Stem Cell Osteosarcoma
Diunggah oleh
Hendrikus Surya Adhi PutraJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Stem Cell Osteosarcoma
Diunggah oleh
Hendrikus Surya Adhi PutraHak Cipta:
Format Tersedia
792 Arch Pathol Lab MedVol 131, May 2007 Primary Osteosarcoma of the BreastBahrami et al
Case Report
Primary Osteosarcoma of the Breast
Report of 2 Cases
Armita Bahrami, MD; Erika Resetkova, MD, PhD; Jae Y. Ro, MD, PhD; Joe D. Iban ez, MD; Alberto G. Ayala, MD
Two distinct histologic variants of primary breast osteo-
sarcoma in 2 elderly women are described. The rst patient
was an 88-year-old woman with a long-standing, slow-
growing, 18-cm mass in her right breast. The second pa-
tient was a 96-year-old woman with a recently self-detect-
ed, painless, 7.5-cm lump in her left breast. Clinically,
there was no evidence of metastasis, and both women un-
derwent simple mastectomy. Histologic features of both
specimens were those of high-grade primary breast osteo-
sarcoma. The rst patients tumor was classied as a chon-
droblastic variant, and the second as an osteoblastic vari-
ant of osteosarcoma. The patients were alive without evi-
dence of local recurrence or hematogenous spread at a 16-
and 4-month follow-up, respectively. Primary mammary
osteosarcoma should be distinguished from metaplastic/
sarcomatoid carcinoma with heterologous osseous/carti-
laginous differentiation or malignant phyllodes tumor be-
cause it has a different biological behavior and requires a
different treatment approach.
(Arch Pathol Lab Med. 2007;131:792795)
P
rimary osteosarcoma of the breast, which is histologi-
cally indistinguishable from conventional osteosar-
coma of the bone and other extraskeletal sites, is an ex-
tremely rare tumor. In comparison, bone-producing spin-
dle cell neoplasms with an epithelial origin, so-called
metaplastic (sarcomatoid) carcinomas, are far more com-
mon. The histogenesis of primary osteosarcoma of the
breast is not clear, but an origin from totipotent mesen-
chymal cells of the breast stroma or a transformation from
a preexisting broadenoma or phyllodes tumor has been
suggested.
14
Primary breast osteosarcomas are considered
highly aggressive tumors associated with early recurrence
and a propensity for hematogenous rather than lymphatic
spread, most commonly to the lungs.
5,6
In this report, we
describe the cases of 2 patients with this rare tumor.
Accepted for publication November 17, 2006.
From the Departments of Pathology, Baylor College of Medicine (Dr
Bahrami); the University of Texas MD Anderson Cancer Center (Dr Re-
setkova); the Methodist Hospital, Cornell University College of Medi-
cine (Drs Ro and Ayala), Houston, Tex. Dr Iban ez is in private practice
in Victoria, Tex.
The authors have no relevant nancial interest in the products or
companies described in this article.
Corresponding author: Alberto G. Ayala, MD, Department of Pathol-
ogy, The Methodist Hospital, Cornell University College of Medicine,
6565 Fannin St, Houston, TX 77030 (e-mail: aayala@tmh.tmc.edu).
Reprints not available from the author.
REPORT OF CASES
Case 1
An 88-year-old woman with a medical history of hypothyroid-
ism presented with shortness of breath and swelling of the legs
secondary to congestive heart failure. When she was 4 years old,
she experienced an accidental burn to her right breast and shoul-
der that resulted in the loss of the right nipple-areola complex.
Physical examination at admission revealed a large mass in her
right breast, estimated to be at least 15 cm. The mass was not
attached to the sternum or the underlying rib. She reported that
this mass had developed slowly over many years without causing
any pain. No axillary lymphadenopathy was detected on physical
examination. The left breast was unremarkable. No mammo-
graphic examination had been performed in the past. Findings
on limited clinical workup, including chest radiography and liver
prole, were within normal limits.
An initial incisional biopsy of the mass revealed a fragment of
skin with an underlying mature cartilaginous neoplasm. On his-
tologic examination, the neoplastic cells had no nuclear atypia or
mitotic activity. The initial differential diagnoses included a be-
nign or malignant cartilaginous neoplasm, metaplastic carcino-
ma, a mixed tumor of salivary gland type, and phyllodes tumor
with cartilaginous metaplasia. The patient underwent simple
mastectomy without postoperative adjuvant chemotherapy or ra-
diation therapy. Sixteen months after mastectomy, the patient
was alive and well with no clinical evidence of local recurrence
or distant metastasis.
Case 2
A 96-year-old woman with a history of chronic mild throm-
bocytopenia and carpal tunnel syndrome presented with a re-
cently self-detected palpable mass in her left breast and 4.5-kg
weight loss during the past year. Mammography done 2 years
previously had shown no suspicion of malignancy. Physical ex-
amination on admission conrmed the presence of a rm mobile
mass in the left breast. No axillary lymphadenopathy was noted.
