Cre 1 Solution PDF
Diunggah oleh
Saints Burner Christopher100%(1)100% menganggap dokumen ini bermanfaat (1 suara)
577 tayangan21 halaman1. The document describes the first-order liquid phase cis-trans isomerization of 2-butene in a tubular reactor with constant volumetric flow rate.
2. It provides equations to relate the reactor volume to the concentrations of reactant A at the inlet and outlet, as well as the rate constant and flow rate.
3. For a given flow rate of 10 dm3/min and rate constant of 0.23 min-1, the required reactor volume is calculated to be 200 dm3 to reduce the exit concentration to 10% of the inlet concentration.
Deskripsi Asli:
Cre 1 Tutorial solution.
Judul Asli
cre 1 solution .pdf
Hak Cipta
© © All Rights Reserved
Format Tersedia
PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen Ini1. The document describes the first-order liquid phase cis-trans isomerization of 2-butene in a tubular reactor with constant volumetric flow rate.
2. It provides equations to relate the reactor volume to the concentrations of reactant A at the inlet and outlet, as well as the rate constant and flow rate.
3. For a given flow rate of 10 dm3/min and rate constant of 0.23 min-1, the required reactor volume is calculated to be 200 dm3 to reduce the exit concentration to 10% of the inlet concentration.
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
100%(1)100% menganggap dokumen ini bermanfaat (1 suara)
577 tayangan21 halamanCre 1 Solution PDF
Diunggah oleh
Saints Burner Christopher1. The document describes the first-order liquid phase cis-trans isomerization of 2-butene in a tubular reactor with constant volumetric flow rate.
2. It provides equations to relate the reactor volume to the concentrations of reactant A at the inlet and outlet, as well as the rate constant and flow rate.
3. For a given flow rate of 10 dm3/min and rate constant of 0.23 min-1, the required reactor volume is calculated to be 200 dm3 to reduce the exit concentration to 10% of the inlet concentration.
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 21
Consider the liquid phase cis trans isomerization of 2butene
which we will write symbolically as
The reaction is first order in A (r
A
= kC
A
) and is carried out in a tubular reactor in which
the volumetric flow rate, v, is constant, i.e., v= v
0
.
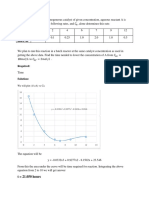
1. Sketch the concentration profile.
2. Derive an equation relating the reactor volume to the entering and exiting
concentrations of A, the rate constant k, and the volumetric flow rate v
0
.
3. Determine the reactor volume necessary to reduce the exiting concentration to 10% of
the entering concentration when the volumetric flow rate is 10 dm
3
/min (i.e.,
liters/min) and the specific reaction rate, k, is 0.23 min
1
.
Solution
1. Sketch C
A
as a function of V.
Species A is consumed as we move down the reactor, and as a result, both the molar
flow rate of A and the concentration of A will decrease as we move. Because the
volumetric flow rate is constant, v = v
0
, one can use Equation (1-8) to obtain the
concentration of A, C
A
= F
A
/v
0
, and then by comparison with Figure 1-12 plot, the
concentration of A as a function of reactor volume, as shown in Figure E1-2.1.
Figure E1-2.1 Concentration profile.
2. Derive an equation relating V, v
0
, k, C
A0
, and C
A
.
For a tubular reactor, the mole balance on species A (j = A) was shown to be given by
Equation (1-11). Then for species A (j = A)
Equation 1-12
For a first-order reaction, the rate law (discussed in Chapter 3) is
Equation E1-2.1
Reactor sizing
Because the volumetric flow rate, v, is constant (v = v0), as it is for most all liquid-
phase reactions,
Equation E1-2.2
Multiplying both sides of Equation (E1-2.2) by minus one and then substituting
Equation (E1-2.1) yields
Equation E1-2.3
Separating the variables and rearranging gives
Using the conditions at the entrance of the reactor that when V = 0, then C
A
= C
A0
,
Equation E1-2.4
Carrying out the integration of Equation (E1-2.4) gives
Equation E1-2.5
We can also rearrange Equation (E1-2.5) to solve for the concentration of A as a
function of reactor volume to obtain
C = C
Ao
exp(kV/v
0
)
Concentration Profile
3. Calculate V. We want to find the volume, V
1
, at which for k = 0.23 min
1
and v
0
= 10 dm
3
/min.
