Autoimmune Hepatitis: Histopathology
Diunggah oleh
Hernan Del CarpioDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Autoimmune Hepatitis: Histopathology
Diunggah oleh
Hernan Del CarpioHak Cipta:
Format Tersedia
REVIEW
Autoimmune Hepatitis: Histopathology
Stephen A. Geller M.D.*
,
Autoimmune hepatitis (AIH), a chronic hepatic necroin-
flammatory disorder, occurs mostly in women. AIH is char-
acterized by prominent interface hepatitis and varying
degrees of lobular hepatitis. Laboratory studies show ele-
vated aminotransferase values, hypergammaglobulinemia,
and serologically demonstrable tissue-directed autoantibod-
ies. The clinical presentation of autoimmune hepatitis has
been reviewed in this edition of Clinical Liver Disease.
1
Liver biopsy is almost always mandated to establish the
diagnosis and estimate the prognosis.
2,3
Immune mechanisms contribute to other liver diseases,
including acute and chronic hepatitis caused by hepatotropic
viruses A, B and C, primary biliary cirrhosis (PBC), primary
sclerosing cholangitis (PSC), and drug-induced chronic hepa-
titis as well as alcoholic liver disease, Wilsons disease, and
perhaps alpha-1-antitrypsin deficiency. However, the condi-
tions principally considered to be autoimmune liver diseases
are autoimmune hepatitis (including drug-induced autoim-
mune hepatitis), PBC, PSC, and autoimmune cholangitis/
cholangiopathy.
AIH should be considered in any patient with unexplained
elevated serum aminotransferase values, particularly because
a timely diagnosis and appropriate therapy can be of great
value in suppressing disease activity. Untreated, AIH univer-
sally leads to cirrhosis and its complications, including death,
and there is a low but significant incidence of hepatocellular
carcinoma. Remission, both clinical and biochemical, is
achieved by as many as 85% of patients, and the need for
transplantation can be significantly reduced.
4
Establishing the
correct diagnosis can be challenging because of the heteroge-
neity of the clinical presentation and the absence of a specific
diagnostic test. Simple scoring systems
5
rely principally on
the clinical history and an evaluation of autoantibodies, but
they also include an evaluation of liver biopsy samples.
Histopathology
Biopsy allows an assessment of the inflammatory activity
and the severity of fibrosis, which is almost always seen,
often with the initial biopsy. Importantly, neither amino-
transferase values nor immunoglobulin G levels reflect the
degree of tissue damage. An adequate tissue sample is vital,
and cores with a total length of at least 2.5 cm and with at
least 10 portal tracts are needed.
6,7
Although the histologi-
cal features listed in the modified staging system are gener-
ally integral to establishing the diagnosis, considerable
variation and other features can be seen.
Differential diagnoses include viral hepatitides (in practice
the most common consideration), other immune liver disor-
ders, drug reactions (in which eosinophils are generally more
prominent), alcoholic liver disease (with fat and Mallory hya-
line), alpha-1-antitrypsin deficiency and Wilson disease (both
of which can be histochemically or biochemically demon-
strated), and other diagnoses. In recent years, it has been rec-
ognized that the biopsy appearance of chronic hepatitis E can
resemble autoimmune hepatitis with a lymphoplasmacytic
portal infiltrate but generally with less prominent interface
and lobular inflammation. It has long been recognized that
biopsy findings for acute hepatitis A can also resemble those
for autoimmune hepatitis, but clinical and serological features
generally establish the correct diagnosis. In most cases, histo-
logical features as well as histochemistry and immunohisto-
chemistry make specific identification feasible (Table 1).
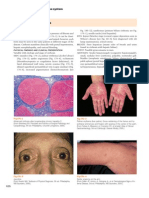
In autoimmune hepatitis, a low-magnification image
strongly suggests the diagnosis because of prominent inter-
face and zone 1 lobular hepatitis (Fig. 1); this picture is
unusual for the various conditions listed previously. When
interface hepatitis is absent or mild, AIH is unlikely, and
Abbreviations: AIC, autoimmune cholangitis/cholangiopathy; AIH, autoimmune hepatitis; LKM, liver/kidney microsome; PBC, primary biliary cirrhosis; PSC,
primary sclerosing cholangitis.