On mammography, the mass was relatively well demarcated and
partially calcied. An incisional biopsy was performed, and poor-
ly differentiated malignancy was diagnosed. The patient declined
to undergo comprehensive metastatic workup. Findings on a lim-
ited study that included measurement of alkaline phosphatase
and chest radiography were negative. The patient underwent a
simple mastectomy without axillary lymph node sampling. She
opted for a conservative approach, and no further postoperative
staging, chemotherapy, or radiation therapy was performed. The
patient made an uneventful recovery; 4 months after the surgery,
she was alive and well with no clinical evidence of local recur-
rence or distant metastasis.
MATERIALS AND METHODS
Hematoxylin-eosinstained histologic sections of formalin-
xed, parafn-embedded tissue of case 1 were prepared at the
Methodist Hospital; sections from case 2 were sent to M. D. An-
Arch Pathol Lab MedVol 131, May 2007 Primary Osteosarcoma of the BreastBahrami et al 793
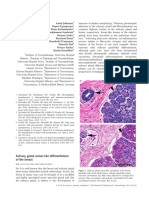
Figure 1. Case 1. Gross appearance of a section from the right breast
mass showing a large, relatively well-circumscribed and predominantly
necrotic tumor with foci of cartilaginous and calcied tissue. The miss-
ing portion of this section was removed for tissue sampling before the
specimen was photographed.
Figure 2. Case 1. Photomicrograph showing a dominant nodule com-
posed of cartilaginous cells surrounded by smaller anaplastic cells,
which in turn are covered by necrotic tissue (hematoxylin-eosin, orig-
inal magnication 40).
Figure 3. Case 1. Photomicrograph displaying atypical cells with rel-
atively round nuclei and prominent nucleoli (upper half of the illustra-
tion) associated with production of irregular lacelike osteoid (lower half
of the illustration) (hematoxylin-eosin, original magnication, 100).
Figure 4. Case 2. Photomicrograph of a high-grade osteosarcoma
composed of osteoblast-like and giant cells within an osteoid matrix
(hematoxylin-eosin, original magnication 200).
derson Cancer Center, Houston, Tex, from an outside institution
for consultation. Immunohistochemical studies were performed
on parafn-embedded tissue by using the standard avidin-biotin
immunoperoxidase technique at the Methodist Hospital and
M. D. Anderson Cancer Center. No ultrastructural studies were
conducted in either case.
PATHOLOGIC FINDINGS
The mastectomy specimen from case 1 contained an 18-
cm relatively well-circumscribed mass, which on cross-
section had a predominance of tan-gray necrotic tissue
with focally gray-white cartilaginous to rm calcied ar-
eas (Figure 1). The tumor was adherent to the overlying
skin. Most of the grossly viable tissue from the tumor was
submitted in 16 blocks for histologic examination.
The mastectomy specimen from case 2 revealed a 7.5-
cm well-delineated oval mass with a focally very rm,
partially calcied cut surface. The mass had no direct ex-
tension onto the chest wall. Representative sections of the
tumor were submitted in 22 blocks for histologic evalua-
tion.
Multiple sections of the mastectomy specimen from case
1 disclosed a predominantly necrotic tumor with a carti-
laginous background (Figure 2). The abundant cartilagi-
nous proliferation varied from mature lacunar cartilage to
poorly differentiated areas displaying myxoid changes
with no lacunar arrangement. In only a few sections was
there transition from cartilaginous proliferation to large
pleomorphic epithelioid cells with high mitotic activity.
The epithelioid cells had a diffuse arrangement and were
intimately associated with production of lacelike to frank-
ly calcied osteoid (Figure 3).
In case 2, the tumor cells were epithelioid, arranged in
a syncytial pattern with nely ramifying calcied and
noncalcied intercellular osteoid matrix. Higher-power
magnication revealed that the tumor was composed of
osteoblast-like cells entrapped within the osteoid matrix
(Figure 4). The cells had a plasmacytoid appearance with
794 Arch Pathol Lab MedVol 131, May 2007 Primary Osteosarcoma of the BreastBahrami et al
enlarged, slightly irregular nuclei and prominent nucleoli.
Mitotic gures were easily found (10 mitoses/10 high-
power microscopic elds). Some areas of the tumor dem-
onstrated a prominent population of bland-appearing os-
teoclast-like multinucleated giant cells associated with the
osteoid matrix. Foci of necrosis and hemorrhage within
the tumor were evident.
In neither case was there histologic evidence of an epi-
thelial or a carcinomatous component, despite extensive
sampling of both tumors. No evidence of a preexisting
cystosarcoma phyllodes was present in any of the exam-
ined sections. The surrounding nonneoplastic breast pa-
renchyma revealed compressed lobular units in case 1 and
focally mild ductal hyperplasia of usual type in case 2.
Additionally, several small hyalinized and calcied bro-
adenomas in the nonneoplastic breast parenchyma of case
2 were noted. The epidermis of the overlying skin was
unremarkable and uninvolved in either case.