Substituting C
A0
, C
A
, v
0
, and k in Equation (E1-2.5), we have
Let's calculate the volume to reduce the entering concentration to C
A
= 0.01 C
A0
.
Again using equation (E1-2.5)
Note: We see that a larger reactor (200 dm
3
) is needed to reduce the exit concentration
to a smaller fraction of the entering concentration (e.g., C
A
= 0.01 C
A0
).
We see that a reactor volume of 0.1 m
3
is necessary to convert 90% of species A
entering into product B for the parameters given.
Question 2
Question 3
Question 4
Problem
The irreversible liquid phase second order reaction
is carried out in a CSTR. The entering concentration of A, C
A0
, is 2 molar and the exit
concentration of A, C
A
is 0.1 molar. The entering and exiting volumetric flow rate, v
o
, is
constant at 3 dm
3
/s. What is the corresponding reactor volume?
Solution
Mole Balance
Rate Law
(2
nd
order)
Combine
What is wrong with this solution?
The entering concentration, C
AO
= 2 mol/dm
3
of A was substituted into the rate law instead of
the exit concentration C
A
= 0.1 mol/dm
3
. Because the CSTR is perfectly mixed, the
concentration inside the CSTR where the reaction is taking place is the same as that in the
exit.
The reactor volume is fairly large at about 5 thousand gallons. (3.785 dm
3
= 1 US gallon)
Question 5
QUESTION 6
Question 7
Anda mungkin juga menyukai
- Ideal Reactors Part 2 Solved ProblemsDokumen15 halamanIdeal Reactors Part 2 Solved ProblemsWaldi SagalaBelum ada peringkat
- Tutorial QuestionsDokumen8 halamanTutorial QuestionsMaame Efua Neizer100% (1)
- Chương 1 - Bài TậpDokumen25 halamanChương 1 - Bài TậpTÍN Phạm Nguyễn TrọngBelum ada peringkat
- Ex0 Questions SolutionsDokumen7 halamanEx0 Questions SolutionsBiniyam haileBelum ada peringkat
- CHFEN 3553 Chemical Reaction Engineering: April 28, 2003 1:00 PM - 3:00 PM Answer All QuestionsDokumen4 halamanCHFEN 3553 Chemical Reaction Engineering: April 28, 2003 1:00 PM - 3:00 PM Answer All QuestionsJiahui TanBelum ada peringkat
- Sample Problems On Ideal Reactor ModelsDokumen7 halamanSample Problems On Ideal Reactor ModelsGirllietopsyBelum ada peringkat
- An Mon2Dokumen5 halamanAn Mon2KHÁNH VÕ ĐĂNGBelum ada peringkat
- A. Answer The Following Questions With Proper ExplanationsDokumen1 halamanA. Answer The Following Questions With Proper ExplanationsRohitBelum ada peringkat
- Sample Problem #1Dokumen2 halamanSample Problem #1Dozdi100% (1)
- Absorption of Carbon Dioxide Into WaterDokumen11 halamanAbsorption of Carbon Dioxide Into WaterEstelle Jean CauilanBelum ada peringkat
- Tutorial 9Dokumen14 halamanTutorial 9Abdimajid MohamedBelum ada peringkat
- Levenspiel C5 Problemas PDFDokumen7 halamanLevenspiel C5 Problemas PDFbete_azmaveteBelum ada peringkat
- 123 Reynolds ApparatusDokumen5 halaman123 Reynolds ApparatusKonem SolutionsBelum ada peringkat
- Tutorial Leaching 2017Dokumen11 halamanTutorial Leaching 2017Victor M. Jaki100% (1)