From the *Department of Pathology and Laboratory Medicine, Weill Cornell Medical College, New York, NY and the
Department of Pathology and Laboratory
Medicine, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA.
Potential conflict of interest: Nothing to report.
View this article online at wileyonlinelibrary.com
VC
2014 by the American Association for the Study of Liver Diseases
doi: 10.1002/cld.301
19 Clinical Liver Disease, Vol 3, No 2, February 2014 An Official Learning Resource of AASLD
care should be taken to prevent unnecessary therapy. An
irregularly distributed and relatively intense portal infiltrate
with either periportal or (in cases with bridging fibrosis
or cirrhosis) paraseptal interface hepatitis is typical (Fig. 1
and 2). Hepatocyte necrosis (acidophilic bodies and apopto-
tic bodies) is seen in periportal areas as well as the rest of
the lobule. Plasma cells with accompanying eosinophils and
lymphocytes are characteristic, but they are not always the
dominant cells, and in the appropriate clinical setting, the
diagnosis can be made with only modest numbers.
8
In
some cases, lymphocytes are dominant instead. When this
occurs, plasma cells in small clusters at the interface sup-
port the diagnosis (Fig. 3). Pseudoacini (rosettes) are seen
with significant lobular involvement and regenerative
Figure 1 AIH. This low-magnification image (hematoxylin-eosin, 340) shows expanded portal tracts with effacement of the interface by a lymphoplasmacytic infil-
trate including many plasma cells. The connective tissue stain shows early fibrosis and regenerative activity with 2-cell-thick liver plates and early nodule formation.
TABLE 1 Some Pathology Clues to Other Chronic Hepatitides in the AIH
Differential Diagnosis
12,13
Hepatitis B Modest interface hepatitis
Ground-glass cells
Immunostain-demonstrable hepatitis B
core and surface antigens
Hepatitis C Modest interface hepatitis
Lymphohistiocytic infiltrate with lymphoid
aggregates, including germinal centers
Few, scattered acidophilic bodies
Hepatitis E Lymphoplasmacytic portal infiltrate
Modest interface hepatitis
Mild or no lobular hepatitis
Alcoholic liver
disease
Steatosis
Balloon hepatocytes
Mallory hyaline
Pericellular, zone 3 (chicken-wire) fibrosis
Wilson disease Increased numbers of glycogenated nuclei
Rarely, visible zone 1 copper granules
Copper-associated protein demonstrable
with Victoria blue or orcein stains
Copper assay for quantitation
Genetic
hemochromatosis
Iron granules visible in zone 1 hepatocytes
Iron stain (e.g., Prussian blue)
Iron assay for quantitation
Alpha-1-antitrypsin
deficiency
Globules potentially seen in zone
1 hepatocytes with hematoxylin-eosin
Globules easily seen with diastaseperiodic
acid Schiff and/or immunostaining
Primary biliary cirrhosis Interface hepatitis minimal
Characteristic bile duct lesion
Progressive bile duct loss
Granulomas
Cholate stasis
Bile ductular reaction (biliary interface
hepatitis)
Increased copper and/or copper-associated
protein
Sclerosing
cholangitis
Interface hepatitis unusual in stage
1 disease
Interface hepatitis can be prominent in
stage 2 disease.
Mild bile duct lesion
Periductal concentric laminar fibrosis
(onion skin)
Bile duct loss with smudgy fibrosis
Variable, often mild ductular reaction
Drug reaction Portal neutrophils and eosinophils
Plasma cells usually not prominent
Fibrosis usually not seen
Figure 2 AIH. This medium-magnification image (hematoxylin-eosin, 3200)
shows a portal tract with an intense lymphoplasmacytic infiltrate effacing the
interface with rosette formation and hepatocyte necrosis (acidophilic bodies).