Immunohistochemical studies in each case were per-
formed on 1 tissue block containing proliferating epithe-
lioid cells. In both cases, the epithelioid cells stained in-
tensely for vimentin, but no immunoreactivity for AE1/
AE3, CAM 5.2, high-molecular-weight cytokeratin 34E12
(CK903), epithelial membrane antigen (EMA), polyclonal
carcinoembryonic antigen, CK5/6, p63, and CD34 was de-
tected. The epithelioid cells in case 1 also did not stain for
smooth muscle actin and S100. The cartilaginous cells,
however, stained strongly for S100, as was expected. The
EMA immunostain in case 1 yielded a focal granular pat-
tern of cytoplasmic staining in cartilaginous cells within
the lacunae without a membranous component, a pattern
that is known to have no diagnostic signicance for epi-
thelial differentiation.
7
Additional keratin stains conducted
in case 2, including CK7, CK8, and CK20, were all nega-
tive, as were immunoassays for estrogen and progesterone
receptors. There was also no overexpression of the HER-
2/neu oncoprotein.
COMMENT
Primary osteosarcoma of the breast has been recognized
as an independent entity in the breast, but the precise fre-
quency of this neoplasm is difcult to determine not only
because of its extreme rarity but also because metaplastic
carcinoma or an underlying phyllodes tumor were not
clearly excluded in some case reports. In general, mam-
mary sarcomas are considered very uncommon and make
up less than 1% of all primary breast malignancies.
1,5,6
Pri-
mary breast osteosarcoma has been reported to account
for 12.5% of mammary sarcomas, reecting its extremely
rare incidence.
1
In a retrospective report of 50 patients
with primary breast osteosarcoma documented in the
Armed Forces Institute of Pathology (AFIP) les, the pa-
tients ages ranged from 27 to 89 years (median, 64.5
years), and the tumor size varied from 1.4 to 13 cm at the
time of diagnosis.
5
The most common presentation was
that of a progressively enlarging mass that was associated
with pain in 18% of the cases.
5
Only 1 patient in that series
had a history of irradiation.
5
In some instances, patients
have had a slow-growing or relatively stable mass for
many years with a sudden increase in size. Occasionally
a history of trauma to the breast has been provided.
1,3,5
Dormant growth was reported in patient 1, who had a
childhood history of burn injury to her breast. Nonethe-
less, there was no evidence that the current tumor could
be related to the past burn or scar tissue. There was no
evidence of squamous atypia in the overlying skin and no
associated supercial brosarcomatous element. The tu-
mor was so large that it occupied almost the entire breast;
however, the tumor did not involve the underlying bone
or overlying epidermis.
Mammographically, these tumors often present as a
well-circumscribed dense lesion within the breast paren-
chyma with focal or extensive coarse calcications.
2,5,8
The
mammographic features may be deceptively benign
2,3
; for
instance, in the AFIP case series, the mammographic im-
pression was that of a benign broadenoma in 33% of
patients.
5
Osteosarcoma of the breast, similar to other ex-
traskeletal osteosarcomas, may have a broad spectrum of
histologic features. The most frequently observed histo-
logic variants in the primary osteosarcomas of the breast
are broblastic, osteoblastic, and osteoclastic (giant cell
rich) variants.
5
In the osteoblastic osteosarcoma, the oste-
oid is deposited in a ne, ramifying, lacelike, or coarsely
trabecular pattern and sometimes in sheaths of osteoid or
bone. Atypical cartilage has been reported in 36% of pri-
mary breast osteosarcomas.
5
Neoplastic cartilage, howev-
er, has rarely been the dominant feature (chondroblastic
osteosarcoma), as observed in case 1. Necrotic foci iden-
tied in 30% of these tumors
5
made up more than 90% of
the mass lesion in case 1.
The diagnosis of metaplastic mammary carcinoma
should be excluded before primary breast osteosarcoma is
diagnosed. Metaplastic mammary carcinoma is a general
term referring to a heterogeneous group of neoplasms
characterized by an admixture of a carcinoma with areas
of squamous, spindle, or heterologous mesenchymal dif-
ferentiation.
5,9
The term spindled or sarcomatoid carcinoma
has been adapted to reect the appearance of a mesen-
chymal neoplasm in these epithelial malignancies. The
term carcinosarcoma has commonly been used to describe
biphasic tumors composed of distinguishable malignant
epithelial and sarcomatoid components with heterologous
elements.
8
For simplicity in this article, however, we have
used sarcomatoid/metaplastic carcinoma as an umbrella term
to encompass carcinosarcoma.
Immunohistochemistry plays a major role in differenti-
ating sarcomatoid carcinoma from primary breast sarco-
ma. The work-up of a tumor suspected to be metaplastic
carcinoma requires the use of more than one epithelial
marker, such as AE1/AE3, CK7, CAM 5.2, EMA, and the
high-molecular-weight cytokeratin 34E12 (K903)
8,9
be-
cause of the varied and seemingly unpredictable cytoker-
atin immunoreactivity in sarcomatoid carcinomas. Of
note, areas with cartilaginous differentiation in breast os-
teosarcoma may be focally immunoreactivate for EMA.
