Calculatinghalflife
Diunggah oleh
api-256503273Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Calculatinghalflife
Diunggah oleh
api-256503273Hak Cipta:
Format Tersedia
Calculating the Half-Life of Twizzlers and M&Mium
This lesson plan includes two labs designed to teach the concept of half-life. The Twizzler lab is
designed to introduce the topic and is best if used before the M&Mium lab.
Primary Learning Outcomes
Students will learn the concept of half-life and how it relates to radioactive material. Students will
determine, with a hands-on experiment, the half-life of Twizzlers and a radioactive element,
M&Mium. Students will create and be able to recognize a graph representing the half-life of an
element. Students will be able to determine how different factors modify the shape of the half-life
graph.
Assessed Georgia Performance Standards
SCSh4. Students will use tools and instruments for observing, measuring, and manipulating scientific
equipment and materials.
SPS1. Students will investigate our current understanding of the atom.
SPS3. Students will distinguish the characteristics and components of radioactivity.
Procedures/Activities (Designed to complete both labs in a 90 minute class period)
Step 1: Duration 15-20 minutes
Prior to class, the instructor should create partitions with 50 M&Ms each.
Step: 2 Duration: 20-30 minutes
Students will learn the concept of half-life in the first lab to determine and the half-life of a Twizzler.
Students will determine the half-life of a Twizzler in terms of number of half-bites and in terms of
time. Students will graph the amount of Twizzler left after each half-bite in order to learn the shape
of a half-life curve. Follow procedure outlined in lab handout.
Step 3: Duration 50-60 minutes
Students will determine the half-life of a radioactive element, M&Mium. The half-life is the number
of shakes that it takes for half of the M&Mium atoms to decay. Students will plot the data and
determine the half-life of M&Mium. Follow procedure outlined in lab handout.
Materials and Equipment
Per student/group: 2 individually wrapped Twizzlers, 50 M&Ms, plastic cup, white paper, graph paper
Total Duration
Teacher prep: 15-20 minutes
In class: 80-90 minutes
Assessment
Students will be assessed on their understanding of half-life via post lab questions and graphs.
Half-life: Determining and Graphing the Half-life of a Twizzler
Background: You should know the term half-life and know how it is related to radioactive
elements. The half-life of a radioactive element is the time it takes for half of its atoms to decay into
something else. For example, iodine-125 (I-125) has a half-life of about 60 days; therefore, in 60 days,
1g of I-125 will turn into half a gram of iodine-125 and half a gram of something else (the radioactive
decay products of radium). After another 60 days have elapsed, only a of the original 1g of I-125
will remain.
Purpose: To determine the half-life of a Twizzler and graph the results.
Materials:
2 Twizzlers (1 for Part I and 1 for Part II)
pencil/pen
2 sheets of graph paper
Procedure: Part I: Amount of Twizzler vs. Bites
1. Hold original Twizzler vertically against the 'y' axis with one end at the origin. Mark the
"length". This represents the beginning amount.
2. Wait for further instructions to Take a bite! You must eat HALF (and only half) the length
of the Twizzler.
3. Repeat step 1, holding the Twizzler a unit from the origin. Mark the new length (this is your y
coordinate).
4. Repeat steps 2 and 3 with the class until the instructor tells you to stop.
5. Draw a smooth Best-Fit line on your graph.
Procedure: Part II: Amount of Twizzler vs. Time
1. This time the procedure in Part I will be repeated except the instructor will tell you to take a
bite every 45 seconds and record your data!
Conclusions and Analysis:
1. Did the Twizzler ever completely disappear? Explain.
2. What was the half-life of the Twizzler in Part II?
3. If you had started with a GIANT Twizzler (2X the normal size) how would this have affected
the shape of the graph? Explain.
Describe the effect on the graph if you took a bite every 90 seconds.