R E V I E W Autoimmune Hepatitis Histopathology Geller
20 Clinical Liver Disease, Vol 3, No 2, February 2014 An Official Learning Resource of AASLD
activity (Fig. 4). Rosettes and plasma cells are typical but
are not pathognomonic or consistently seen. Furthermore,
plasma cells and rosettes occur with other liver diseases.
Zone 1 (periportal) emperipolesis, the engulfing of lympho-
cytes by hepatocytes, occurs in the interface hepatitis area.
There may be giant-cell transformation (giant-cell hepatitis).
Severe inflammation extends beyond the periportal zone
with parenchymal collapse and, not uncommonly, bridging
necrosis, especially with acute clinical relapse and when
AIH is acute and fulminant.
9
Portal-to-portal or portal-to-
central fibrosis and cirrhosis are seen (Fig. 5).
Key histological features contributing positively to a score
establishing the diagnosis of AIH according to the revised
International Autoimmune Hepatitis Group modified staging
Figure 3 AIH. This high-magnification image (hematoxylin-eosin, 3400)
shows a predominantly lymphocytic portal infiltrate with clusters of plasma
cells at the interface.
Figure 4 AIH. This image (hematoxylin-eosin, 3400) shows rosettes.
Figure 5 AIH. This first-biopsy image (Masson trichrome, 3200) shows
fibrosis with early bridge formation (arrow).
Figure 6 AIH with overlap syndrome. This image (hematoxylin-eosin, 3200)
shows nonsuppurative cholangitis consistent with PBC.
R E V I E W Autoimmune Hepatitis Histopathology Geller
21 Clinical Liver Disease, Vol 3, No 2, February 2014 An Official Learning Resource of AASLD
system
4
are (1) interface hepatitis (13), which is the most
important; (2) a lymphoplasmacytic infiltrate (11); and (3)
rosette formation (11). Other features also help to establish
the diagnosis. For example, AIH differs from chronic hepati-
tis C in having more severe lobular inflammation and
necrosis as well as greater numbers of plasma cells, more
marked interface hepatitis, and broad areas of parenchymal
collapse (Table 2). Zone 3 (centrilobular) necrosis is well
described in AIH but is often inadequately recognized.
10
Zone 3 necrosis without fulminant hepatitis can lead to
erroneous diagnoses such as ischemia/hypoxia and toxic/
drug injury. Biliary changes are uncommon in AIH and are
almost always indicative of some other disorder. Isolated
bile duct injury, however, can be seen and does not exclude
AIH.
11
When anti-nuclear antibody values are significantly
increased in association with biopsy-demonstrable bile duct
injury (Fig. 6), the diagnosis of AIC is likely. Negative score
findings are the absence of these three findings (25), biliary
changes (23), and features suggesting an alternative
etiology (23).
There are no known direct microscopic correlates for the
various identifiable autoantibodies. For example, the
biopsy findings for liver/kidney microsome (LKM)associ-
ated AIH are similar to those for other forms (Fig. 7).
When overlap syndromes (e.g., AIH1PBC, AIH1PSC,
and AIH1AIC) occur, atypical histological changes can be
seen (Fig. 6).
12,13
Rarely, granulomas are seen. Other coinci-
dental disorders, such as hepatitis C, alcoholic liver disease,
human immunodeficiency virus positivity, and iron storage
disease, affect morphology. Increasingly, drug effects must be
considered. AIH-like microscopic changes caused by drugs
are generally resolved with the cessation of medication, but
chronic drug-induced AIH also occurs. Fibrosis and cirrhosis
are distinctly unusual in drug-induced AIH, but cholestasis,
portal neutrophils, and eosinophils are likely. The experience
of the reviewing pathologist can also affect the ability to estab-
lish the diagnosis.
Fulminant AIH is uncommon and is morphologically
indistinguishable from other forms of massive/submassive
necrosis.
12,13
Cirrhosis in AIH generally shows a greater degree of
inflammation than cirrhosis due to other causes. Septa are
easily recognized, as are areas of prior parenchymal collapse,
Figure 7 LKM AIH. Similar to Fig. 1, this low-magnification image (hematoxylin-eosin, 320) shows a marked lymphoplasmacytic infiltrate with effacement of the
interface.