5,8
In case 1, as previously mentioned, the EMA immunostain
showed only a nonspecic granular cytoplasmic staining
in cartilaginous cells. Caution should be exercised with the
use of cytokeratins because a positive cytokeratin result
cannot be considered a specic feature of carcinomas: for
example, aberrant cytokeratin expression may be rarely
seen in sarcomas, including osteosarcoma.
10
Therefore, a
generous sampling of the specimen is still a crucial step
in documenting any missing component of carcinoma.
Fine-needle aspiration specimens of primary osteosar-
coma of the breast may show pleomorphic spindle cells,
osteoclast-like giant cells, and plaques of osteoid;
3,11
how-
ever, similar cytologic patterns may be seen in sarcoma-
toid carcinoma. If spindle and giant cells are the predom-
inant elements, the distinction between primary sarcoma,
Arch Pathol Lab MedVol 131, May 2007 Primary Osteosarcoma of the BreastBahrami et al 795
malignant phyllodes tumor, and sarcomatoid carcinoma
cannot be made with condence.
8
Pettinato et al
11
sug-
gested that epithelial components may be identied im-
munocytochemically by applying a spectrum of cytoker-
atins, which might help distinguish a sarcomatoid carci-
noma from a true sarcoma.
A metastatic lesion from a primary osteosarcoma of the
bone should be considered in the differential diagnosis. In
addition, the diagnosis of primary breast osteosarcoma
similar to that of other extraskeletal tumors requires the
absence of a direct connection between the tumor and the
underlying skeleton. These possibilities were excluded in
our cases because of the lack of evidence of a primary
osseous osteosarcoma, and no connection between the tu-
mors and the underlying skeleton. Osteosarcomas of the
bone chiey occur during the rst 2 decades of life.
12
In
contrast, extraskeletal osteosarcomas are rarely seen in pa-
tients younger than 40 years of age. When arising in older
patients, osteosarcoma of the bone is generally secondary
to irradiation, Paget disease of bone, or a dedifferentiated
chondrosarcoma.
Treatment should include complete surgical removal of
the tumor with an adequate margin. Although no nal
conclusion on the optimal treatment approach for these
lesions has been made, most authors have included mas-
tectomy in the standard therapy for breast sarcomas to
ensure a complete excision with adequate margins.
5,6,13
Ax-
illary lymph node dissection is essentially not indicated
because axillary node involvement is exceptional.
6,13
Sar-
comatoid carcinomas, in contrast, often require axillary
node dissection, as do primary breast carcinomas. In a
recent report, however, Carter et al
9
raised the question of
benet from axillary dissection in the absence of adenop-
athy in a subset of metaplastic carcinoma with a predom-
inant spindle component, given the rarity of lymph node
metastasis in their 29 cases. Although adjuvant combina-
tion chemotherapy has dramatically increased the survival
of patients with primary osteosarcomas of the bone, the
response of osteosarcomas of the breast is still unclear be-
cause of the limited data.
Although sarcomas of the breast usually appear to be
associated with a better prognosis than conventional
breast carcinomas of the same size,
6
reports on the prog-
nosis of patients with osteogenic sarcomas of the breast in
general indicate a poor outcome.
6,14
Exceptions to this gen-
erally aggressive behavior do occur, based on the reports
of long-term survivals among some of these patients.
15
The
5-year survival rate of primary breast osteosarcoma
among the AFIP case series was 38%, and metastases de-
veloped in 42% of cases, most commonly to the lung.
5
The
most signicant predictor of survival was tumor size.
5
Al-
though most metastases developed within 3 years of di-
agnosis,
5
late-onset metastasis to the lung, up to 9 years
after diagnosis, has been reported.
6
Among the AFIP case
series, patients with broblastic osteosarcoma had a sig-
nicantly better survival outcome than those with osteo-
blastic or osteoclastic subtypes; however, no case in that
series was classied as chondroblastic osteosarcoma.
5
Al-
though our patients have not shown any evidence of local
recurrence or hematogenous spread after mastectomy, our
follow-up is too short to allow us to predict the true be-
havior of these tumors.
In summary, we reported 2 rare examples of primary
osteosarcoma of the breast occurring in elderly patients.
This tumor must be differentiated from metaplastic car-
cinoma because these neoplastic entities have different bi-
ological behaviors and require different treatments.
The authors thank Del Rainosek, MD, for contributing case 2
and the clinical and follow-up information.
References
1. Jernstrom P, Lindberg AL, Meland ON. Osteogenic sarcoma of the mam-
mary gland. Am J Clin Pathol. 1963;40:521526.
2. Remadi S, Doussis-Anagnostopoulu I, Mac GW. Primary osteosarcoma of
the breast. Pathol Res Pract. 1995;191:471474.
3. Mertens HH, Langnickel D, Staedtler F. Primary osteogenic sarcoma of the
breast. Acta Cytol. 1982;26:512516.
