The Calorimetric Determination of Manganese in Paper Clips
Diunggah oleh
Onkarabile MatomeHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
The Calorimetric Determination of Manganese in Paper Clips
Diunggah oleh
Onkarabile MatomeHak Cipta:
Format Tersedia
The Calorimetric Determination of Manganese in Paper Clips
Aim
The objectives of this experiment is to determine the mass percentage of Manganese in two
paper clips sample by visible spectroscopy, This is achieved by preparing standards solutions
which will be used to plot the calibration curve and later the calibration curve is used to
determine the manganese content in solutions of unknown Mn.
Method
The method outlined in the CEM2007F was followed closely with no alterations.
1. First you prepare the standards solutions of varying concentration such that the total
final solution of 5, 10, 15, 20 and 25 ppm of Mn will results. Note that the same
procedure performed with the standard solutions is followed with the blank and
unknown solutions of Mn we are going to test.
2. Add volumes of 100 ppm Mn as specified in (1) in 5 conical flasks.
3. Add 25 cm
3
of HNO
3
in each flask and include the sixth one with just 25 cm
3
HNO
3
for the blank solution in it.
4. Add two more flasks with paper clips in them, this will be the unknown solutions and
repeat (3).
5. Cover all the 8 flask with class lids and boil them until most of the paper clip has
dissolved
6. Allow cooling after all the paper clip has dissolved
7. Add 0.5 g of ammonium perodisulfate and repeat (5) and (6) except this time you
count time ( 5-15 min)
8. Add 5 cm
3
of concentrated phosphoric acid, 25 cm
3
of water and 0.4 g cm
3
KlO
4
to each flask
and repeat (5 and 6)
9. Now fill the volumetric flask to the mark with this solutions and measure absorbance using
spectrophotometer at the wavelength of 525.3 nm.
Results
Mass of paper clips
Mass1 = 0.3597 g
Mass2 = 0.3210 g
Table 1: Concentration of Mn and Absorbance Measured
Concentration of Mn in
100ml solution (ppm)
Absorbance at 525 nm
5 0.223
10 0.432
15 0.614
20 0.860
25 1.062
Table 2: Absorbance of Sample Measured
Sample 1 0.636 g
Sample 2 0.480 g
Calculations
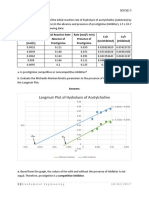
Graph plot of absorbance vs concentration at the wavelength of 525.3 nm
y = 0.0421x + 0.0064
R = 0.9983
0.000
0.200
0.400
0.600
0.800
1.000
1.200
0 5 10 15 20 25 30
A
b
s
o
r
b
a
n
c
e
Concentration (ppm)
Graph of Absorbance Vs Concentration at the
wavelength of 525.3 nm
From the graph using excel we get the calibration curve equation of the straight line to be
y = 0.0421x + o.oo64
Where: y ~ absorbance
x ~ concentration (ppm)
Calculation of concentration of the samples
Sample1
x1 =
x1 =
= 11.2 ppm = 11.2 mg/l
Mass Mn in sample 1 = 11.2 x 0.1 = 1.12 mg
Sample2
x2 =
x2 =
= 14.96 ppm = 14.96 mg/l = 15 mg/l
Mass of Mn in sample 2 = 15 x 0.1 = 1.5 mg
Mass % of Mn in paper clips
Sample1
Mass1 % = Mass of Mn in sample1/Mass1 = (1.12mg)/(0.3597g) = 0.35%
Mass2% = Mass of Mn in sample1/Mass1 = (1.50mg)/(0.3210g) = 0.42%
Discussion and Conclusion
525.3 nm a Wavelength obtained from absorption spectrum of KMnO
4
was set . Standard
solution was used to zero the spectrophotometer each time a new samples was tested.
Hence a calibration curve was obtained to compare the absorbance of the unknown
solution of Mn. Paper clip we labelled 1 had 0.35% of Mn and paper clip 2 had 0.42% of
Mn.