TABLE 2 Histopathological Features Most Useful in Differentiating AIH
From Chronic Hepatitis C Virus
Feature AIH Hepatitis C Virus
Lobular inflammation/necrosis 12111 1/2
Plasma cells 12111 021
Interface hepatitis 12111 0211
Parenchymal collapse 12111 0
Steatosis 021 12111*
Portal lymphoid aggregates 021 12111
Germinal center formation in
lymphoid aggregates
0 0211
Bile duct injury
021 1211
This table has been adapted with permission from Biopsy Interpreta-
tion of the Liver, 2nd ed.
12
*Steatosis is particularly seen with genotype 3 hepatitis C virus.
Bile duct injury is rarely seen in AIH but is characteristic of AIC.
11
0 5 no change, 15 minimal or mild change, 115 moderate
change, 1115 marked change.
TABLE 3 Key Histopathology Features of AIH
1. Liver biopsy shows a moderate to severe necroinflammatory process with
prominent portal inflammation, interface hepatitis, a lymphoplasmacytic
infiltrate including many plasma cells, and acinar transformation of
hepatocytes (rosettes).
2. Plasma cells are not always the dominant inflammatory cells and may be
prominent only at the interface.
3. Fibrosis/cirrhosis is often seen on first biopsy.
4. It may present as acute, fulminant liver failure with massive or submassive
necrosis, including centrilobular (zone 3) necrosis.
5. Autoimmune liver disease variants may show features of more than one
immune disorder (overlap syndromes).
6. AIC is a distinct disorder histologically resembling PBC (without anti-
mitochondrial antibodies in serum and with anti-nuclear antibodies).
R E V I E W Autoimmune Hepatitis Histopathology Geller
22 Clinical Liver Disease, Vol 3, No 2, February 2014 An Official Learning Resource of AASLD
and the developing nodules vary greatly in size. Dysplastic
nodules can be seen, as can small hepatocellular carcinomas.
Key features of AIH are summarized in Table 3. n
CORRESPONDENCE
Stephen A. Geller, M.D., Department of Pathology and Laboratory Medicine,
Weill Cornell Medical College, 1300 York Avenue, New York, NY 10065.
E-mail: stg2013@med.cornell.edu.
References
1. Lucey MR, Vierling J. Clinical presentation and natural history of autoim-
mune hepatitis. Clin Liver Dis 2014;9-11.
2. Liberal R, Grant CR, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: a
comprehensive review. J Autoimmun 2013;41:126-139.
3. Weiler-Norman C, Sebode M, Lohse AW. Autoimmune hepatitis 2103 and
beyond. Minerva Gastroenterol Dietol 2013;59:133-141.
4. Strassburg CP. Autoimmune hepatitis. Dig Dis 2013;31:155-163.
5. Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al.
International Autoimmune Hepatitis Group report: a review of criteria for
diagnosis of autoimmune hepatitis. J Hepatol 1999;31:929-938.
6. Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in
chronic hepatitis C. Hepatology 2003;38:1449-1457.
7. Rousselet MC, Michalak S, Dupre F, Croue A, Bedossa P, Saint-Andre JP, et al.
Sources of variability in histological scoring of chronic viral hepatitis. Hepato-
logy 2005;41:257-264.
8. Vergani D, Longhi MS, Bogdanos DP, Ma Y, Mieli-Vergani G. Autoimmune
hepatitis. Semin Immunopathol 2009;31:421-435.
9. Czaja AJ. Acute and acute severed (fulminant) autoimmune hepatitis. Dig Dis
Sci 2013;58:897-914.
10. Zen Y, Notsumata K, Tanaka N, Nakanuma Y. Hepatic centrilobular zonal
necrosis with positive antinuclear antibody: a unique subtype or early disease
in autoimmune hepatitis. Hum Pathol 2007;38:1669-1675.
11. Ludwig J, Czaja AJ, Dickson ER, LaRusso NF, Weisner RH. Manifestations of
nonsuppurative cholangitis in chronic hepatobiliary disease: morphologic
spectrum, clinical correlations and terminology. Liver 1984;4:105-116.