- S.no. Name of The Experiment Date of Conduction Date of Submission P2 Cascade CSTR 4 February, 2021 9 February, 2021Dokumen14 halamanS.no. Name of The Experiment Date of Conduction Date of Submission P2 Cascade CSTR 4 February, 2021 9 February, 2021DEEPSHIKA DUTTABelum ada peringkat
- CSTRDokumen20 halamanCSTRSharing Caring100% (1)
- Isothermal CSTR PDFDokumen9 halamanIsothermal CSTR PDFprashant_cool_4_uBelum ada peringkat
- RXN CH 5Dokumen68 halamanRXN CH 5Yonas AddamBelum ada peringkat
- Kinetics (Gjjkkkgty)Dokumen5 halamanKinetics (Gjjkkkgty)Chrysler Kane DepnagBelum ada peringkat
- Lab6-Tubular Flow ReactorDokumen11 halamanLab6-Tubular Flow ReactorNurtasha Atikah100% (1)
- Final Report PFRDokumen12 halamanFinal Report PFRmark_ancotBelum ada peringkat
- Chapter 2 - Lle EditedDokumen60 halamanChapter 2 - Lle EditedSiti Nurshahira100% (1)
- Gate 1993 PDFDokumen11 halamanGate 1993 PDFVammsy Manikanta SaiBelum ada peringkat
- Isothermal Semi-Batch Reactor PPT RJC SirDokumen16 halamanIsothermal Semi-Batch Reactor PPT RJC Sirsdjdsf100% (1)
- Chapter 5 - Absorption (Part 1)Dokumen41 halamanChapter 5 - Absorption (Part 1)La Casa Jordan100% (1)
- PDFDokumen88 halamanPDFMuralidharanBelum ada peringkat
- Mass Transfer CoefficientDokumen5 halamanMass Transfer CoefficientPinjala AnoopBelum ada peringkat
- Results and Discussion of CSTR in SeriesDokumen3 halamanResults and Discussion of CSTR in SeriesleenzalalBelum ada peringkat
- Rr320802chemicalreactionengineeringiDokumen8 halamanRr320802chemicalreactionengineeringiSanthosh KumarBelum ada peringkat
- Tutorial 1Dokumen4 halamanTutorial 1Hanee Farzana HizaddinBelum ada peringkat
- Heat TransferDokumen3 halamanHeat TransferAlbert Junior EvangelistaBelum ada peringkat
- Tutorial-7 SolDokumen3 halamanTutorial-7 SolAvengerBelum ada peringkat
- CHE201ch12Dokumen25 halamanCHE201ch12chandro57Belum ada peringkat
- Topic 3.2 - Internal Diffusion and ReactionDokumen36 halamanTopic 3.2 - Internal Diffusion and ReactionHamdan Azman100% (1)
- Diffusion 2.1: A AB A AB A A AB A ADokumen35 halamanDiffusion 2.1: A AB A AB A A AB A AManish MahadevwalaBelum ada peringkat
- 10.2 Thermo ProbsetDokumen20 halaman10.2 Thermo ProbsetJan Rommel DuterteBelum ada peringkat
- Try MeDokumen9 halamanTry MeKrizzete HernandezBelum ada peringkat
- Sample Problem #17Dokumen10 halamanSample Problem #17Dozdi100% (10)
- Week 4 - Vapor-Liquid Separation (Multicomponent Distillation)Dokumen19 halamanWeek 4 - Vapor-Liquid Separation (Multicomponent Distillation)psychopassBelum ada peringkat
- Experiment No. 7 Measurement of Reaction ConversionDokumen8 halamanExperiment No. 7 Measurement of Reaction ConversionHoneylet Recaña TayactacBelum ada peringkat
- Experiment Chemical ReactorDokumen4 halamanExperiment Chemical ReactorIboniks Beponpiks DabondatskiBelum ada peringkat
- SedimentationDokumen9 halamanSedimentationAutumn JohnsonBelum ada peringkat