References
1. Colorimetric Determination of Manganese in Steel." 123HelpMe.com. 30 Mar 2014
<http://www.123HelpMe.com/view.asp?id=148733>.
2. Skoog, D. A, West, D M and Holler, Fundamentals of Analytical Chemistry, 6
th
edition,
Saunders College Publishing, Fort Worth, 1992 chp 20C 2 to 7.
Anda mungkin juga menyukai
- Lab Activity 6 - Modelling Molecular ShapesDokumen3 halamanLab Activity 6 - Modelling Molecular ShapesCharles Reginald K. HwangBelum ada peringkat
- Chem26.1 ATQ6 Double Indicator TitrationDokumen2 halamanChem26.1 ATQ6 Double Indicator TitrationAlexander Gordon InesBelum ada peringkat
- Improved Synthesis of Basic Zinc AcetateDokumen2 halamanImproved Synthesis of Basic Zinc Acetatejinzo88Belum ada peringkat
- JrrysnDokumen4 halamanJrrysnEureca Parra100% (2)
- Acetylation Ferrocene 2012Dokumen3 halamanAcetylation Ferrocene 2012VargasArn50% (2)
- Long Report Exp 6Dokumen6 halamanLong Report Exp 6Mxokzah Cmoh100% (1)
- Determination of Nitrate in Municipal Waste Water by UV SpectrosDokumen7 halamanDetermination of Nitrate in Municipal Waste Water by UV SpectrosNeal CuaBelum ada peringkat
- Grignard ExperimentDokumen1 halamanGrignard ExperimentNigel YeoBelum ada peringkat
- CUSO4 PostlabDokumen8 halamanCUSO4 PostlabRuwanthika Fernando100% (1)
- From Final ExamDokumen9 halamanFrom Final ExamThrishnaa BalasupurManiamBelum ada peringkat
- Chem 112.1 - Exer 2 PostlabDokumen7 halamanChem 112.1 - Exer 2 PostlabGerry Mark GubantesBelum ada peringkat
- Full Report Exer 1Dokumen8 halamanFull Report Exer 1marinella100% (1)
- Preparing Standard Acid and BaseDokumen7 halamanPreparing Standard Acid and Basebrittany obrienBelum ada peringkat
- Spectrophotometric Determination of The Acid Dissociation Constant of Methyl RedDokumen3 halamanSpectrophotometric Determination of The Acid Dissociation Constant of Methyl Red7063673nasBelum ada peringkat
- 4500-B Boron (2000) PDFDokumen3 halaman4500-B Boron (2000) PDFAntoninaPontes100% (1)
- Reading Phase Diagrams and ILAR University of The Philippines DilimanDokumen5 halamanReading Phase Diagrams and ILAR University of The Philippines DilimanAcademicBMBelum ada peringkat
- Water Treatment Water Treatment: Lecture 7: DisinfectionDokumen17 halamanWater Treatment Water Treatment: Lecture 7: DisinfectionAbo-Khaled MohammedBelum ada peringkat
- FP Assignment 2Dokumen1 halamanFP Assignment 2Austin Phua Yun HockBelum ada peringkat
- Formal Lab ReportDokumen5 halamanFormal Lab ReportAlfred ContadoBelum ada peringkat
- Experiment No 18Dokumen4 halamanExperiment No 18Suvrasoumya Mohanty100% (2)
- Expt. #3 - FRDokumen9 halamanExpt. #3 - FRClarice Mae DacasinBelum ada peringkat
- Nickel Experiment XWDokumen4 halamanNickel Experiment XWKhairul Anwar Abd HamidBelum ada peringkat
- EXp-26 Mini ProjectDokumen16 halamanEXp-26 Mini ProjectHazem Al-hazmi50% (2)
- Exp. 2 c230 S - 07 Keq of FeSCNDokumen10 halamanExp. 2 c230 S - 07 Keq of FeSCNdenciopoBelum ada peringkat
