat
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- Taking History in MedicineDokumen20 halamanTaking History in Medicineprincemasr6Belum ada peringkat
- K10 - ISK AtasDokumen39 halamanK10 - ISK AtasfelixBelum ada peringkat
- Certificate of Health: SurnameDokumen2 halamanCertificate of Health: SurnameNK productionBelum ada peringkat
- AAO Network GlaucomaDokumen59 halamanAAO Network Glaucomasafasayed100% (1)
- Haemaroids CaseDokumen11 halamanHaemaroids CaseYugesvari_Thir_1690Belum ada peringkat
- Norovirus - CDCDokumen1 halamanNorovirus - CDCDibyajyoti RabhaBelum ada peringkat
- Medical IdiomsDokumen14 halamanMedical IdiomsLeidy RodriguezBelum ada peringkat
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Critical SkillsDokumen5 halamanCritical SkillsJosiah MwashitaBelum ada peringkat
- Adrian Jess Galindo: DefinitionDokumen2 halamanAdrian Jess Galindo: DefinitionAdrian MangahasBelum ada peringkat
- Updates On Management of Gastric CancerDokumen9 halamanUpdates On Management of Gastric CancerestherBelum ada peringkat
- Dilatation & Curettage: DR Ayswarya NarayanDokumen19 halamanDilatation & Curettage: DR Ayswarya NarayanPrajwal Kp0% (1)
- Cancer Gastrico AvancesDokumen48 halamanCancer Gastrico AvancesSeal MachucaBelum ada peringkat
- Micro para The IntroductionDokumen51 halamanMicro para The IntroductionknotstmBelum ada peringkat
- Bino - Lec - Midterm Exam - Yellow - PadDokumen11 halamanBino - Lec - Midterm Exam - Yellow - PadDanielle SangalangBelum ada peringkat
- Related Teenshealth Links: Health Problems SeriesDokumen13 halamanRelated Teenshealth Links: Health Problems SeriesMario BadayosBelum ada peringkat
- What Are Glycerol Suppositories?Dokumen2 halamanWhat Are Glycerol Suppositories?Delia TuguiBelum ada peringkat
- Andrés Felipe Cardona: Eduardo Obando)Dokumen3 halamanAndrés Felipe Cardona: Eduardo Obando)Zarit Diseños CaliBelum ada peringkat
- Lifestyle Diseases Affecting The Nursing Faculty at Tarlac State University During Covid 19 PandemicDokumen19 halamanLifestyle Diseases Affecting The Nursing Faculty at Tarlac State University During Covid 19 PandemicDeinielle Magdangal RomeroBelum ada peringkat
- Formula For Calculating The IV Flow RateDokumen16 halamanFormula For Calculating The IV Flow Rateblythe RiveroBelum ada peringkat
- SaravanaDokumen21 halamanSaravanaRasu Kutty100% (1)
- Adverse Childhood Experiences (Aces) : © 2022 Elsevier Ltd. All Rights ReservedDokumen16 halamanAdverse Childhood Experiences (Aces) : © 2022 Elsevier Ltd. All Rights ReservedDiamondChuBelum ada peringkat
- Nbme 16Dokumen35 halamanNbme 16osara M78% (49)
- MCQ Question SheetDokumen6 halamanMCQ Question SheethappyhappylandBelum ada peringkat
- Forensic Midterm MCQsDokumen4 halamanForensic Midterm MCQsNikhil TyagiBelum ada peringkat
- Agent JohnsonDokumen1 halamanAgent JohnsonJulio Cesar PerezBelum ada peringkat
- Oral Halitosis: Definitions: Breath Malodor, Defined As Foul or Offensive Odor of Expired Air, May BeDokumen7 halamanOral Halitosis: Definitions: Breath Malodor, Defined As Foul or Offensive Odor of Expired Air, May BeSnowBelum ada peringkat
- Trisomies: Abnormal Number of ChromosomesDokumen10 halamanTrisomies: Abnormal Number of ChromosomesNaumanBelum ada peringkat
- Case Study About Type II Diabetes MellitusDokumen82 halamanCase Study About Type II Diabetes MellitusKristine YoungBelum ada peringkat
- Gastrointestinal Quiz AnswersDokumen5 halamanGastrointestinal Quiz AnswersFranciskhokhyx III100% (5)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsDari EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsBelum ada peringkat
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDDari EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDPenilaian: 5 dari 5 bintang5/5 (3)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionDari EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionPenilaian: 4 dari 5 bintang4/5 (404)
- The Age of Magical Overthinking: Notes on Modern IrrationalityDari EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityPenilaian: 4 dari 5 bintang4/5 (32)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDari EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BePenilaian: 2 dari 5 bintang2/5 (1)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaDari EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaPenilaian: 4.5 dari 5 bintang4.5/5 (266)