Half-life: Determining and Graphing the Half-life of M&Mium
Background: You should know the term half-life and know how it is related to radioactive
elements. The half-life of a radioactive element is the time it takes for half of its atoms to decay into
something else. For example, iodine-125 (I-125) has a half-life of about 60 days; therefore, in 60 days,
1g of I-125 will turn into half a gram of iodine-125 and half a gram of something else (the radioactive
decay products of radium). After another 60 days have elapsed, only a of the original 1g of I-125
will remain.
Purpose: To determine the half-life of the element M&Mium.
Materials:
Bag of M&Mium Isotopes
**Radioactive members of this isotope family are easily distinguished via a bold m on
the front surface of the atom.**
1 plastic cup
pencil/pen
white piece of paper
1 sheet of graph paper
Procedure:
1. Count the number of M&Mium atoms as you place them in the cup. Record the total number of
radioactive atoms you start with in your data table (on the back of your graph paper).
2. Cover and shake/rattle the cup.
3. Carefully pour your atoms onto your white paper. You will see that several of the previously
radioactive atoms in the group have decayed, and the m is no longer visible. This means that
they are now considered "safe" and, since they are no longer radioactive, may actually be eaten
without fear of any harm to you! Please do so, and as you remove the edible atoms, count
them so you may determine the number of atoms that have decayed in that particular
shake. (NOTE: You should not eat any of the decayed M&Mium atoms until you are on
your 3
rd
trial)
4. Now you need to continue this pattern until no more radioactive members remain. Remember
to record the number of decayed atoms after each shake!
Analysis:
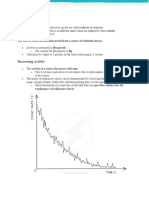
Using the graph paper provided, construct a graph of N (Number of decayed atoms) as a function of
the number of shakes. Use the average of the 3 trials to construct this graph. (Remember to label your
x-axis, y-axis, and indicate a title for your graph.)
Conclusions:
1. Calculate the half-life of M&Mium? (i.e., What number of shakes are necessary to reduce the
radioactive members to one-half?)
M&Mium Data Table
Shake #
Trial 1
# Decayed
Trial 2
# Decayed
Trial 3
# Decayed
Total Average
_________________________________________________________
_____________________
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
_
Anda mungkin juga menyukai
- Student Exploration: Half-Life: Prior Knowledge Questions (Do These BEFORE Using The Gizmo.)Dokumen5 halamanStudent Exploration: Half-Life: Prior Knowledge Questions (Do These BEFORE Using The Gizmo.)Kristina33% (3)
- 7.5 Halflife SEDokumen5 halaman7.5 Halflife SEWyatt KesterBelum ada peringkat
- Student Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationDokumen5 halamanStudent Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationJahel HerculesBelum ada peringkat
- Half lifeSEDokumen4 halamanHalf lifeSERusty WynderBelum ada peringkat
- Report Sheet Half Life 2Dokumen6 halamanReport Sheet Half Life 2Ayhan AbdulAzizBelum ada peringkat
- 08.01 Half-Life and Radioactive DecayDokumen3 halaman08.01 Half-Life and Radioactive DecayAnonymous 8VJhV1eI2yBelum ada peringkat