- Common Ion Effect and Buffers: M. de LeonDokumen8 halamanCommon Ion Effect and Buffers: M. de LeondeleonmatthewreiBelum ada peringkat
- Kinetics of Ester HydrolysisDokumen24 halamanKinetics of Ester HydrolysisNick OnuskaBelum ada peringkat
- Institute of Space Technology BS-5 (MS&E)Dokumen4 halamanInstitute of Space Technology BS-5 (MS&E)Osama Aadil SaadiBelum ada peringkat
- Q Method of Shelf Life Estimation: Example Protocol For Drug And/Or Drug Product Stability StudiesDokumen2 halamanQ Method of Shelf Life Estimation: Example Protocol For Drug And/Or Drug Product Stability StudiesAccung Buccu100% (1)
- Pset TakeHomeFinalExam Part1 - 1stsem1617Dokumen3 halamanPset TakeHomeFinalExam Part1 - 1stsem1617Shara Jane DelmoBelum ada peringkat
- Experiment No 1 PDFDokumen3 halamanExperiment No 1 PDFVaid RahulBelum ada peringkat
- 3 Determination of Complex Ion by Jobs MethodDokumen2 halaman3 Determination of Complex Ion by Jobs Methodvishwanathz47Belum ada peringkat
- Hydrolysis of Tert-Butyl Chloride and Solvent EffectDokumen7 halamanHydrolysis of Tert-Butyl Chloride and Solvent EffectangelbenavidezBelum ada peringkat
- Post Lab Discussion Chem 26.1Dokumen48 halamanPost Lab Discussion Chem 26.1Coleen SalvadorBelum ada peringkat
- PH CH 126.1 Fischer Esterification of Methyl Benzoate 2Dokumen6 halamanPH CH 126.1 Fischer Esterification of Methyl Benzoate 2Tammy CacnioBelum ada peringkat
- Exp 2 - DilutionDokumen6 halamanExp 2 - DilutionSiti FatimahBelum ada peringkat
- Unit 5 Formal Lab ReportDokumen5 halamanUnit 5 Formal Lab Reportapi-359422860Belum ada peringkat
- Macro Cyclic SynthesisDokumen5 halamanMacro Cyclic SynthesisBrenda DelgadoBelum ada peringkat
- MC 2021-073Dokumen6 halamanMC 2021-073Jovilyn De LeonBelum ada peringkat
- Laboratory Report 2.1 Grain Size Analysis Sieve Test and Hydrometer TestDokumen8 halamanLaboratory Report 2.1 Grain Size Analysis Sieve Test and Hydrometer TestM MBelum ada peringkat
- Test 14 Total HardnessDokumen7 halamanTest 14 Total HardnessshahjahanhashimaliBelum ada peringkat
- Format of Lab Report Example 8609Dokumen14 halamanFormat of Lab Report Example 8609herrk167% (3)
- Chemistry Practicals First YearsDokumen65 halamanChemistry Practicals First Yearskokimesh0% (1)
- 3500 PBDokumen3 halaman3500 PBpollux23Belum ada peringkat
- Formal Report Experiment 3Dokumen6 halamanFormal Report Experiment 3Rafael Lee100% (2)
- Gravimetric Determination of Barium SulfateDokumen3 halamanGravimetric Determination of Barium SulfateArmiee InfiniteBelum ada peringkat
- Tutorial 5Dokumen6 halamanTutorial 5hsiegler2Belum ada peringkat
- Institute of Space Technology BS-5 (MS&E)Dokumen3 halamanInstitute of Space Technology BS-5 (MS&E)Osama Aadil Saadi100% (1)
- QC1Lec ReviewerDokumen10 halamanQC1Lec Reviewerprinz1mendezBelum ada peringkat
- Mass Transfer OperationsDokumen77 halamanMass Transfer OperationsNhã UyênBelum ada peringkat