- Sample Problem #3Dokumen2 halamanSample Problem #3Dozdi80% (5)
- LeachingDokumen22 halamanLeachingRenu Sekaran0% (1)
- Stoichiometric TableDokumen22 halamanStoichiometric Table伟铭Belum ada peringkat
- H. W (2) (Chapter-01)Dokumen9 halamanH. W (2) (Chapter-01)Asmaa ObiedBelum ada peringkat
- Assig 2 Che422 Spring 2012Dokumen3 halamanAssig 2 Che422 Spring 2012ⵃⴰⵎⵣⴰ ⵖⵉⵢⵜBelum ada peringkat
- Hydrodynamics of Packed ColomnDokumen6 halamanHydrodynamics of Packed ColomnDhananjay KadamBelum ada peringkat
- Experiment No: 6: Feed Tanks Batch ReactorDokumen5 halamanExperiment No: 6: Feed Tanks Batch Reactorfareeha saeedBelum ada peringkat
- Collection and Analysis of Rate DataDokumen24 halamanCollection and Analysis of Rate DataAfs IkhlasBelum ada peringkat
- CRE-II - Chapter-04 Fluid-Particle Systems - HKB 2.10.18Dokumen42 halamanCRE-II - Chapter-04 Fluid-Particle Systems - HKB 2.10.18Ananya DaveBelum ada peringkat
- 3K4 2013 Assignment 2 Solutions PDFDokumen9 halaman3K4 2013 Assignment 2 Solutions PDFHanjin SeoBelum ada peringkat
- Ps1-Che171 Chemical Reaction Engineering 1Dokumen2 halamanPs1-Che171 Chemical Reaction Engineering 1Cha CanceranBelum ada peringkat
- Solution:: Cosh Cosh 1 Cosh CoshDokumen2 halamanSolution:: Cosh Cosh 1 Cosh CoshVaibhav GuptaBelum ada peringkat
- Chapter 2 - Data InterpretationDokumen24 halamanChapter 2 - Data InterpretationPHƯƠNG ĐẶNG YẾNBelum ada peringkat
- Chapter 3 - ExerciseDokumen24 halamanChapter 3 - ExerciseNguyễn Văn HòaBelum ada peringkat
- Exercise TRK 1Dokumen14 halamanExercise TRK 1Ananda CahyaBelum ada peringkat
- 5895223Dokumen14 halaman5895223DeneshVijayBelum ada peringkat
- A B R KC K 0.5 Min: Tutorial 3Dokumen2 halamanA B R KC K 0.5 Min: Tutorial 3shikharBelum ada peringkat
- Garbage Enzyme University SarawakDokumen6 halamanGarbage Enzyme University SarawakSaints Burner ChristopherBelum ada peringkat
- Tutorial 5Dokumen7 halamanTutorial 5Saints Burner ChristopherBelum ada peringkat
- UEMK4353 Assignment - Mech DesignDokumen2 halamanUEMK4353 Assignment - Mech DesignSaints Burner ChristopherBelum ada peringkat
- Visio Stream Table Part3Dokumen1 halamanVisio Stream Table Part3Saints Burner ChristopherBelum ada peringkat
- Industrial Training Report UtarDokumen29 halamanIndustrial Training Report UtarSaints Burner Christopher100% (1)
- Visio IndexDokumen1 halamanVisio IndexSaints Burner ChristopherBelum ada peringkat
- HAZOP TemplateDokumen12 halamanHAZOP TemplateSaints Burner ChristopherBelum ada peringkat
- P&IDDokumen1 halamanP&IDSaints Burner ChristopherBelum ada peringkat
- Introduction For Batch Reactor ExperimentDokumen5 halamanIntroduction For Batch Reactor ExperimentSaints Burner Christopher25% (4)
- Chemical Reaction Engineering Catalyst ResearchDokumen4 halamanChemical Reaction Engineering Catalyst ResearchSaints Burner ChristopherBelum ada peringkat
- Fluidiesed BedDokumen14 halamanFluidiesed BedSaints Burner ChristopherBelum ada peringkat