12. Geller SA. Autoimmune hepatitis and related disorders. In: Geller SA, Pet-
rovic LM, eds. Biopsy Interpretation of the Liver. 2nd ed. Philadelphia, PA:
Lippincott Williams & Wilkins; 2009:120-135.
13. Washington MK, Manns MP. Autoimmune hepatitis. In: Burt A, Portmann B,
Ferrell L, eds. MacSweens Pathology of the Liver. 6th ed. Edinburgh, United
Kingdom: Churchill Livingstone; 2012:467-490.
R E V I E W Autoimmune Hepatitis Histopathology Geller
23 Clinical Liver Disease, Vol 3, No 2, February 2014 An Official Learning Resource of AASLD
Anda mungkin juga menyukai
- Autoimmune Hepatitis - SLE Overlap SyndromeDokumen2 halamanAutoimmune Hepatitis - SLE Overlap SyndromeMulyono Aba AthiyaBelum ada peringkat
- Feline Cholangiohepatitis: AbstractDokumen7 halamanFeline Cholangiohepatitis: AbstractibnuzikrillahBelum ada peringkat
- NEJM LiverDokumen7 halamanNEJM LiverRinova DevindaBelum ada peringkat
- Chronic Hepatitis: Progress ReportDokumen17 halamanChronic Hepatitis: Progress ReportIntan Nursiani AgnurBelum ada peringkat
- Protein Losing EnteropathyDokumen9 halamanProtein Losing EnteropathyrajibBelum ada peringkat
- AIH - SLE DifferentiateDokumen8 halamanAIH - SLE DifferentiateFanny PritaningrumBelum ada peringkat
- Hepatitis, Autoimmune - StatPearls - NCBI BookshelfDokumen4 halamanHepatitis, Autoimmune - StatPearls - NCBI BookshelfMiguel RomeroBelum ada peringkat
- Chronic Liver DiseaseDokumen75 halamanChronic Liver DiseasebitspurBelum ada peringkat
- Henoch-Schonlein PurpuraDokumen5 halamanHenoch-Schonlein PurpuraPramita Pramana100% (1)
- 54 Shivraj EtalDokumen3 halaman54 Shivraj EtaleditorijmrhsBelum ada peringkat
- Surgery Osce PrepDokumen94 halamanSurgery Osce PrepSamara AliBelum ada peringkat
- Colangita SclerozantaDokumen12 halamanColangita SclerozantaChoi DongYiBelum ada peringkat
- PR03671Dokumen4 halamanPR03671Dalia ExtrapolisBelum ada peringkat
- Autoimmune Hepatitis - Primary Biliary Cirrhosis Overlap SyndromeDokumen3 halamanAutoimmune Hepatitis - Primary Biliary Cirrhosis Overlap Syndromesusanto kusumaBelum ada peringkat
- Autoimmune HepatitisDokumen3 halamanAutoimmune HepatitisMohammed FaragBelum ada peringkat
- Chronic Hepatitis: Progress ReportDokumen17 halamanChronic Hepatitis: Progress ReportAdriana Margarita María Trejos TenorioBelum ada peringkat
- Acute Liver Failure Pathophysiologic Basis and The Current and Emerging TherapiesDokumen9 halamanAcute Liver Failure Pathophysiologic Basis and The Current and Emerging TherapiesIonut GrozavBelum ada peringkat
- Sepsis Induced CholestasisDokumen12 halamanSepsis Induced CholestasisYusuf Hakim AjiBelum ada peringkat
- Autoimmune Hepatitis: Diagnostic Criteria and Serological TestingDokumen4 halamanAutoimmune Hepatitis: Diagnostic Criteria and Serological TestingHernan Del CarpioBelum ada peringkat
- Ischemic Colitis: A Clinical Case and Concise ReviewDokumen15 halamanIschemic Colitis: A Clinical Case and Concise ReviewDevi AmaraBelum ada peringkat
- AF SeedingDokumen6 halamanAF SeedingdonkeyendutBelum ada peringkat