- C H O + A O + B NH C C H NO + D H O+eCO: InstructionsDokumen4 halamanC H O + A O + B NH C C H NO + D H O+eCO: InstructionsJohn Paul Jandayan33% (3)
- Lab+Manual+2014 Cbe 2207Dokumen63 halamanLab+Manual+2014 Cbe 2207Krishnan Mohan100% (1)
- Lecture-7 (Measurement of KLa) )Dokumen12 halamanLecture-7 (Measurement of KLa) )Babu PonnusamiBelum ada peringkat
- Precipitation Methods Compleximetry Methods Gravimetric Methods PDFDokumen42 halamanPrecipitation Methods Compleximetry Methods Gravimetric Methods PDFYanika HontoriaBelum ada peringkat
- Spectrophotometric Determination of An Equilibrium ConstantDokumen6 halamanSpectrophotometric Determination of An Equilibrium ConstantJett CanoyBelum ada peringkat
- Experiment 3 Che 314Dokumen11 halamanExperiment 3 Che 314Seele TlhagaBelum ada peringkat
- Kappa Number AnalysisDokumen3 halamanKappa Number AnalysiskudaBelum ada peringkat
- Experiment 1 The Potentiometric Titration of Hydrogen PeroxideDokumen10 halamanExperiment 1 The Potentiometric Titration of Hydrogen PeroxideAfiqah SamanBelum ada peringkat
- Lbych29 HandoutDokumen24 halamanLbych29 HandoutKyle LatayanBelum ada peringkat
- Oxalate LabDokumen5 halamanOxalate LabtylerBelum ada peringkat
- Stream 1. Slurry 2.00 3. Acid Make Up 4. Oxygen 5 6 7 8: Control Molar Flows (Kmol/h)Dokumen32 halamanStream 1. Slurry 2.00 3. Acid Make Up 4. Oxygen 5 6 7 8: Control Molar Flows (Kmol/h)Onkarabile MatomeBelum ada peringkat
- XSteam Excel v2.6 USDokumen12 halamanXSteam Excel v2.6 USOnkarabile MatomeBelum ada peringkat
- Sibu HXDokumen3 halamanSibu HXOnkarabile MatomeBelum ada peringkat
- BVPDokumen2 halamanBVPOnkarabile Matome100% (1)
- NLEQDokumen6 halamanNLEQOnkarabile MatomeBelum ada peringkat
- Selectivity Vs ConversionDokumen1 halamanSelectivity Vs ConversionOnkarabile MatomeBelum ada peringkat
- Mass of Tube 1 2 3 4 Before Tippping (G) After Tipping (G) Mass of BoraxDokumen1 halamanMass of Tube 1 2 3 4 Before Tippping (G) After Tipping (G) Mass of BoraxOnkarabile MatomeBelum ada peringkat
- University of The Witwatersrand, Johannesburg School of Mechanical, Industrial & Aeronautical EngineeringDokumen3 halamanUniversity of The Witwatersrand, Johannesburg School of Mechanical, Industrial & Aeronautical EngineeringOnkarabile MatomeBelum ada peringkat
- This Is The Financial Model That Calculates The New Interest and Expected New Balance PROVIDED That The Interest Rate and Capital Cost Are GivenDokumen2 halamanThis Is The Financial Model That Calculates The New Interest and Expected New Balance PROVIDED That The Interest Rate and Capital Cost Are GivenOnkarabile MatomeBelum ada peringkat
- MMPDS-01: Intro AppendiciesDokumen1 halamanMMPDS-01: Intro AppendiciesOnkarabile MatomeBelum ada peringkat
- GATE Metrology QuestionsDokumen14 halamanGATE Metrology QuestionsWilson Kumar33% (3)
- Mechanical MeasurementDokumen48 halamanMechanical MeasurementNaman Dave100% (2)
- SACAA Comm NavigationDokumen4 halamanSACAA Comm NavigationOsa AigBelum ada peringkat