- Disc Bowl Flow ChartDokumen3 halamanDisc Bowl Flow ChartSaints Burner ChristopherBelum ada peringkat
- Assignment 1 (CRE 2)Dokumen1 halamanAssignment 1 (CRE 2)Saints Burner ChristopherBelum ada peringkat
- Experiment 1: Batch Reactor: Experiment 1: 6.1. Calibration Curve - Conductivity Vs ConversionDokumen2 halamanExperiment 1: Batch Reactor: Experiment 1: 6.1. Calibration Curve - Conductivity Vs ConversionSaints Burner ChristopherBelum ada peringkat
- Chapter 3 - Tutorial SolutionDokumen8 halamanChapter 3 - Tutorial SolutionSaints Burner ChristopherBelum ada peringkat
- Part 1 (A)Dokumen7 halamanPart 1 (A)Saints Burner ChristopherBelum ada peringkat
- Gas Absorption Report PDFDokumen13 halamanGas Absorption Report PDFSaints Burner Christopher100% (1)
- Foodchapter 1 PDFDokumen1 halamanFoodchapter 1 PDFSaints Burner ChristopherBelum ada peringkat
- Microsoft Word - Cpci Assignment NewDokumen25 halamanMicrosoft Word - Cpci Assignment NewSaints Burner ChristopherBelum ada peringkat
- Table 10.4 Food Packaging ConsiderationsDokumen1 halamanTable 10.4 Food Packaging ConsiderationsSaints Burner ChristopherBelum ada peringkat
- For Temperature Changes Per TimeDokumen1 halamanFor Temperature Changes Per TimeSaints Burner ChristopherBelum ada peringkat
- Part 1 (A)Dokumen7 halamanPart 1 (A)Saints Burner ChristopherBelum ada peringkat
- For Temperature Changes Per TimeDokumen1 halamanFor Temperature Changes Per TimeSaints Burner ChristopherBelum ada peringkat
- Chemical Engineering Compulsory SubjectDokumen1 halamanChemical Engineering Compulsory SubjectSaints Burner ChristopherBelum ada peringkat
- Eis AssignmentDokumen4 halamanEis AssignmentSaints Burner ChristopherBelum ada peringkat
- For Temperature Changes Per TimeDokumen1 halamanFor Temperature Changes Per TimeSaints Burner ChristopherBelum ada peringkat
- Ni Hao Hi Chi Le Ma? Have You Eaten? Zai Jian Good ByeDokumen1 halamanNi Hao Hi Chi Le Ma? Have You Eaten? Zai Jian Good ByeSaints Burner ChristopherBelum ada peringkat
- Flash Card FrenchDokumen6 halamanFlash Card FrenchSaints Burner ChristopherBelum ada peringkat
- French Flash Card 18Dokumen1 halamanFrench Flash Card 18Saints Burner ChristopherBelum ada peringkat
- Fluids Secondaires PDFDokumen11 halamanFluids Secondaires PDFmohand_mindietaBelum ada peringkat
- Separation Science - Chromatography Unit Thomas Wenzel Department of Chemistry Bates College, Lewiston ME 04240 Twenzel@bates - EduDokumen69 halamanSeparation Science - Chromatography Unit Thomas Wenzel Department of Chemistry Bates College, Lewiston ME 04240 Twenzel@bates - EduthecriticBelum ada peringkat
- A. Wipf - Path IntegralsDokumen164 halamanA. Wipf - Path Integralsnom nomBelum ada peringkat
- Concrete SyllabusDokumen4 halamanConcrete SyllabusBibudh DwaBelum ada peringkat
- A4-P 4.0 enDokumen32 halamanA4-P 4.0 enmkpqBelum ada peringkat