4. Going JJ, Lumsden AB, Anderson TJ. A classical osteogenic sarcoma of the
breast: histology, immunohistochemistry and ultrastructure. Histopathology. 1986;
10:631641.
5. Silver SA, Tavassoli FA. Primary osteogenic sarcoma of the breast: a clini-
copathologic analysis of 50 cases. Am J Surg Pathol. 1998;22:925933.
6. Barnes L, Pietruszka M. Sarcomas of the breast: a clinicopathologic analysis
of ten cases. Cancer. 1977;40:15771585.
7. Dabbs DJ. Diagnostic Immunohistochemistry. 2nd ed. Philadelphia, Pa:
Churchill Livingstone; 2006:70.
8. Rosen PP. Rosens Breast Pathology. 2nd ed. Philadelphia, Pa: Lippincott
William & Wilkins; 2001:425453, 818819.
9. Carter MR, Hornick JL, Lester S, Fletcher CD. Spindle cell (sarcomatoid)
carcinoma of the breast: a clinicopathologic and immunohistochemical analysis
of 29 cases. Am J Surg Pathol. 2006;30:300309.
10. Okada K, Hasegawa T, Yokoyama R, Beppu Y, Itoi E. Osteosarcoma with
cytokeratin expression: a clinicopathological study of six cases with an emphasis
on differential diagnosis from metastatic cancer. J Clin Pathol. 2003;56:742746.
11. Pettinato G, Manivel JC, Petrella G, De Chiara A, Cali A. Primary osteo-
genic sarcoma and osteogenic metaplastic carcinoma of the breast: immunocy-
tochemical identication in ne needle aspirates. Acta Cytol. 1989;33:620626.
12. Sordillo PP, Hajdu SI, Magill GB, Golbey RB. Extraosseous osteogenic sar-
coma: a review of 48 patients. Cancer. 1983;51:727734.
13. Ciatto S, Bonardi R, Cataliotti L, Cardona G. Sarcomas of the breast: a
multicenter series of 70 cases. Neoplasma. 1992;39:375379.
14. Azzopardi JG. Problems in Breast Pathology. Vol 11. Philadelphia, Pa: WB
Saunders; 1979:366367.
15. Kennedy T, Biggart JD. Sarcoma of the breast. Br J Cancer. 1967;21:635
644.
Reproducedwith permission of thecopyright owner. Further reproductionprohibited without permission.
Anda mungkin juga menyukai
- The Anatomy and The Ultimate Cure of CancerDari EverandThe Anatomy and The Ultimate Cure of CancerPenilaian: 5 dari 5 bintang5/5 (2)
- 2013-Oncology New QuestionsDokumen5 halaman2013-Oncology New QuestionsHarley Justiniani Dela CruzBelum ada peringkat
- Woc Osteosarcoma WidyaDokumen1 halamanWoc Osteosarcoma WidyaWidya Agustiani0% (1)
- Meigs' Syndrome and Pseudo-Meigs' Syndrome: Report of Four Cases and Literature ReviewsDokumen6 halamanMeigs' Syndrome and Pseudo-Meigs' Syndrome: Report of Four Cases and Literature ReviewsAlfa FebriandaBelum ada peringkat
- 10 18017-Iuitfd 308495-296849Dokumen3 halaman10 18017-Iuitfd 308495-296849asshagab04Belum ada peringkat
- Askin TumorDokumen6 halamanAskin TumorGaluh Kresna BayuBelum ada peringkat
- Management Fam 1998Dokumen5 halamanManagement Fam 1998Envhy WinaBelum ada peringkat
- Nej M CPC 079007Dokumen10 halamanNej M CPC 079007Pandu Nugroho KantaBelum ada peringkat
- Cotyledonoid Dissecting Leiomyoma of The Uterus:: A Case Report and Review of The LiteratureDokumen5 halamanCotyledonoid Dissecting Leiomyoma of The Uterus:: A Case Report and Review of The Literaturedr.putra888Belum ada peringkat
- Articulo de Serie de CasoDokumen8 halamanArticulo de Serie de CasoJairo Lino BBelum ada peringkat
- Idiopathic Granulomatous Mastitis: Case Report and Review of The LiteratureDokumen4 halamanIdiopathic Granulomatous Mastitis: Case Report and Review of The LiteratureZaireen TahirBelum ada peringkat
- Lipoma of The Ovary: A Case Report and Review of The LiteratureDokumen2 halamanLipoma of The Ovary: A Case Report and Review of The LiteratureBill HarmanBelum ada peringkat
- ManuscriptDokumen5 halamanManuscriptAnisa Mulida SafitriBelum ada peringkat
- Jurnal Kedokteran Dan Kesehatan Indonesia: Indonesian Journal of Medicine and HealthDokumen7 halamanJurnal Kedokteran Dan Kesehatan Indonesia: Indonesian Journal of Medicine and HealthNio DoodohBelum ada peringkat