- Atlas of Liver Pathology - Chapter 8 Biliary Tree DiseaseDokumen45 halamanAtlas of Liver Pathology - Chapter 8 Biliary Tree DiseaseShabir HussainBelum ada peringkat
- Chronic Liver DiseasesDokumen24 halamanChronic Liver DiseasestabarekBelum ada peringkat
- 3 s2.0 B9780702066962000230 MainDokumen14 halaman3 s2.0 B9780702066962000230 MainKate ClarksonBelum ada peringkat
- Otoimmun Hepatitis and Skin LesionDokumen19 halamanOtoimmun Hepatitis and Skin LesionVedat KacarBelum ada peringkat
- (03241750 - Acta Medica Bulgarica) Non-Invasive Diagnostics of Liver FibrosisDokumen7 halaman(03241750 - Acta Medica Bulgarica) Non-Invasive Diagnostics of Liver FibrosisTeodorBelum ada peringkat
- Hepaticencephalopathy: Adam G. GowDokumen15 halamanHepaticencephalopathy: Adam G. GowJuan DuasoBelum ada peringkat
- Cirrhosis of LiverDokumen6 halamanCirrhosis of LiverpakdejackBelum ada peringkat
- Kolestasis SepsisDokumen21 halamanKolestasis SepsisLilik NatasubrataBelum ada peringkat
- College of Medicine: Thi Qar UniversityDokumen30 halamanCollege of Medicine: Thi Qar Universityhussain AltaherBelum ada peringkat
- Systemic Disease and The Liver: Maria Isabel Fiel,, Thomas D. SchianoDokumen14 halamanSystemic Disease and The Liver: Maria Isabel Fiel,, Thomas D. Schianokhuyennguyenhmu080692Belum ada peringkat
- Biliary CirrhosisDokumen11 halamanBiliary CirrhosisradistryaBelum ada peringkat
- Assessment of Jaundice in The Hospitalized Patient PDFDokumen16 halamanAssessment of Jaundice in The Hospitalized Patient PDFlaura canoBelum ada peringkat
- Cytomegalovirus Triggering Autoimmune Hepatitis: Case Report and Literature ReviewDokumen3 halamanCytomegalovirus Triggering Autoimmune Hepatitis: Case Report and Literature ReviewRabiatul 'raney' AdawiyahBelum ada peringkat
- Falla Hepatica GastroDokumen12 halamanFalla Hepatica GastrolouispekasBelum ada peringkat
- Article Casey LIVER Hepatic CirrhosisDokumen8 halamanArticle Casey LIVER Hepatic CirrhosisMaría José Muñoz PalaciosBelum ada peringkat
- ClassificationDokumen16 halamanClassificationpieterinpretoria391Belum ada peringkat
- Case Report: Twelve-Year-Old Girl With Primary Biliary CirrhosisDokumen4 halamanCase Report: Twelve-Year-Old Girl With Primary Biliary Cirrhosisapi-318749549Belum ada peringkat
- Liver DiseaseDokumen19 halamanLiver Diseasenishi kBelum ada peringkat
- Jessica Reid-Adam: Department of Pediatrics, Icahn School of Medicine at Mount Sinai, New York, NYDokumen3 halamanJessica Reid-Adam: Department of Pediatrics, Icahn School of Medicine at Mount Sinai, New York, NYAnastasia Widha SylvianiBelum ada peringkat
- Antiphospholipid Syndrome - StatPearls - NCBI BookshelfDokumen10 halamanAntiphospholipid Syndrome - StatPearls - NCBI BookshelfDika KibasBelum ada peringkat
- Fmge MCQDokumen4 halamanFmge MCQgrreddy8364320Belum ada peringkat
- B9781416049197X50014 B978141604919750178X MainDokumen4 halamanB9781416049197X50014 B978141604919750178X MainMANGBelum ada peringkat
- Liver Diseases: Lecture On Pathological Anatomy For The 3-rd Year StudentsDokumen27 halamanLiver Diseases: Lecture On Pathological Anatomy For The 3-rd Year StudentsRodriguez Vivanco Kevin DanielBelum ada peringkat