- Zaundre Pusey - Half-lifeSEDokumen8 halamanZaundre Pusey - Half-lifeSEJBE legendsBelum ada peringkat
- Student Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationDokumen5 halamanStudent Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationSai83% (6)
- Gizmo Half-LifeDokumen5 halamanGizmo Half-Lifeskyler29% (7)
- Teacher Guide: Half-Life: Learning ObjectivesDokumen4 halamanTeacher Guide: Half-Life: Learning ObjectivesPeyton100% (1)
- Chem Lab 2Dokumen2 halamanChem Lab 2Samuel ArzadonBelum ada peringkat
- School Base Assessment-1Dokumen48 halamanSchool Base Assessment-1Marissa FreemanBelum ada peringkat
- Half-lifeSEDokumen6 halamanHalf-lifeSEJBE legends100% (1)
- sheniblog-VIII - Phy&Chem - All Units (EM)Dokumen58 halamansheniblog-VIII - Phy&Chem - All Units (EM)battlegroundmobileindia815Belum ada peringkat
- Activities OpticsDokumen20 halamanActivities OpticsPrince Jaspher De TorresBelum ada peringkat
- M2 Unit 3 G8Dokumen8 halamanM2 Unit 3 G8Julia Geonzon LabajoBelum ada peringkat
- Strength of Materials: Tensile TestDokumen9 halamanStrength of Materials: Tensile Test270329Belum ada peringkat
- Half-Life Student Lab Sheet-REVDokumen6 halamanHalf-Life Student Lab Sheet-REVElPerritoSapeeBelum ada peringkat
- 13c Candyium LabDokumen2 halaman13c Candyium Labapi-285693263Belum ada peringkat
- MEC101 ASSIG.2 (Group)Dokumen2 halamanMEC101 ASSIG.2 (Group)Syed AzzizBelum ada peringkat
- Modelling Half-Life: M&Mium: Background InformationDokumen2 halamanModelling Half-Life: M&Mium: Background InformationNicole LouiseBelum ada peringkat
- Units and MeasurementDokumen23 halamanUnits and MeasurementIftita SelvianaBelum ada peringkat
- Experiment 3 GRP5Dokumen5 halamanExperiment 3 GRP5Sharmaine MedelBelum ada peringkat
- PHYS 3884 Lab ManualDokumen289 halamanPHYS 3884 Lab ManualSoad faresBelum ada peringkat
- Half lifeSEDokumen5 halamanHalf lifeSENatalie YoungBelum ada peringkat
- Lab 2 Charpy Impact TestDokumen8 halamanLab 2 Charpy Impact TestSyahir Zulkifli0% (1)
- The Simple Pendulum 2007Dokumen4 halamanThe Simple Pendulum 2007Xhruster XobileBelum ada peringkat
- 6.01 Pendulum Lab: PurposeDokumen3 halaman6.01 Pendulum Lab: PurposeKing Everest0% (1)
- 1 221 Measurement LabDokumen4 halaman1 221 Measurement LabArzu KerimBelum ada peringkat
- Half Life Introduction LabDokumen4 halamanHalf Life Introduction Labapi-251355123Belum ada peringkat
- ECE584 Lab Manual04Dokumen33 halamanECE584 Lab Manual04Samiksha AhlawatBelum ada peringkat
- Syllabus General Chemistry Lab SKDokumen43 halamanSyllabus General Chemistry Lab SKamvooijsBelum ada peringkat
- Microwave Lab ManualDokumen30 halamanMicrowave Lab Manualscribdsurya4uBelum ada peringkat
- PTG Chapter 3 Asal PhysicsDokumen9 halamanPTG Chapter 3 Asal PhysicslohiyanityaBelum ada peringkat
- PTG Chapter 3 Asal PhysicsDokumen9 halamanPTG Chapter 3 Asal Physicszzrnwdzpsmhs951003Belum ada peringkat
- Vanessa Rodriguez - Collins Half-Life GizmoDokumen5 halamanVanessa Rodriguez - Collins Half-Life GizmoVanessa RodriguezBelum ada peringkat
- Half LifeDokumen2 halamanHalf LifeJULIO CANIPASBelum ada peringkat
- Physics SBA 1Dokumen2 halamanPhysics SBA 1Donae LyonsBelum ada peringkat
- Half LifeDokumen11 halamanHalf LifeMarshell JonesBelum ada peringkat