- Cystic Hygroma of The Neck: Single Center Experience and Literature ReviewDokumen6 halamanCystic Hygroma of The Neck: Single Center Experience and Literature ReviewLiv InkBelum ada peringkat
- Dematos 1997Dokumen6 halamanDematos 1997Ali AmokraneBelum ada peringkat
- 1 s2.0 S1930043321006816 MainDokumen5 halaman1 s2.0 S1930043321006816 MainFatimah AssagafBelum ada peringkat
- Jin-Young Lee, Dae-Bong Kim, Beom Seok Kwak,* 김어진**: Primary fibrosarcoma of the breast: a case reportDokumen12 halamanJin-Young Lee, Dae-Bong Kim, Beom Seok Kwak,* 김어진**: Primary fibrosarcoma of the breast: a case reporteosfieldBelum ada peringkat
- Adenomatoid Tumors of The Uterus: An Analysis of 60 CasesDokumen7 halamanAdenomatoid Tumors of The Uterus: An Analysis of 60 CasesGabriela OliveiraBelum ada peringkat
- Breast Carcinoma in Axillary Tail of Spence: A Rare Case ReportDokumen4 halamanBreast Carcinoma in Axillary Tail of Spence: A Rare Case Reportrajesh domakuntiBelum ada peringkat
- 49 Kishor EtalDokumen4 halaman49 Kishor EtaleditorijmrhsBelum ada peringkat
- Yang Et Al. - 2020 - Blunt Abdominal Trauma Resulting in Ovarian Mucinous Cystadenoma RuptureDokumen2 halamanYang Et Al. - 2020 - Blunt Abdominal Trauma Resulting in Ovarian Mucinous Cystadenoma RuptureJamesLeeBelum ada peringkat
- Clinical Presentation of Peritoneal MesotheliomaDokumen5 halamanClinical Presentation of Peritoneal Mesotheliomamalvina.sekolonik911Belum ada peringkat
- Fam New 4Dokumen5 halamanFam New 4novaBelum ada peringkat
- Dec24 16 PDFDokumen8 halamanDec24 16 PDFijasrjournalBelum ada peringkat
- Saimura 1999Dokumen6 halamanSaimura 1999Mariela Judith UgarteBelum ada peringkat
- 1 s2.0 S0002937816308225 MainDokumen1 halaman1 s2.0 S0002937816308225 MainowusuesselBelum ada peringkat
- Cancer de MamaDokumen5 halamanCancer de MamaRuth Lisbet Chara CharaBelum ada peringkat
- Casereports 18 125 PDFDokumen6 halamanCasereports 18 125 PDFAstrianti Kusuma WardaniBelum ada peringkat
- Lipoma of The Uterine Corpus Exceptional Eventuality Combined With An Ovarian ThecomaDokumen5 halamanLipoma of The Uterine Corpus Exceptional Eventuality Combined With An Ovarian ThecomaAlex HydronBelum ada peringkat
- Liang 2016Dokumen4 halamanLiang 2016danielleclimacoBelum ada peringkat
- Mastitis 2Dokumen4 halamanMastitis 2lolytofarisaBelum ada peringkat
- OR Case Scenario 2 - Breast CADokumen2 halamanOR Case Scenario 2 - Breast CAVictor P. DegamoBelum ada peringkat
- Jgo 21 129 PDFDokumen3 halamanJgo 21 129 PDFJuan Carlos Parraga SanclementeBelum ada peringkat
- LeíomaDokumen6 halamanLeíomaStephanie OdriozolaBelum ada peringkat
- Modern Pathol 2006 p75Dokumen8 halamanModern Pathol 2006 p75Handris SupriadiBelum ada peringkat
- Lipid Rich CarcinomaDokumen4 halamanLipid Rich CarcinomaDoctorcookies MalinauBelum ada peringkat
- Weissferdt 2015Dokumen6 halamanWeissferdt 2015gabrielnunesBelum ada peringkat
- Giant Uterine Leiomyoma - Case Report and Review of LiteratureDokumen3 halamanGiant Uterine Leiomyoma - Case Report and Review of LiteratureMan ManuelBelum ada peringkat
- A Case Report On Liposarcoma of BreastDokumen6 halamanA Case Report On Liposarcoma of BreastSunilKumar CNBelum ada peringkat
- Pinacetal Primary Uterine AngiosarcomaDokumen3 halamanPinacetal Primary Uterine AngiosarcomaIlincaBelum ada peringkat
- Acino in BreastDokumen2 halamanAcino in BreastDima PathBelum ada peringkat
- Imagenes Citologicas, Benignas, de MamaDokumen19 halamanImagenes Citologicas, Benignas, de MamaPauloRosalesBelum ada peringkat
- Relato Caso de Hpe Intra CapsularDokumen5 halamanRelato Caso de Hpe Intra Capsularalbertofigueiredo_14Belum ada peringkat
- Eyelid TattoingDokumen15 halamanEyelid Tattoing小島隆司Belum ada peringkat
- Case Report 2Dokumen6 halamanCase Report 2ancillaagraynBelum ada peringkat
- Diagnosis in Oncology: UnusualaspectsofbreastcancerDokumen5 halamanDiagnosis in Oncology: UnusualaspectsofbreastcancerLìzeth RamìrezBelum ada peringkat