- B-64504en - 02 Fanuc Series 30i-Pb, Series 31i-Pb Operator's ManualDokumen1.216 halamanB-64504en - 02 Fanuc Series 30i-Pb, Series 31i-Pb Operator's ManualGiang Nguyen VanBelum ada peringkat
- 03 - ICSE9th - Physics BookLet 27 May 21Dokumen99 halaman03 - ICSE9th - Physics BookLet 27 May 21Vivaan GandhiBelum ada peringkat
- Rubik's Revenge (4x4x4)Dokumen5 halamanRubik's Revenge (4x4x4)Febbi Abdul MuktiBelum ada peringkat
- 8.03 Fall 2004 Problem Set 2 SolutionsDokumen15 halaman8.03 Fall 2004 Problem Set 2 SolutionseviroyerBelum ada peringkat
- QPDokumen3 halamanQPlandscapesinthemistBelum ada peringkat
- Final School TranscriptDokumen3 halamanFinal School TranscriptSteven TeguhBelum ada peringkat
- Performance Under Cyclic Load of Built-Up T-Stubs For Double T Moment ConnectionsDokumen14 halamanPerformance Under Cyclic Load of Built-Up T-Stubs For Double T Moment ConnectionsMilmxmenBelum ada peringkat
- Paper 2 2001Dokumen20 halamanPaper 2 2001DisturbedPotatoBelum ada peringkat
- Agar Extraction Process For Gracilaria CliftoniiDokumen7 halamanAgar Extraction Process For Gracilaria CliftoniiAdaBelum ada peringkat
- Worksheet 1 131 2021W2 HydrostaticsDokumen8 halamanWorksheet 1 131 2021W2 HydrostaticseBelum ada peringkat
- SL 2.8-2.10 Exponents and LogarithmsDokumen26 halamanSL 2.8-2.10 Exponents and LogarithmsZeynep Aysu İskenderBelum ada peringkat
- 970803B Meter Fact. LinearDokumen4 halaman970803B Meter Fact. Linearsyed jeelani ahmedBelum ada peringkat
- Solution Collins Cap 8Dokumen460 halamanSolution Collins Cap 8Hamid MasoodBelum ada peringkat
- A Model For Traffic SimulationDokumen5 halamanA Model For Traffic Simulationraci78Belum ada peringkat
- Proceedings of Spie: Rapid, Automated, Quality Control of Diffraction Grating EfficiencyDokumen6 halamanProceedings of Spie: Rapid, Automated, Quality Control of Diffraction Grating EfficiencyironbatjediBelum ada peringkat
- SI-E1-2009-R001-Structural Calculation-15-05-20 PDFDokumen138 halamanSI-E1-2009-R001-Structural Calculation-15-05-20 PDFரதி சுரேஷ்100% (1)
- Pu 00011931Dokumen481 halamanPu 00011931Eduardo Salgado EnríquezBelum ada peringkat
- Phy-153 Course OutlineDokumen26 halamanPhy-153 Course OutlinealdricBelum ada peringkat
- Centrifugal PumpsDokumen0 halamanCentrifugal PumpsAbsar MamunBelum ada peringkat
- Deney 1 SonDokumen6 halamanDeney 1 Songizem.gelekciBelum ada peringkat
- Special Types of Matrices: By: Engr. Glenda Alega - de MesaDokumen22 halamanSpecial Types of Matrices: By: Engr. Glenda Alega - de Mesasairin parkBelum ada peringkat
- CH 10 Lecture 3 Angular Overlap: I. Ligand Field Theory and Square Planar ComplexesDokumen16 halamanCH 10 Lecture 3 Angular Overlap: I. Ligand Field Theory and Square Planar ComplexesIsrael PobleteBelum ada peringkat
- Radar ReceiversDokumen15 halamanRadar Receiversmas3565560100% (2)
- Manual PDFDokumen200 halamanManual PDFEddie grassgunter100% (1)
- 2SK2847Dokumen6 halaman2SK2847jsalinas78Belum ada peringkat