- PTG Chapter 10 Asal PhysicsDokumen10 halamanPTG Chapter 10 Asal Physicszzrnwdzpsmhs951003Belum ada peringkat
- Mos 2Dokumen19 halamanMos 2Abdullah HaroonBelum ada peringkat
- Lab Report I, Practical Work I A01654147 Samantha Monserrat Hernandez HerreraDokumen8 halamanLab Report I, Practical Work I A01654147 Samantha Monserrat Hernandez HerreraSamantha Monserrat Hernández HerreraBelum ada peringkat
- Mech of Materials - Lab 1Dokumen15 halamanMech of Materials - Lab 1shihabsultanBelum ada peringkat
- Física Carenalga 2.0Dokumen11 halamanFísica Carenalga 2.0Juan Felipe Pedraza CiceroBelum ada peringkat
- Simple Pendulum CourseworkDokumen7 halamanSimple Pendulum Courseworkbdg8266a100% (3)
- Pendulum LabDokumen5 halamanPendulum LabdanahansenBelum ada peringkat
- Investigating The Factors Affecting A Simple Pendulum: Experiment 5Dokumen4 halamanInvestigating The Factors Affecting A Simple Pendulum: Experiment 5ProDiJai 321Belum ada peringkat
- Labex1 PDFDokumen2 halamanLabex1 PDFsujetagottraBelum ada peringkat
- VII - 11.1 - Transportation in Animals and Plants - V-1 - Panchali - Stu (4 Files Merged)Dokumen10 halamanVII - 11.1 - Transportation in Animals and Plants - V-1 - Panchali - Stu (4 Files Merged)alatexgamingBelum ada peringkat
- Hall EffectDokumen71 halamanHall EffectMRIGAANK JASWALBelum ada peringkat
- Metallography Exp.Dokumen6 halamanMetallography Exp.Nedjmah LechehebBelum ada peringkat
- As PhysicsDokumen227 halamanAs PhysicsGhadaYousefAwad100% (5)
- Student Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationDokumen11 halamanStudent Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, Radiationalex rodriguezBelum ada peringkat
- Chapter 1 MaterialsDokumen31 halamanChapter 1 MaterialsFahmi AmiBelum ada peringkat
- Cambridge Ordinary Level: Cambridge Assessment International EducationDokumen16 halamanCambridge Ordinary Level: Cambridge Assessment International EducationMomin FayzanBelum ada peringkat
- General Physics Lesson Plan 2D Motion: ObjectiveDokumen5 halamanGeneral Physics Lesson Plan 2D Motion: ObjectiveEjurango JhaBelum ada peringkat
- Tcnjfinalevalkkennedysp14 5 4Dokumen2 halamanTcnjfinalevalkkennedysp14 5 4api-256503273Belum ada peringkat
- Kennedy Krystyne Fingerprint Results 09 03 13Dokumen1 halamanKennedy Krystyne Fingerprint Results 09 03 13api-256503273Belum ada peringkat
- Diadema Lesson PlansDokumen2 halamanDiadema Lesson Plansapi-256503273Belum ada peringkat
- Kennedy Krystyne TCNJ DiplomaDokumen1 halamanKennedy Krystyne TCNJ Diplomaapi-256503273Belum ada peringkat
- Education ResumeDokumen2 halamanEducation Resumeapi-256503273Belum ada peringkat
- Diadema Case StudyDokumen6 halamanDiadema Case Studyapi-256503273Belum ada peringkat
- Mmium DataDokumen1 halamanMmium Dataapi-256503273Belum ada peringkat
- Spont Gen ResultsDokumen2 halamanSpont Gen Resultsapi-256503273Belum ada peringkat
- Spontaneously Testing Spont GenDokumen2 halamanSpontaneously Testing Spont Genapi-256503273Belum ada peringkat
- Spont Gen Lesson PlanDokumen2 halamanSpont Gen Lesson Planapi-256503273Belum ada peringkat
- Mmium IonDokumen4 halamanMmium Ionapi-256503273Belum ada peringkat
- LinusDokumen20 halamanLinusShiang ChinBelum ada peringkat
- Homework EssaysDokumen2 halamanHomework EssaysDascBelum ada peringkat