- ASHRAE Units and ConversionsDokumen2 halamanASHRAE Units and ConversionsvitaliskcBelum ada peringkat
- Pressureconversion PDFDokumen3 halamanPressureconversion PDFShaikhMazharAhmedBelum ada peringkat
- 3.4 LandSurveyingDokumen37 halaman3.4 LandSurveyingHundeejireenyaBelum ada peringkat
- Metonic Cycle PDFDokumen2 halamanMetonic Cycle PDFavinashBelum ada peringkat
- Approximate Conversions From SI/Metric Units To Standard/Imperial UnitsDokumen4 halamanApproximate Conversions From SI/Metric Units To Standard/Imperial Unitssadna fernandoBelum ada peringkat
- Survey NotesDokumen166 halamanSurvey Notesglttcgamer100% (1)
- Process Industries A Study GuideDokumen160 halamanProcess Industries A Study GuideElcan AlmammadovBelum ada peringkat
- How To Read MicrometerDokumen18 halamanHow To Read MicrometerSaravana PandiyanBelum ada peringkat
- Report TacheometryDokumen11 halamanReport Tacheometryhibby_tomey33% (3)
- Tacheometric Surveying Study Notes: Unit - IIDokumen11 halamanTacheometric Surveying Study Notes: Unit - IIimcivil15Belum ada peringkat
- Accuracy, Trueness, Error, Bias, Precision, and Uncertainty: What Do These Terms Mean?Dokumen3 halamanAccuracy, Trueness, Error, Bias, Precision, and Uncertainty: What Do These Terms Mean?Aaron Chris GonzalesBelum ada peringkat
- Units and MeasurementsDokumen18 halamanUnits and MeasurementsLord Siva75% (4)
- Thermo Spectronic Genesys 6 Manual: Read/DownloadDokumen3 halamanThermo Spectronic Genesys 6 Manual: Read/DownloadaseelBelum ada peringkat
- Catalog Amc Tesa 2014 15Dokumen419 halamanCatalog Amc Tesa 2014 15Andrei-ViorelBelum ada peringkat
- Grade 5 Measuring Mass WorksheetsDokumen3 halamanGrade 5 Measuring Mass WorksheetsAndres100% (1)
- Rahukaalam CalculatorDokumen2 halamanRahukaalam CalculatorTNBKPCLBelum ada peringkat
- Equation SheetDokumen2 halamanEquation SheetJohn DoeBelum ada peringkat
- Direct Reading Theodolite Wild T16Dokumen2 halamanDirect Reading Theodolite Wild T16Marcelo Alberto RechBelum ada peringkat
- Engineering Metrology and Measurements Lab ME6513Dokumen26 halamanEngineering Metrology and Measurements Lab ME6513Ramanathan DuraiBelum ada peringkat
- Texto en Ingles TopografiaDokumen3 halamanTexto en Ingles TopografiaAngelita ZamsanBelum ada peringkat
- Nabl 122 12 PDFDokumen21 halamanNabl 122 12 PDFmaheshBelum ada peringkat
- Sounding Boat EnggDokumen2 halamanSounding Boat EnggRaja VelBelum ada peringkat
- PROC-TC-012 Procedure For Calibration Flask, Cylinder SOP (PROC - C)Dokumen19 halamanPROC-TC-012 Procedure For Calibration Flask, Cylinder SOP (PROC - C)Ban ZanganaBelum ada peringkat
- Coordinates and TimeDokumen8 halamanCoordinates and TimedhritimohanBelum ada peringkat
- Assignment No. 1 PhysicsDokumen6 halamanAssignment No. 1 PhysicsObi MagsombolBelum ada peringkat
- 05-9719!34!37 (With Frosted Filter)Dokumen10 halaman05-9719!34!37 (With Frosted Filter)ElvisBelum ada peringkat