- 7cholestatic Liver DiseaseDokumen9 halaman7cholestatic Liver DiseaseDaniel Fernando LozanoBelum ada peringkat
- Acute Liver Injury and FailureDokumen14 halamanAcute Liver Injury and FailureWeslei ChaconBelum ada peringkat
- Chapter 49Dokumen6 halamanChapter 49Melissa Aina Mohd YusofBelum ada peringkat
- ACVIM Consensus Statement On The Diagnosis and TreatmentDokumen28 halamanACVIM Consensus Statement On The Diagnosis and TreatmentBê LagoBelum ada peringkat
- Fiziopatologie (English (British) )Dokumen22 halamanFiziopatologie (English (British) )Alina VoineaBelum ada peringkat
- Venesection: Phlebotomy orDokumen20 halamanVenesection: Phlebotomy orVera June RañesesBelum ada peringkat
- Pathology of Alcoholic Liver DiseaseDokumen7 halamanPathology of Alcoholic Liver DiseasehghBelum ada peringkat
- Infectious Mononucleosis: A Case ReportDokumen3 halamanInfectious Mononucleosis: A Case ReportSerghei CojocariBelum ada peringkat
- 1 s2.0 S1357303923000816 MainDokumen6 halaman1 s2.0 S1357303923000816 MaincamiloBelum ada peringkat
- Pi Is 0012369215372330Dokumen7 halamanPi Is 0012369215372330avnish sharmaBelum ada peringkat
- Halothane HepatotoxicityDokumen11 halamanHalothane Hepatotoxicityarifjo7999Belum ada peringkat
- Pathophysiology: Henoch-Schönlein Purpura (HSP Also Known As "Anaphylactoid Purpura"Dokumen5 halamanPathophysiology: Henoch-Schönlein Purpura (HSP Also Known As "Anaphylactoid Purpura"nikki_fuentes_1Belum ada peringkat
- Anaemia in Dogs and Cats (Part 2) : Continuing EducationDokumen6 halamanAnaemia in Dogs and Cats (Part 2) : Continuing EducationAchmad NugrohoBelum ada peringkat
- Amoebic Liver Abscess: Clinical MedicineDokumen5 halamanAmoebic Liver Abscess: Clinical MedicineAizat KamalBelum ada peringkat
- HP y CancerDokumen14 halamanHP y CancerHernan Del CarpioBelum ada peringkat
- Gastro Colopatia HTPDokumen18 halamanGastro Colopatia HTPHernan Del CarpioBelum ada peringkat
- Fisiopato HTPDokumen11 halamanFisiopato HTPHernan Del CarpioBelum ada peringkat
- Overlap Syndrome: A Real Syndrome?: Underlying ConceptsDokumen5 halamanOverlap Syndrome: A Real Syndrome?: Underlying ConceptsHernan Del CarpioBelum ada peringkat
- Autoimmune Hepatitis: Diagnostic Criteria and Serological TestingDokumen4 halamanAutoimmune Hepatitis: Diagnostic Criteria and Serological TestingHernan Del CarpioBelum ada peringkat
- Desordenes Testiculares AgudosDokumen9 halamanDesordenes Testiculares AgudosHernan Del CarpioBelum ada peringkat
- HCSP Fact Sheet: Pruritus (Itching)Dokumen3 halamanHCSP Fact Sheet: Pruritus (Itching)jovian danikoBelum ada peringkat
- JawapanLengkap Modul Biology PDFDokumen32 halamanJawapanLengkap Modul Biology PDFT-specialist SBelum ada peringkat
- T He 153 Human Organ Matching and Labelling Activity Ver 2Dokumen3 halamanT He 153 Human Organ Matching and Labelling Activity Ver 2Ly Nguyen0% (1)
- Fast Fed Cycle 2016 MJHDokumen11 halamanFast Fed Cycle 2016 MJHGoran MaliBelum ada peringkat