- Embryonal Rhabdomyosarcoma of The Uterine Cervix: Two Cases Report and Literature ReviewDokumen7 halamanEmbryonal Rhabdomyosarcoma of The Uterine Cervix: Two Cases Report and Literature ReviewPhn StanleyBelum ada peringkat
- 2017 Laparoscopic Excision of A Uterine Adenomatoid TumorDokumen5 halaman2017 Laparoscopic Excision of A Uterine Adenomatoid Tumorpaula rangelBelum ada peringkat
- Coincidence of Thymoma and Breast Cancer in A 56 Year Old FemaleDokumen7 halamanCoincidence of Thymoma and Breast Cancer in A 56 Year Old FemaleOscar HalumBelum ada peringkat
- Abdominal Inflammatory Myofibroblastic Tumor: Report On Four Cases and Review of LiteratureDokumen6 halamanAbdominal Inflammatory Myofibroblastic Tumor: Report On Four Cases and Review of LiteratureLisma EkayantiBelum ada peringkat
- 10.1164@ajrccm Conference.2011.183.1 Meetingabstracts.a6458Dokumen2 halaman10.1164@ajrccm Conference.2011.183.1 Meetingabstracts.a6458Luis Gutierrez GarciaBelum ada peringkat
- LeiomyomaDokumen3 halamanLeiomyomacandiddreamsBelum ada peringkat
- Journal On CancerDokumen5 halamanJournal On CancerChriz LechBelum ada peringkat
- Sister Mary Joseph NoduleDokumen3 halamanSister Mary Joseph NoduleNazir KhanBelum ada peringkat
- Krukenberg Tumour Simulating Uterine Fibroids and Pelvic Inflammatory DiseaseDokumen3 halamanKrukenberg Tumour Simulating Uterine Fibroids and Pelvic Inflammatory DiseaseradianrendratukanBelum ada peringkat
- Chondrosarcoma of Breast - A Case ReportDokumen2 halamanChondrosarcoma of Breast - A Case ReportInternational Journal of Innovative Science and Research TechnologyBelum ada peringkat
- Finger Clubbing and A Lung MassDokumen5 halamanFinger Clubbing and A Lung MassSeptinaAyuSamsiatiBelum ada peringkat
- Tumeurs Ovariennes A Cellulles de La Granulosa Juveniles (1) DjeltiDokumen7 halamanTumeurs Ovariennes A Cellulles de La Granulosa Juveniles (1) DjeltiDjelti SihemBelum ada peringkat
- BREAST - Surg Quiz Rationale From BookDokumen3 halamanBREAST - Surg Quiz Rationale From BookLady ElocinBelum ada peringkat
- Breast, General PathologyDokumen3 halamanBreast, General PathologyKavi SelvanBelum ada peringkat
- Walked out of the New Road to Conquer Cancer: Walked out of the New Way of Cancer Treatment with Immune Regulation and Control of Combination of Chinese and Western MedicineDari EverandWalked out of the New Road to Conquer Cancer: Walked out of the New Way of Cancer Treatment with Immune Regulation and Control of Combination of Chinese and Western MedicineBelum ada peringkat
- Black Box Warnings: Renal Failure Patients: Masters SB (2012)Dokumen16 halamanBlack Box Warnings: Renal Failure Patients: Masters SB (2012)Hendrikus Surya Adhi PutraBelum ada peringkat
- Single Versus Multi-Incisional Video-Assisted Thoracic Surgery: A Systematic Review and Meta-AnalysisDokumen12 halamanSingle Versus Multi-Incisional Video-Assisted Thoracic Surgery: A Systematic Review and Meta-AnalysisHendrikus Surya Adhi PutraBelum ada peringkat
- Acute Pain Management in Patients With Opioid ToleranceDokumen6 halamanAcute Pain Management in Patients With Opioid ToleranceHendrikus Surya Adhi PutraBelum ada peringkat
- Development at Any Phase Is Always Linked With Technology and TechnologyDokumen1 halamanDevelopment at Any Phase Is Always Linked With Technology and TechnologyHendrikus Surya Adhi PutraBelum ada peringkat
- Severe Sepsis and Septic Shock: Taking Advantage of Window of Opportunity. CMAJDokumen1 halamanSevere Sepsis and Septic Shock: Taking Advantage of Window of Opportunity. CMAJHendrikus Surya Adhi PutraBelum ada peringkat
- 4 DapusDokumen1 halaman4 DapusHendrikus Surya Adhi PutraBelum ada peringkat
- Glion StudentsExcercise TemplateDokumen49 halamanGlion StudentsExcercise TemplateHendrikus Surya Adhi PutraBelum ada peringkat
- Enuresis DataDokumen9 halamanEnuresis DataHendrikus Surya Adhi PutraBelum ada peringkat