- Type Conditions and StyleDokumen18 halamanType Conditions and StyleAmir ChohanBelum ada peringkat
- Stephen Karcher - Great Vessel Blog PostsDokumen60 halamanStephen Karcher - Great Vessel Blog PostsKenneth AndersonBelum ada peringkat
- Holiday Homework - Class VIII PDFDokumen7 halamanHoliday Homework - Class VIII PDFDua FatimaBelum ada peringkat
- Asthma Nursing Care Plans - LippincottDokumen45 halamanAsthma Nursing Care Plans - LippincottDyllanoBelum ada peringkat
- Why I Think Students Should Cheat AnalysisDokumen3 halamanWhy I Think Students Should Cheat AnalysisH.MBelum ada peringkat
- Front Fin All Print LDokumen10 halamanFront Fin All Print LRichelle TanBelum ada peringkat
- KPRIET Deep Learning BrochureDokumen2 halamanKPRIET Deep Learning BrochureJuan Daniel Garcia VeigaBelum ada peringkat
- How Does Psychotherapy WorkDokumen450 halamanHow Does Psychotherapy Workpeoplecangrow100% (4)
- Managerial Counselling Module IIDokumen50 halamanManagerial Counselling Module IIPrerana AroraBelum ada peringkat
- Datavision OCDDokumen6 halamanDatavision OCDYashwanth Varma100% (1)
- Impact of Motivation On Organizational PerformanceDokumen6 halamanImpact of Motivation On Organizational Performancetewodros gashaw0% (1)
- Different Approaches To Industrial RelationsDokumen8 halamanDifferent Approaches To Industrial RelationsAnkit Vira100% (1)
- Amazon Leadership PrinciplesDokumen2 halamanAmazon Leadership PrinciplesvarelayorganonBelum ada peringkat
- Bradley Quillen: 308 Shadow Ridge Way Cave Springs, AR 72718 (479) 531-5745Dokumen2 halamanBradley Quillen: 308 Shadow Ridge Way Cave Springs, AR 72718 (479) 531-5745api-4062470820% (1)
- Broken Open-Elizabeth Lesser - Book Review by Bosco OdongoDokumen2 halamanBroken Open-Elizabeth Lesser - Book Review by Bosco OdongoKiwalabye OsephBelum ada peringkat
- NSTP FormatDokumen3 halamanNSTP FormatJason PanganBelum ada peringkat
- Lesson Plan in MythologyDokumen4 halamanLesson Plan in MythologyLeelani Sabio JasarenoBelum ada peringkat
- Retrograde PlanetsDokumen2 halamanRetrograde PlanetsthavaBelum ada peringkat
- NEF Advanced SB Answer KeyDokumen98 halamanNEF Advanced SB Answer KeyMaria Blasco86% (7)
- CTDT GR 9-12 Searching For SummerDokumen22 halamanCTDT GR 9-12 Searching For SummergashunardiBelum ada peringkat
- Redemptive Leadership ModelDokumen10 halamanRedemptive Leadership ModelPeter RichardsonBelum ada peringkat
- The View of The Invisible World: Ninian Smart's Analysis of The Dimensions of Religion and of Religious ExperienceDokumen8 halamanThe View of The Invisible World: Ninian Smart's Analysis of The Dimensions of Religion and of Religious ExperienceeastwestculturalBelum ada peringkat
- Song Outline Worksheet Using Figurative LanguageDokumen5 halamanSong Outline Worksheet Using Figurative LanguageCathPang-MakBelum ada peringkat
- Biography BanduraDokumen1 halamanBiography BanduraEdrianne J.Belum ada peringkat
- Ap Psych SyllabusDokumen30 halamanAp Psych Syllabusapi-261300427Belum ada peringkat
- Tracie PeateDokumen12 halamanTracie PeateAbdullah KilawiBelum ada peringkat
- Running Head: Cloud and Townsend Critique 1Dokumen8 halamanRunning Head: Cloud and Townsend Critique 1cayden2009Belum ada peringkat
- Class 12 ENGLISH PROJECTDokumen5 halamanClass 12 ENGLISH PROJECTCricket ClutchBelum ada peringkat