- Drug Addiction - Biology Science Fair Project IdeasDokumen11 halamanDrug Addiction - Biology Science Fair Project IdeasSwaraj KhedekarBelum ada peringkat
- What? Who?: DR - Mabel Sihombing Sppd-Kgeh DR - Ilhamd SPPD Dpertemen Ilmu Penyakit Dalam Rs - Ham/Fk-Usu MedanDokumen45 halamanWhat? Who?: DR - Mabel Sihombing Sppd-Kgeh DR - Ilhamd SPPD Dpertemen Ilmu Penyakit Dalam Rs - Ham/Fk-Usu MedanM Rizky Assilmy LubisBelum ada peringkat
- 1ST Term S3 BiologyDokumen36 halaman1ST Term S3 BiologyFaith Opadele100% (2)
- Treatment of Liver and Spleen Illnesses by HerbsDokumen16 halamanTreatment of Liver and Spleen Illnesses by HerbsZachary LeeBelum ada peringkat
- Klinofeed PresentationDokumen90 halamanKlinofeed Presentationthanh ba mat100% (1)
- Alagille SyndromeDokumen16 halamanAlagille SyndromeMuhammad ShiddiqBelum ada peringkat
- Pathophysiology of Alcoholic Liver DiseaseDokumen4 halamanPathophysiology of Alcoholic Liver Diseaseshailendra tripathiBelum ada peringkat
- Bile (Report in Biochemistry)Dokumen2 halamanBile (Report in Biochemistry)Luci BernasBelum ada peringkat
- DR Max Gerson, Charlotte Gerson - Gerson Therapy - Gerson Therapy Manual Training For Professionals (Gerson Insitute) - Gerson Therapy (2022)Dokumen482 halamanDR Max Gerson, Charlotte Gerson - Gerson Therapy - Gerson Therapy Manual Training For Professionals (Gerson Insitute) - Gerson Therapy (2022)Décio Leme100% (1)
- How To Detox Your Liver NaturallyDokumen27 halamanHow To Detox Your Liver NaturallyKajsa100% (1)
- Gallstone DiseaseDokumen104 halamanGallstone DiseaseTan DanBelum ada peringkat
- IridologyDokumen18 halamanIridologyMadhu mithaBelum ada peringkat
- Anatomy of LiverDokumen22 halamanAnatomy of Liversiewv_1Belum ada peringkat
- Ghana CNTDokumen155 halamanGhana CNTRoy VermaBelum ada peringkat
- 1 LiverDokumen3 halaman1 LiverJonance YeeBelum ada peringkat
- Pettenkofer's TestDokumen13 halamanPettenkofer's TestPrincess Lie Rizo AquinoBelum ada peringkat
- Parenchymatous AND Stromal-Vascular Degenerations: Lecturer - prof.G.I.Hubina-VakulikDokumen59 halamanParenchymatous AND Stromal-Vascular Degenerations: Lecturer - prof.G.I.Hubina-Vakulikсветлый поповBelum ada peringkat
- Daniel FastDokumen19 halamanDaniel FastRon KrisBelum ada peringkat
- 637a1d3f56493 - Anatomy Fun Easy Summary - of - AbdomenDokumen64 halaman637a1d3f56493 - Anatomy Fun Easy Summary - of - AbdomenPA inbox100% (2)
- MELD-Plus Wiki Nov 24 2019Dokumen3 halamanMELD-Plus Wiki Nov 24 2019UKBelum ada peringkat
- Diarrhea Crampy Abdominal Pain Nausea OmitingDokumen14 halamanDiarrhea Crampy Abdominal Pain Nausea OmitingLynne CammayoBelum ada peringkat
- Dr. Ernst Ziegler-1stEd PDFDokumen392 halamanDr. Ernst Ziegler-1stEd PDFcasBelum ada peringkat
- NLS Diagnostics of LiverDokumen5 halamanNLS Diagnostics of LiverAbdu Ben HallamBelum ada peringkat
- Chronic Transaminitis 07Dokumen39 halamanChronic Transaminitis 07Jessica GarletsBelum ada peringkat
- HB Degradation and Related ProblemDokumen23 halamanHB Degradation and Related ProblemHatim AlnourBelum ada peringkat
- Open CholecystectomyDokumen15 halamanOpen CholecystectomyjimdioBelum ada peringkat