- Kidney Stones & Uretral Stones: What Are Stones and The Difference Between Kidney Stones and Ureteral Stones?Dokumen21 halamanKidney Stones & Uretral Stones: What Are Stones and The Difference Between Kidney Stones and Ureteral Stones?Hendrikus Surya Adhi PutraBelum ada peringkat
- Blood Group IncompatibilityDokumen7 halamanBlood Group IncompatibilityHendrikus Surya Adhi PutraBelum ada peringkat
- Attention Deficit Hyperactivity Disorder and Enuresis in Children and AdolescentsDokumen8 halamanAttention Deficit Hyperactivity Disorder and Enuresis in Children and AdolescentsHendrikus Surya Adhi PutraBelum ada peringkat
- Resep ObatDokumen1 halamanResep ObatHendrikus Surya Adhi PutraBelum ada peringkat
- Teaching Photo Radiology: Thorax EditionDokumen0 halamanTeaching Photo Radiology: Thorax EditionHendrikus Surya Adhi PutraBelum ada peringkat
- Bone TumorsDokumen15 halamanBone Tumorssarguss1450% (2)
- Endometrial CancerDokumen2 halamanEndometrial CancerfionalausBelum ada peringkat
- Esophageal CancerDokumen66 halamanEsophageal Canceroezeamuzie100% (1)
- Histopathology Photos - Part 1Dokumen86 halamanHistopathology Photos - Part 1solom islamBelum ada peringkat
- OBGYN Invasive Cervical Cancer ArticleDokumen7 halamanOBGYN Invasive Cervical Cancer ArticleVanessa HermioneBelum ada peringkat
- Clinical Oncology AssignmentDokumen10 halamanClinical Oncology Assignmentapi-635186395Belum ada peringkat
- Oral Squamous Cell CarcinomaDokumen31 halamanOral Squamous Cell CarcinomaAli Rahimi100% (1)
- Athymic Nude MouseDokumen2 halamanAthymic Nude MouseFCiênciasBelum ada peringkat
- Comparing Breast-Conserving Surgery With Radical MastectomyDokumen6 halamanComparing Breast-Conserving Surgery With Radical MastectomyRonald Cariaco FlamesBelum ada peringkat
- Pediatric Brain TumorsDokumen19 halamanPediatric Brain TumorsTezza DinayantiBelum ada peringkat
- Handbook of Ovarian CancerDokumen264 halamanHandbook of Ovarian CancerGina R100% (1)
- 702 1383 1 PB PDFDokumen7 halaman702 1383 1 PB PDFEko RistiyantoBelum ada peringkat
- Trabajo 3. BRAF GeneDokumen15 halamanTrabajo 3. BRAF GenejordyeeBelum ada peringkat
- Colorectal CancerDokumen27 halamanColorectal CancerDwi P SugiartiBelum ada peringkat
- Pathology Outlines - Staging-CarcinomaDokumen1 halamanPathology Outlines - Staging-Carcinomayolanda sukarmaBelum ada peringkat
- Neoplasms of Nose & para Nasal SinusesDokumen171 halamanNeoplasms of Nose & para Nasal SinusesJulian HobbsBelum ada peringkat
- Breast Case ProformaDokumen2 halamanBreast Case ProformaSyed FirdozBelum ada peringkat
- Kanker Ovarium: Dr. Dino Rinaldy, SP - OG (K) Onk RSUD Dr. H. Abdul MoeloekDokumen16 halamanKanker Ovarium: Dr. Dino Rinaldy, SP - OG (K) Onk RSUD Dr. H. Abdul MoeloekIlham HarsyaBelum ada peringkat
- Epithelial Cell Abnormalities - GlandularDokumen34 halamanEpithelial Cell Abnormalities - Glandularsarguss14Belum ada peringkat
- Kolorektal & Anus: Dr. Yusmaidi, SP.BDokumen104 halamanKolorektal & Anus: Dr. Yusmaidi, SP.BAsmorowatiBelum ada peringkat
- Degenerasi Hialin Pada: Pyelonefritis KronikDokumen16 halamanDegenerasi Hialin Pada: Pyelonefritis KronikNininghrBelum ada peringkat
- Mutation of The PIK3CA Oncogene in Human Cancers: MinireviewDokumen5 halamanMutation of The PIK3CA Oncogene in Human Cancers: Minireviewmilenerato2240Belum ada peringkat
- Lord'S Angels Montessori School, Inc. M - /20 A - /20 H - /20Dokumen4 halamanLord'S Angels Montessori School, Inc. M - /20 A - /20 H - /20Rose Ann VillanuevaBelum ada peringkat
- SolidtumorsinchildrenDokumen24 halamanSolidtumorsinchildrenPriyaBelum ada peringkat
- Cancer Research and WebquestDokumen4 halamanCancer Research and Webquestapi-418022315Belum ada peringkat
- Chapter 29 Child With CancerDokumen9 halamanChapter 29 Child With CancerMARCERA JERALDINE ALESSA P.Belum ada peringkat
- Ovarian Cancer DissertationDokumen5 halamanOvarian Cancer DissertationHelpWritingPaperYonkers100% (1)
- Breast LumpDokumen17 halamanBreast LumpgaganBelum ada peringkat